Electron localization in negatively charged formamide clusters studied by photodetachment spectroscopy
Abstract
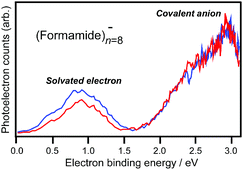
Size-dependent features of the electron localization in negatively charged formamide clusters (FAn−, n
= 5–21) have been studied by photodetachment

 Please wait while we load your content...
Please wait while we load your content...