Nickel(iii) oxidation of its glycylglycylhistamine complex†
Abstract
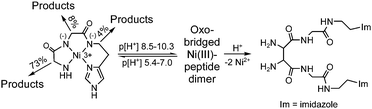
The doubly-deprotonated Ni(III) complex of Gly2Ha (where Ha is histamine) undergoes base-assisted oxidative self-decomposition of the peptide. At ≤ p[H+] 7.0, a major pathway is a two-electron oxidation at the α-carbon of the N-terminal glycyl residue. Major products (up to 73%) of this two-electron oxidation are glyoxylglycylhistamine and ammonia. Glyoxylglycylhistamine will decay to give isocyanatoacetylhistamine and formaldehyde. Two-electron oxidations of the second glycyl and histamine residues occur as minor pathways (12% of the total possible reaction). Above p[H+] 8.5, two Ni(III)–peptide complexes form an oxo bridge in the axial positions to give a reactive dimer species. This proximity allows the resulting Ni(II)–peptide radical intermediates to undergo peptide–peptide cross-linking at the N-terminal glycyl residues. The products found below p[H+] 7.0 are observed above p[H+] 8.5 as well, although in lower yields. In contrast to this work, NiIII(H−2Gly2HisGly) undergoes a four-electron oxidation at the N-terminal glycyl residue. Oxidation at the internal glycyl and histidyl residues are not observed. The reactivity of NiIII(H−2Gly2Ha)+ is also different than CuIII(H−2Gly2Ha)+, which undergoes a two-electron oxidation at the histamine group with no peptide–peptide cross-linking in basic solution.

 Please wait while we load your content...
Please wait while we load your content...