New examples of metalloaromatic Al-clusters: (Al4M4)Fe(CO)3 (M = Li, Na and K) and (Al4M4)2Ni: rationalization for possible synthesis†
Abstract
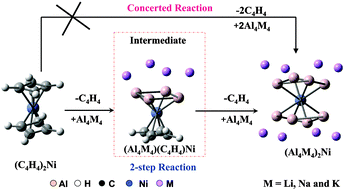
Ab initio calculations reveal that all-metal antiaromatic molecules like Al4M4 (M = Li, Na and K) can be stabilized in half sandwich (Al4M4)Fe(CO)3 and full sandwich (Al4M4)2Ni complexes. The formation of the full sandwich complex [(Al4M4)2Ni] from its organometallic precursor depends on the stability of the organic–inorganic hybrid (C4H4)Ni(Al4Li4).

 Please wait while we load your content...
Please wait while we load your content...