Synthesis of organochalcogens stabilized by intramolecular non-bonded interactions of sterically unhindered 2-phenyl-2-oxazoline†
Abstract
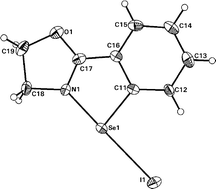
The synthesis and characterization of low-valent organoselenium and -sulfur compounds incorporating sterically unhindered

 Please wait while we load your content...
Please wait while we load your content...