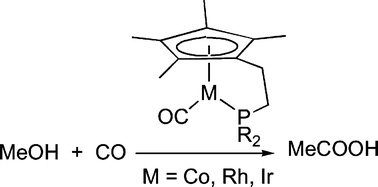
2-Phosphanylethylcyclopentadienyl lithium compounds, Li[C5R′4(CH2)2PR2] (R = Et, R′ = H or Me, R = Ph, R′ = Me), have been prepared from the reaction of spirohydrocarbons C5R′4(C2H4) with LiPR2. C5Et4HSiMe2CH2PMe2, was prepared from reaction of Li[C5Et4] with Me2SiCl2 followed by Me2PCH2Li. The lithium salts were reacted with [RhCl(CO)2]2, [IrCl(CO)3] or [Co2(CO)8] to give [M(C5R′4(CH2)2PR2)(CO)] (M = Rh, R = Et, R′ = H or Me, R = Ph, R′ = Me; M = Ir or Co, R = Et, R′ = Me), which have been fully characterised, in many cases crystallographically as monomers with coordination of the phosphorus atom and the cyclopentadienyl ring. The values of νCO for these complexes are usually lower than those for the analogous complexes without the bridge between the cyclopentadienyl ring and the phosphine, the exception being [Rh(Cp′(CH2)2PEt2)(CO)] (Cp′ = C5Me4), the most electron rich of the complexes. [Rh(C5Et4SiMe2CH2PMe2)(CO)] may be a dimer. [Co2(CO)8] reacts with C5H5(CH2)2PEt2 or C5Et4HSiMe2CH2PMe2 (L) to give binuclear complexes of the form [Co2(CO)6L2] with almost linear PCoCoP skeletons. [Rh(Cp′(CH2)2PEt2)(CO)] and [Rh(Cp′(CH2)2PPh2)(CO)] are active for methanol carbonylation at 150 °C and 27 bar CO, with the rate using [Rh(Cp′(CH2)2PPh2)(CO)] (0.81 mol dm−3 h−1) being higher than that for [RhI2(CO)2]− (0.64 mol dm−3 h−1). The most electron rich complex, [Rh(Cp′(CH2)2PEt2)(CO)] (0.38 mol dm−3 h−1) gave a comparable rate to [Cp*Rh(PEt3)(CO)] (0.30 mol dm−3 h−1), which was unstable towards oxidation of the phosphine. [Rh(Cp′(CH2)2PEt2)I2], which is inactive for methanol carbonylation, was isolated after the methanol carbonylation reaction using [Rh(Cp′(CH2)2PEt2)(CO)]. Neither of [M(Cp′(CH2)2PEt2)(CO)] (M = Co or Ir) was active for methanol carbonylation under these conditions, nor under many other conditions investigated, except that [Ir(Cp′(CH2)2PEt2)(CO)] showed some activity at higher temperature (190 °C), probably as a result of degradation to [IrI2(CO)2]−. [M(Cp′(CH2)2PEt2)(CO)] react with MeI to give [M(Cp′(CH2)2PEt2)(C(O)Me)I] (M = Co or Rh) or [Ir(Cp′(CH2)2PEt2)Me(CO)]I. The rates of oxidative addition of MeI to [Rh(C5H4(CH2)2PEt2)(CO)] and [Rh(Cp′(CH2)2PPh2)(CO)] are 62 and 1770 times faster than to [Cp*Rh(CO)2]. Methyl migration is slower, however. High pressure NMR studies show that [Co(Cp′(CH2)2PEt2)(CO)] and [Cp*Rh(PEt3)(CO)] are unstable towards phosphine oxidation and/or quaternisation under methanol carbonylation conditions, but that [Rh(Cp′(CH2)2PEt2)(CO)] does not exhibit phosphine degradation, eventually producing inactive [Rh(Cp′(CH2)2PEt2)I2] at least under conditions of poor gas mixing. The observation of [Rh(Cp′(CH2)2PEt2)(C(O)Me)I] under methanol carbonylation conditions suggests that the rhodium centre has become so electron rich that reductive elimination of ethanoyl iodide has become rate determining for methanol carbonylation. In addition to the high electron density at rhodium.

 Please wait while we load your content...
Please wait while we load your content...