Kinetics of oxidation of ascorbic acid and 1,4-dihydroxybenzene by semiquinone radical bound to ruthenium(ii)
Abstract
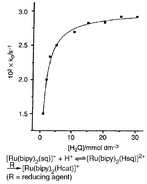
In 40% (v/v) MeOH–H2O media, containing [H+] (0.001–0.038 mol dm−3), the semiquinone (sq) radical, bound to Ru(II) in [Ru(bpy)2(sq)]+1, oxidises ascorbic acid (H2A) to dehydroascorbic acid (A), and 1,4-dihydroxybenzene (H2Q) to p-benzoquinone (Q); 1 is itself reduced to [Ru(bpy)2(Hcat)]+2H. The reactions are centred at sq not Ru(II). The sq/cat couple in 1 is reversible and its E1/2 increases with increasing [H+]; rate of chemical reduction of 1 to 2H increases in parallel. Rate increases also with increasing mole percent of D2O in the solvent suggesting a preliminary protonation equilibrium producing 1H, in which a H+ binds to the π-electron cloud of Ru(II)-bound sq. Under the experimental conditions, the kinetically significant species are 1H, H2Q, H2A and HA−. The kinetic activity of HA− ion is only ≈200 times more than that of H2A. This testifies against a purely outer-sphere mechanism and suggests significant electronic interaction between the redox partners. Increased percentage of MeOH in the solvent decreases λmax for the LMCT band; reaction rate for ascorbic acid decreases in parallel.

 Please wait while we load your content...
Please wait while we load your content...