Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Active site exploration of core–corona structured bifunctional cobalt ferrite-containing nitrogen-doped carbon nanotubes for rechargeable zinc–air battery application†
Shankar
Baskaran
,
E. A. Anook
Nazer
and
Azhagumuthu
Muthukrishnan
 *
*
School of Chemistry, Indian Institute of Science Education and Research Thiruvananthapuram, Maruthamala P.O., Vithura 695551, Kerala, India. E-mail: muthukrishnan@iisertvm.ac.in
First published on 15th March 2024
Abstract
First-row transition metal-containing bifunctional electrocatalysts are essential in developing metal–air batteries with high cycling efficiency. Core–corona structured porous N-doped carbon with tangled carbon nanotubes was synthesised by pyrolysing CoFe2O4 encapsulated ZIF-8. Structural characterisation indicates the presence of the CoFe metal alloy, which catalyses the formation of nanotubes. Besides, the CoFe alloy nanoparticles encapsulated by graphitic carbon improve the oxygen reduction and evolution reactions, corroborated by control samples. The bifunctional activity of the catalysts was analysed using the zinc–air battery, which exhibits an excellent power density of 162 mW cm−2 and a high specific capacity of 900 mA h gZn−1. The real-time application was evaluated using the solid-state zinc–air battery, which exhibits the highest open circuit potential of 1.53 V. The CoFe alloy encapsulated N-doped carbon should be an efficient air-electrode catalyst for metal–air battery application.
Introduction
Rechargeable metal–air batteries have attracted much attention and are promising potential alternatives to the dominant Li-ion batteries in portable electronic devices. Zinc–air batteries (ZAB) are rapidly growing due to their high theoretical energy density and air-stable electrodes.1–3 The development of low-cost and durable bifunctional electrocatalysts is one of the bottlenecks for the ZAB application in portable and wearable electronic devices. Although the combination of precious metals/metal oxides is used as catalysts, the development of electrocatalysts based on first-row transition metal elements is reported with higher efficiency.4–6 Bifunctional activity of catalytic materials is preferred due to the two different reactions in ZABs, namely the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) during charging and discharging, respectively. First-row transition metal oxides,7 chalcogenides,8–10 nitrides,11 metal alloys,12 and bimetallic oxides (spinels and perovskites) can bring bifunctional activity.13–17 Fe–N–C catalysts are well-developed for their ORR activity, whereas metal oxides are mostly known for their OER activity. Hence, bimetallic electrocatalysts are preferred as bifunctional catalysts in ZABs due to the different metal active sites for the ORR and OER.Bimetallic spinel oxides are widely studied for their ORR and OER activity and their applications in fuel cells and ZABs.18–21 The presence of two metal ions in spinel oxides brought bifunctional catalytic activity (ORR and OER). Although several spinel compounds were reported for the ORR and OER activity, Co and Fe-based spinel materials are primarily known.15,22,23 It may be attributed to the two metal active sites Fe and Co responsible for ORR and OER activity, respectively.23 Besides, the activity can be improved by oxygen vacancies in spinel oxides. Bimetallic alloys on carbon supports (synthesised using the typical heat-treatment method) improve the ORR activity. The CoFe alloy was reported for its enhanced bifunctional activity due to the synergistic effect of both metals.15,24,25 The role of carbon supports along with the metal active sites is very important. Carbon supports help to improve the conductivity and stability of metal/metal-oxide nanoparticles. Porous carbon materials derived from zeolitic imidazolate metal–organic frameworks could be perfect N-doped carbon materials.26–28 ZIF-8 is a single carbon and nitrogen source to synthesise N-doped carbon, and is more active than pure carbon supports. Further, the ZIF-8 material yields porous N-doped carbon, which helps to improve mass transport.29–31 The CoFe alloy was synthesised on the ZIF, followed by pyrolysis which leads to the CoFe–N/C materials.
