Synthesis of (R)-2-methyl-4-deoxy and (R)-2-methyl-4,5-dideoxy analogues of 6-phosphogluconate as potential inhibitors of 6-phosphogluconate dehydrogenase†
Abstract
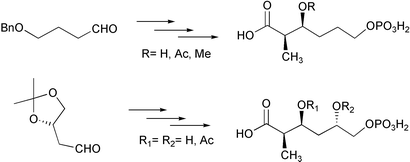
The synthesis of (2R)-2-methyl-4,5-dideoxy and (2R)-2-methyl-4-deoxy analogues of 6-phosphogluconate is described. The synthetic strategy relies on the Evans aldol reaction for the installation of the chiral centres in the 2- and 3-positions. The selective phosphorylation at the primary alcohol function of

 Please wait while we load your content...
Please wait while we load your content...