Selective catalytic oxidation of benzyl alcohol and alkylbenzenes in ionic liquids
Abstract
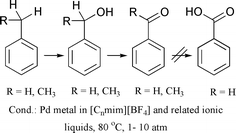
Industrially performed catalytic oxidation reactions often suffer from drawbacks such as poor conversion and selectivity due to over-oxidation, corrosive reaction media, lack of solvent and catalyst recycling, and negative environmental impact due to evaporation of the solvents. In order to provide a methodology that addresses these problems, ionic liquids have been investigated as reaction media. For the example of the oxidation of benzyl alcohol to benzaldehyde (dehydrogenation), it was shown that the palladium metal catalysed oxidation can be brought about, leading to better TOFs than those observed in dimethyl sulfoxide, with the added advantage of facile catalyst and solvent recycling. It was found that the selectivity to benzaldehyde is strongly dependent on the level of chloride ion, which leads to the formation of dibenzyl ether. Secondly, the amount of water present in the ionic liquid determines the extent of benzoic acid formation. Interestingly, ionic liquids are able to deactivate the water formed in the oxidation of benzyl alcohol and thus prevent it from further reaction to benzoic acid. The oxidation of toluene and ethylbenzene showed that the introduction of oxygen into the molecule is feasible using the same methodology.

 Please wait while we load your content...
Please wait while we load your content...