Co and Fe-containing bimetallic compounds yield promising bifunctional activity, reported in recent days. Due to the poor conductivity of spinel oxides, conductive carbon supports were used along with spinel oxides to ensure facile electron transfer. CoFe2O4 carbon composites have been extensively studied as ORR electrocatalysts.32 The CoFe2O4 on graphitic carbon nitride support exhibits an onset potential of 0.90 V (vs. RHE) towards the ORR.33 Polypyrrole-based N-doped carbon nanotubes were used as carbon supports for CoFe2O4, which show excellent ORR activity.34 Hollow-structured CoFe–CoFe2O4 supported by N-doped carbon demonstrated excellent bifunctional activity in the zin–air battery.35 N-doped carbon nanotubes derived from the g-C3N4 encapsulated CoFe alloy show excellent ORR and OER activity in zinc–air batteries.22 The carbon matrix obtained from MOFs upon carbonisation shows better electrical conductivity, high surface area and permanent porosity. The ZIF-8 MOF is specially used to synthesise porous N-doped carbon, wherein the pores are generated due to the evaporation of metallic zinc.29 Though several materials were synthesised based on Co and Fe metal/metal oxides, the ambiguity in discerning the real active sites has been seen throughout the literature.
Herein, ZIF-8 encapsulated CoFe2O4 was pyrolysed to synthesise CoFe2O4/NC catalysts, wherein the CoFe2O4 partially reduced to CoFe alloy nanoparticles with the formation of a core–corona structured N-doped carbon nanotube spikes. The ORR and OER activities were found to be excellent. The CoFe–N/C materials exhibit excellent ORR activity compared with Fe–N/C or Co–N/C catalysts synthesised using the same procedure. Besides, the CoFe–N/C catalyst-coated carbon air electrode in the liquid ZAB exhibits excellent power density and specific capacity.
Methodology
The CoFe2O4 was synthesised by using the previous synthetic protocol.36 Briefly, 0.3 mole of Fe(NO3)3·9H2O (98%, Merck, India) was added to the 0.15 mole aqueous solution of Co(NO3)2·6H2O (98%, Merck, India) with constant stirring (35 mL) followed by the addition of 3 M NaOH (97%, Spectrochem, India) until the solution pH became 11.5 with continuous stirring for 6 hours. The content was transferred to a Teflon autoclave and heated to 180 °C for 12 hours. The precipitate was washed with DI water, ethanol and acetone, centrifuged and dried at 80 °C overnight. A desired amount of CoFe2O4 (CF) was sonicated in 40 mL methanol, and 0.587 g of Zn(NO3)2·6H2O (98%, Avra Synthesis, India) was added. With this mixture, 1.298 g of 2-methyl imidazole (2-MIM) (98%, Avra Synthesis, India) methanolic solution was added and sonicated to maintain the uniform dispersion. The dispersion was kept for 21 hours without disturbance. A pale brownish precipitate of the CoFe2O4–ZIF-8 composite was obtained and dried at 60 °C in an oven. Thus-synthesised composites were pyrolysed at 900 °C under a N2-atmosphere to synthesise CoFe2O4-containing porous N-doped carbon (CF–N/C, CF indicates CoFe2O4 and N/C referred the N-doped carbon). CF10–N/C4, CF25–N/C4 and CF50–N/C4 catalysts were synthesised using the above protocol (the numbers 10, 25 and 50 refer to the initial amount of CoFe2O4 taken in milligrams, and 4 refers to the pyrolysis time in hours). The CF10–N/C4 was washed with 0.1 M HCl to remove the free excess metal oxides to analyse the role of spinel oxide, and it was referred to as CF10–N/C4–AW. The Fe3O4 and Co3O4 encapsulated ZIF-8 composites were synthesised individually using a similar protocol and were named F10–N/C4 (only Fe) and C10–N/C4 (only Co) and analysed for ORR activity to understand the role of individual oxides.The ZIF-8 was synthesised as reported in the literature.37 0.05 mole of Zn(NO3)2·6H2O and 0.4 mole of 2-MIM were dissolved in 40 mL methanol and sonicated for 20 minutes, followed by stirring for 21 hours at room temperature. The obtained precipitate was washed with methanol and centrifuged.
