A metal–organic framework material that functions as an enantioselective catalyst for olefin epoxidation†
Abstract
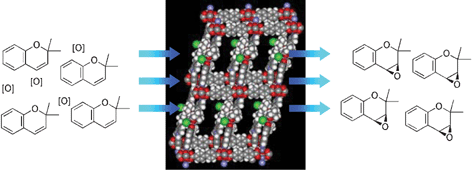
A new microporous metal–organic framework compound featuring chiral (salen)Mn struts is highly effective as an asymmetric
- This article is part of the themed collection: ChemComm 60th Anniversary Historic Papers from North America

 Please wait while we load your content...
Please wait while we load your content...