The cyclisation of substituted phthalanilic acids in acetic acid solution. A kinetic study of substituted N-phenylphthalimide formation
Abstract
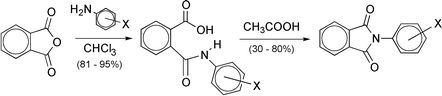
One novel and ten known substituted 3′- and 4′-phthalanilic acids have been prepared. These have been cyclised to two novel and nine known substituted N-phenylphthalimides by heating with

 Please wait while we load your content...
Please wait while we load your content...