Asymmetric synthesis of β-haloaryl β-amino acid derivatives
Abstract
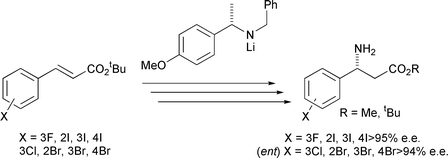
Lithium N-benzyl-N-α-methyl-4-methoxybenzylamide may be employed as a homochiral ammonia equivalent for the synthesis of homochiral β-haloaryl β-amino acid derivatives via a strategy involving its

 Please wait while we load your content...
Please wait while we load your content...