The electrochemical experiments were carried out using a four-electrode bi-potentiostat (PARSTAT PMC 1000/1500 electrochemical workstation with the PINE INSTRUMENTS rotator) in a custom-made electrochemical cell. The catalytic ink was prepared from the 5 mg sample with 150 μL of isopropanol, 150 μL of Millipore water and 50 μL of Nafion (binder) mixture, as reported in our recent studies.38 The uniformly dispersed catalytic ink was coated on a 5.6 mm glassy carbon (GC) disk of the rotating ring (Pt)-disk (GC) electrode (RRDE) with a theoretical collection efficiency of 0.37 (model: E7R9 RRDE tips). Previously, the electrode was polished with various grades of alumina slurry to mirror-finish and the particles were removed by ultrasonication. The catalytic ink (6.9 μL) was drop-coated on the GC disk of the RRDE, and the loading density of the catalyst was 0.4 mg cm−2. Finally, the electrode was dried under a N2 atmosphere. The Ag/AgCl (sat. KCl) and carbon cloth with a graphite rod were used as the reference and counter electrodes. The potentials were corrected to a reversible hydrogen electrode from the pH of the electrolyte. The linear sweep voltammetry (LSV) experiment was performed by scanning the potential from 1.2 to 0.1 V in an O2-saturated 0.1 M KOH electrolyte. The blank experiment was conducted to subtract the charging current in the N2-saturated electrolyte. During the ORR measurement, the ring electrode was kept at a constant potential (0.25 V) to oxidise the H2O2 intermediate formed on the disc. The number of electrons and percentage of H2O2 were calculated using the reported equations (ESI†). The OER was evaluated using the rotating disk electrode voltammetry in 1 M KOH electrolyte.
A self-made aqueous zinc–air battery was assembled using CF10-N/C4 catalyst coated (loading density is 1 mg cm−2) air electrode, as described in previous work.39,40 The carbon paper (Toray) was used as an air electrode, and the zinc plate (0.5 mm thickness) was used as the anode. The electrolyte is a mixture of KOH (6 M) and Zn(CH3COO)2 (0.2 M) solution. The air-accessible surface area of the air electrode is 1.539 cm2.
Results and discussion
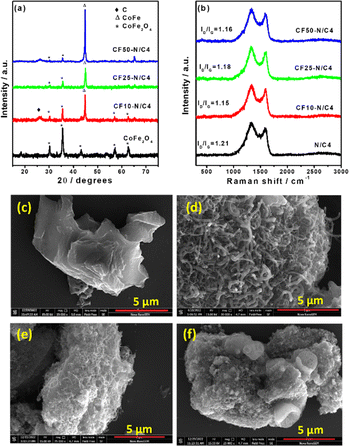
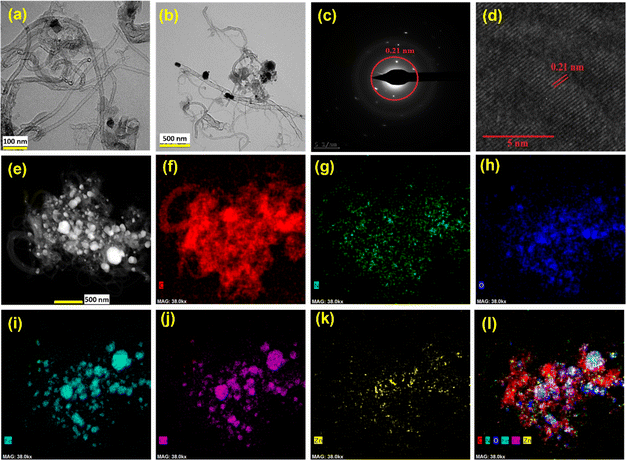
The ZIF-8 and CoFe2O4 materials are separately characterised by XRD patterns, and the synthesised ZIF-8 in the presence of CoFe2O4 (CoFe2O4 encapsulated ZIF-8) has a similar XRD pattern, which may be due to the highly crystalline and larger quantity of ZIF-8 compared with the CoFe2O4 (Fig. S1†). Subsequent pyrolysis of the composite at 900 °C leads to the decomposition of the ZIF-8, followed by the formation of the graphitisation. Interestingly, the new peaks in the XRD pattern after pyrolysis indicate the CoFe alloy formation (Fig. 1a). The ZIF-8 provides the carbon and nitrogen source for graphitisation, leading to the N-doped carbon. The pyrolysis time was optimised where the XRD pattern demonstrates the presence of both the CoFe alloy and CoFe2O4 (Fig. S2†) after four hours of pyrolysis. During the high temperature pyrolysis, the metallic zinc forms and evaporates at ∼900 °C. The evaporation of Zn after the graphitisation created the pores, producing the porous N-doped carbon material. The increasing content of CoFe2O4 in the initial composite material leads to the disappearance of CoFe2O4 peaks due to the thick formation of graphitic layers catalysed by the CoFe alloy, which hinders the exposure of CoFe2O4 particles. Fig. 1b shows the Raman spectra of synthesised materials. The defect density was estimated using the ID/IG value and was 1.21 for N/C4 catalysts. Incorporating CoFe2O4 induces more graphitisation, resulting in a decrease in the ID/IG value.41 The value is not decreased while increasing the CoFe2O4 content beyond 10 wt% in the