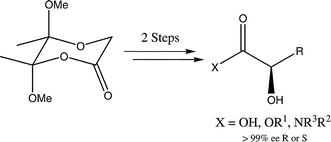
Preparation of enantiopure butane-2,3-diacetals of glycolic acid and alkylation reactions leading to α-hydroxyacid and amide derivatives
Abstract
The preparation of butane-2,3-diacetal protected glycolic acid and related systems is described together with highly selective alkylation reactions of (R,R) and (S,S) butanediacetal protected glycolic acid. These compounds are readily deprotected to give enantiopure α-hydroxyacids, α-hydroxyesters or α-hydroxyamides by suitable choice of conditions.

 Please wait while we load your content...
Please wait while we load your content...