Receptor versatility of tris(pyridin-1-ium-2-ylmethyl)amine in anion binding through hydrogen bonding†
Abstract
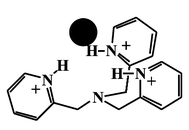
The receptor ability of tris(pyridin-1-ium-2-ylmethyl)amine (triprotonated tris(2-pyridylmethyl)amine, H3TPA3+) toward inorganic anions such as PF6−, CF3SO3−, Br−, and Cl− was investigated. Several spectroscopic studies revealed that H3TPA3+ offered characteristic receptor selectivity in the anion complexation. Structural analysis of the receptor-anion complexes [H3TPA(PF6)](PF6)2, [H3TPA(CF3SO3)](CF3SO3)(PF6), [H3TPA(Br)](PF6)2, and [H3TPA(Cl)](PF6)2 indicated that the H3TPA3+ receptor nicely caught each anion guest (X−) in its three dimensional cavity via hydrogen bonding (X⋯H⋯N) with pyridinium groups. Treatment of the [H3TPA(X)]2+ (X = PF6− or CF3SO3−) complex with a large excess of KBr in the solid state led to the replacement of PF6− or CF3SO3− with Br− to give [H3TPA(Br)]2+, whereas Cl− in the [H3TPA(Cl)]2+ complex was not replaced. The PF6− anions located in and out of the H3TPA3+ cavity were replaced stepwise with the added Cl− or Br−anion. The strength of the hydrogen bonding increased as the pKb value of the anion decreased in the series Cl− > Br− > CF3SO3− > PF6−.

 Please wait while we load your content...
Please wait while we load your content...