Optically pure β-substituted β-hydroxy aspartates as glutamate transporter blockers
Abstract
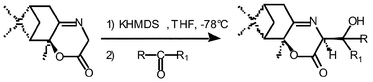
A short asymmetric synthesis of optically pure β-substituted β-hydroxy aspartates is described. The key step is an aldol reaction between a glycine enolate derived from an oxazinone intermediate used as chiral auxiliary and various α-keto

 Please wait while we load your content...
Please wait while we load your content...