initial mixture.The SEM images of synthesised materials are shown in Fig. 1c–f. The bare N/C4 shows a highly porous morphology, as reported in the literature (Fig. 1c). However, the CoFe2O4 incorporated carbon (CF10–N/C4) leads to fibril structures on the top of the porous N-doped carbon particles (like core–corona morphology), as shown in Fig. 1d. Upon increasing the CoFe2O4 content, bulk porous structures were seen significantly, with occasional fibril structures (Fig. 1e and f). This may be attributed to the decreasing carbon content to the metal ratio, further confirmed by energy-dispersive X-ray analysis (EDAX) (Fig. S3†). The CoFe2O4–N-doped porous carbon composite was further analysed using transmission electron microscopy (TEM), as shown in Fig. 2a and b. The TEM images reveal that the fibril structure corresponds to the N-doped carbon nanotubes (N-CNT), whose average diameter is 50 nm. The length of the nanotubes varies from a few hundred nanometers to a few micrometres. The high-angle annular dark-field scanning transmission electron microscopic (HAADF-STEM) images indicate the presence of Co and Fe, some of which are present at the edges of the nanotubes. The selected area electron diffraction (SAED) pattern from the metal nanoparticles and its lattice fringes indicate that the d-spacing was estimated to be 0.21 nm, matching the 110 plane of the CoFe alloy (Fig. 2c and d). This strongly suggests that the CoFe alloy was formed during the pyrolysis, catalysing the N-CNT formation. Further, the electron energy loss spectroscopy (EELS) elemental mapping of the compound shows that metals were present in the bulk region along with carbon and nitrogen as shown in Fig. 2f–l. This indicates that Co and Fe were wrapped with graphitic layers derived from ZIF-8. The nanotubes comprised carbon and nitrogen, and no metallic traces were present on the tube. A trace amount of Zn was also observed due to the ZIF-8 precursor.
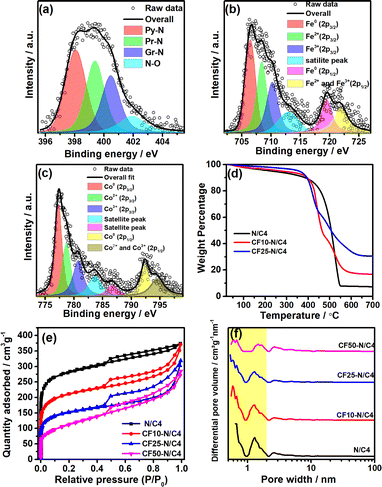
The compounds were characterised using X-ray photoelectron spectroscopy (XPS) to analyse the nature of the atomic species, as shown in Fig. 3. The C-1s spectra indicate that the carbon is present in the form of sp2–C (284.3 eV), sp3–C (285.3 eV), C–N (286.4 eV), C–O (288 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (290 eV) (Fig. S4a†). Similarly, O-1s spectra were deconvoluted into many peaks; among them, the peaks at 528.9 and 529.6 eV are attributed to the oxygen in the crystal lattice. The peaks at 530.4, 531.6, 532.2, and 533.2 eV referred to the different oxygen functional groups in the graphitic carbon (Fig. S4b†). The N-1s spectra (Fig. 3a) reveal that the nitrogen was present in its typical forms, such as pyridinic (398.1 eV), pyrrolic (399.6 eV), graphitic (401 eV) and nitrogen oxides (403.5 eV). The pyridinic and pyrrolic forms of nitrogen are present in major portions, which play crucial roles in the ORR activity.42,43 The XPS peaks of Fe and Co species in the composite materials were analysed, and the Co and Fe contents were observed as 0.43% and 0.62% from the survey spectrum. These values correspond to the surface concentrations of Co and Fe whereas the bulk concentrations are much higher than the surface content, which is analysed later. The deconvoluted Fe-2p XPS peaks confirm the metallic Fe (706.4 eV) as a major portion, followed by the Fe2+ and Fe3+ in equal amounts (Fig. 3b). Co-2p3/2 and Co-2p1/2 were deconvoluted, and the major peak at 777.4 eV refers to the metallic cobalt (Fig. 3c). The other peaks refer to the higher oxidation states of cobalt (Co2+ and Co3+). Similarly, the significant amount of the metallic cobalt and iron in the sample confirms the formation of the CoFe alloy, which has already been confirmed by the intense 110 peak (44.8°) in the powder XRD pattern.
