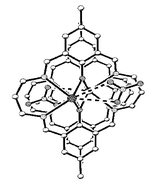
The syntheses and crystal structures of the lead(II) complexes [Pb(LH2)(ClO4)][ClO4], [Pb(LH2)(NO3)2] and [Pb2L(NO3)2] of the tetraiminodiphenol macrocyclic ligand (H2L) derived from a [2+2] condensation reaction between 2,6-diformyl-4-methylphenol and 1,3-diaminopropane are reported. In the mononuclear complexes, the two uncoordinated imino nitrogens are protonated and are hydrogen bonded to the phenolate oxygens. A supramolecular assembly occurs for [Pb(LH2)(ClO4)][ClO4], due to weak interactions between the metal and three oxygen atoms of three different symmetry-related perchlorates, thereby forming a hexameric species with a propeller structure. [Pb(LH2)(NO3)2], however, is a monomer with normal bidentate binding modes for the nitrates. By contrast, [Pb2L(NO3)2]
exhibits a 2-D structural network comprising parallel chains from two independent [Pb2L]2+ units, to which the nitrate anions are associated rather unconventionally: one oxygen is coordinated to two symmetry-related metal centres, another oxygen to a single lead, while the third oxygen remains free. The structural features of the complexes in solution have been investigated by 1H NMR spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...