Precise structure activity relationships in asymmetric catalysis using carbohydrate scaffolds to allow ready fine tuning: dialkylzinc–aldehyde additions†
Abstract
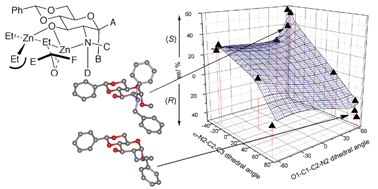
The ready construction of 24 stereochemically and functionally diverse carbohydrate ligand structures from a core D-glucosamine scaffold has allowed the evaluation of broad ranging structure activity relationships in ligand accelerated zincate additions to aldehydes, with variations in ΔΔG‡(R-S) of up to 5650 J mol−1 that create opposing senses of asymmetric induction and that are consistent with models based on several ligand X-ray structures and molecular mechanics analysis. Factorial analysis of enantioselectivity using key dihedral angles and steric volume on N-2 also highlight the potential for the use of factorial design in ligand construction.

 Please wait while we load your content...
Please wait while we load your content...