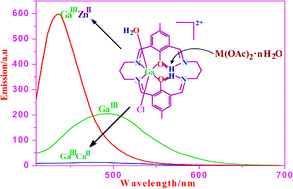
Mononuclear aluminium(III), gallium(III) and indium(III) complexes [MIII(LH2)(H2O)Cl](ClO4)2·nH2O (1–3) of the tetraiminodiphenolate macrocyclic ligand L2− have been synthesized. The two uncoordinated imine nitrogens of these compounds are protonated and hydrogen bonded to the metal-bound phenolate oxygens to stabilize them against hydrolytic cleavage. The gallium(III) compound, 2, forms the heterodinuclear complexes [GaIIIMIIL(μ-OAc)(OAc)(H2O)][ClO4] (M = Co, 4; Ni, 5) and [GaIIIMIIL(μ-OAc)(OAc)][ClO4]·2H2O (M = Cu, 6; Zn, 7). The presence of the core unit [GaIIIMIIL(μ-OAc)(OAc)]+ in all the compounds has been ascertained by ESI-MS measurements. Complexes 1–3 show luminescence at room temperature between 505 and 490 nm. Spectrofluorimetric titrations of 2 with Zn(OAc)2·2H2O and Cu(OAc)2·H2O in acetonitrile have shown that the formation of a 1 ∶ 1 GaIIIZnII complex occurs with the growth of luminescence intensity, while the formation of a GaIIICuII complex leads to the complete quenching of luminescence. Complexes 1–7 have been further characterized by their proton NMR spectra.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...