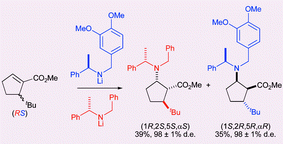
Preparation of methyl (1R,2S,5S)- and (1S,2R,5R)-2-amino-5-tert-butyl-cyclopentane-1-carboxylates by parallel kinetic resolution of methyl (RS)-5-tert-butyl-cyclopentene-1-carboxylate
Abstract
Comparison of the kinetic and parallel kinetic resolutions of

 Please wait while we load your content...
Please wait while we load your content...