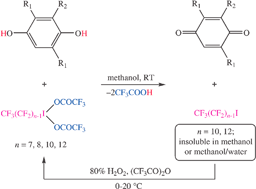
Reactions of commercial fluorous alkyl iodides RfnI (1-Rfn; Rfn = CF3(CF2)n−1; n = 7, 8, 10, 12) with 80% H2O2 and trifluoroacetic anhydride give RfnI(OCOCF3)2 (2-Rfn; 89–97%). These rapidly oxidize 1,4-hydroquinones in methanol. Subsequent additions of CF3C6F11 or FC-72 give liquid/liquid biphase systems. The product quinones are generally isolated in ≥95% yields from the methanol phases, and 1-Rfn in ≥95% yields from the fluorous phases. Alternatively, the very low solubilities of 1-Rf10 in 10 ∶ 1 v/v methanol ∶ water or 1-Rf12 in methanol allow efficient recovery via solid/liquid phase separations without recourse to fluorous solvents. The recovered 1-Rfn may be reoxidized to 2-Rfn and reused.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...