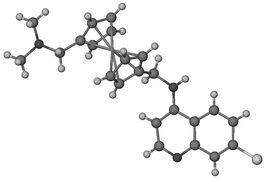
The synthesis of the new compounds (7-chloroquinolin-4-yl)-N′-(1′-dimethylaminomethylferrocen-1-ylmethyl)-amine (4a) and N-(7-chloroquinolin-4-yl)-N′-(1′-dimethylaminomethylferrocen-1-ylmethyl)-ethane-1,2-diamine (6a) is reported. The key step in the synthesis is the cleavage of a ferrocene–Sn bond with n-BuLi to give a lithiumferrocenide species (10), which is then treated with an electrophile. Thus, 1′-dimethylaminomethyl-1-tri-n-butylstannyl-ferrocene (11) and subsequently 1′-dimethylaminomethylferrocene-1-carbaldehyde (7a) were synthesised from 1,1′-bis(tri-n-butylstannyl)ferrocene, employing [CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe2]I and DMF to introduce the amine and then the aldehyde functionalities. In addition, the compound 1′-dimethylaminomethyl-1-lithiumferrocenide was isolated and the 1H and 13C NMR data are reported. X-Ray crystal and molecular structures are reported for compound 4a and the related compound N-(7-chloroquinolin-4-yl)-N′-(2-dimethylaminomethylferrocen-1-ylmethyl)-ethane-1,2-diamine (5a). The antiplasmodial activity in vitro against chloroquine sensitive and resistant strains of Plasmodium falciparum is reported and compared to a series of ferrocene, ruthenocene and phenylene analogues.
NMe2]I and DMF to introduce the amine and then the aldehyde functionalities. In addition, the compound 1′-dimethylaminomethyl-1-lithiumferrocenide was isolated and the 1H and 13C NMR data are reported. X-Ray crystal and molecular structures are reported for compound 4a and the related compound N-(7-chloroquinolin-4-yl)-N′-(2-dimethylaminomethylferrocen-1-ylmethyl)-ethane-1,2-diamine (5a). The antiplasmodial activity in vitro against chloroquine sensitive and resistant strains of Plasmodium falciparum is reported and compared to a series of ferrocene, ruthenocene and phenylene analogues.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe2]I and DMF to introduce the
NMe2]I and DMF to introduce the 

 Please wait while we load your content...
Please wait while we load your content...