Mercury(ii) partitioning from aqueous solutions with a new, hydrophobic ethylene-glycol functionalized bis-imidazolium ionic liquid†
Abstract
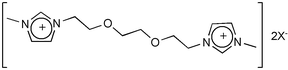
A room temperature ionic liquid containing a bis-imidazolium cation incorporating a short ethylene-glycol spacer, 1,1′-[1,2-ethanediylbis(oxy-1,2-ethanediyl)] bis[3-methyl-1H-imidazolium-1-yl]bis(trifluoromethanesulfonyl)imide, has been prepared from the corresponding chloride salt, and the X-ray crystal structure of the low-melting hexafluorophosphate salt has been determined. The crystal structure reveals the ether linkage to be quite flexible and to participate in strong C2–H⋯O hydrogen bonds leading to asymmetry. The crystal structure of the bis-imidazolium salt incorporating a decyl-spacer, 1,1′-[1,10-decyl]bis[3-methyl-1H-imidazolium-1-yl] hexafluorophosphate, has also been determined and displays an all-trans (symmetric) conformation except at the beta carbon positions where a characteristic kink is observed. Introducing the ethylene-glycol functionality dramatically increases the distribution ratio of mercury ions, but not caesium, from aqueous solution to the hydrophobic ionic liquid, and from basic solution. This is the first example of pH dependent partitioning and stripping of mercury from ionic liquid/aqueous two-phase systems. The crystal structure of the related mercury(II) carbene complex, obtained from the reaction of mercury(II) acetate with 1,1′-[oxybis(2,1-ethanediyloxy-2,1-ethanediyl)]bis[3-methyl-1H-imidazolium-1-yl] tosylate, containing a three-ether spacer, in acetonitrile, reveals the possibility of a carbene extraction mechanism.

 Please wait while we load your content...
Please wait while we load your content...