Thermodynamic and kinetic hydricity of transition metal hydrides†
Abstract
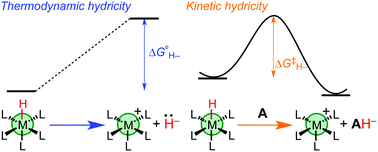
The prevalence of transition metal-mediated hydride transfer reactions in chemical synthesis, catalysis, and biology has inspired the development of methods for characterizing the reactivity of transition metal hydride complexes. Thermodynamic hydricity represents the free energy required for heterolytic cleavage of the metal–hydride bond to release a free hydride ion, H−, as determined through equilibrium measurements and thermochemical cycles. Kinetic hydricity represents the rate of hydride transfer from one species to another, as measured through kinetic analysis. This tutorial review describes the common methods for experimental and computational determination of thermodynamic and kinetic hydricity, including advice on best practices and precautions to help avoid pitfalls. The influence of solvation on hydricity is emphasized, including opportunities and challenges arising from comparisons across several different solvents. Connections between thermodynamic and kinetic hydricity are discussed, and opportunities for utilizing these connections to rationally improve catalytic processes involving hydride transfer are highlighted.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...
