The Lycopodium alkaloids
Abstract
Covering: 1993–2004
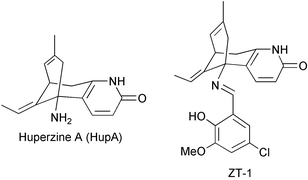
Lycopodium alkaloids are quinolizine, or pyridine and α-pyridone type alkaloids. Some Lycopodium alkaloids are potent inhibitors of acetylcholinesterase (AChE). Huperzine A (HupA) is reported to increase efficiency for learning and memory in animals, and it shows promise in the treatment of Alzheimer's disease (AD). 201 Lycopodium alkaloids from 54 species of Lycopodium (sensu lato) have been reported so far. This review is intended to cover the chemical, pharmacological and clinical research on Lycopodium alkaloids reported in the literature from the spring of 1993 to August 2004. Structures of 81 new Lycopodium alkaloids are presented, classified and analyzed. The structural characters and biogenetic relationships of the four major Lycopodium alkaloid groups (lycopodine, lycodine, fawcettimine and miscellaneous) are discussed. Bioactivities of Lycopodium alkaloids, especially HupA, are summarized. In particular, the effect of HupA and other cholinesterase inhibitors (anti-AD drugs) on acetylcholine esterase (AChE) activity in the rat cortex and butylcholine esterase activity are compared. Structure–activity relationships and structure modifications of HupA and its analogs are described. Information on clinical trials with HupA and its derivative ZT-1 is presented. The state of HupA availability and recent advances in in vitro propagation of HupA producing plants are outlined. Finally, hypotheses about Lycopodium alkaloid biosynthetic pathways are discussed.

 Please wait while we load your content...
Please wait while we load your content...