O (290 eV) (Fig. S4a†). Similarly, O-1s spectra were deconvoluted into many peaks; among them, the peaks at 528.9 and 529.6 eV are attributed to the oxygen in the crystal lattice. The peaks at 530.4, 531.6, 532.2, and 533.2 eV referred to the different oxygen functional groups in the graphitic carbon (Fig. S4b†). The N-1s spectra (Fig. 3a) reveal that the nitrogen was present in its typical forms, such as pyridinic (398.1 eV), pyrrolic (399.6 eV), graphitic (401 eV) and nitrogen oxides (403.5 eV). The pyridinic and pyrrolic forms of nitrogen are present in major portions, which play crucial roles in the ORR activity.42,43 The XPS peaks of Fe and Co species in the composite materials were analysed, and the Co and Fe contents were observed as 0.43% and 0.62% from the survey spectrum. These values correspond to the surface concentrations of Co and Fe whereas the bulk concentrations are much higher than the surface content, which is analysed later. The deconvoluted Fe-2p XPS peaks confirm the metallic Fe (706.4 eV) as a major portion, followed by the Fe2+ and Fe3+ in equal amounts (Fig. 3b). Co-2p3/2 and Co-2p1/2 were deconvoluted, and the major peak at 777.4 eV refers to the metallic cobalt (Fig. 3c). The other peaks refer to the higher oxidation states of cobalt (Co2+ and Co3+). Similarly, the significant amount of the metallic cobalt and iron in the sample confirms the formation of the CoFe alloy, which has already been confirmed by the intense 110 peak (44.8°) in the powder XRD pattern.
The metal content was estimated using the thermogravimetric analysis (TGA), as shown in Fig. 3d. The materials were heated in the air atmosphere to remove the carbon content. A significant weight loss was observed above 400 °C, indicating the carbon matrix removal from the composite.44 The left-over materials were analysed using XRD, indicating the CoFe2O4 structure (Fig. S5b†). The TGA of N/C4 (without CoFe2O4) shows 7.2 wt% of ZnO, the same for all the compounds. After the ZnO correction, the TGA curves indicate that the CF10–N/C4 and CF25–N/C4 samples have 9.7% and 23.2% CoFe2O4, respectively. The TGA curve of acid-washed CF10–N/C4 shows 2 wt% metal oxide content (Fig. S6†), and the EELS mapping confirms that the remaining material consists of oxides of Zn, Co and Fe (Fig. S7†).
The surface area and porosity of the materials were analysed using Brunauer–Emmett–Teller (BET) adsorption isotherms, demonstrating the Type IV adsorption (Fig. 3e). However, the materials consist of micropores predominantly due to the evaporation of zinc during the heat treatment.29,45,46 The N/C4 (synthesised from ZIF-8 without CoFe2O4) exhibits the highest surface area (1004 m2 g—1), whereas the incorporation of CoFe2O4 decreases the surface area due to the formation of the bulk CoFe2O4 structure. Besides, it increases the slit narrow mesopores, demonstrated by the H4 hysteresis loop.47 The non-local density functional theoretical (NLDFT) analysis reveals that CF10–N/C4 and CF25–N/C4 consist of narrow mesopores whose diameter varies from 2 to 3 nm (Fig. 3f). The role of micropores and narrow mesopores was discussed in the literature, and their presence is indispensable for carbon-based materials to improve ORR activity.48 The surface area of CF50–N/C4 is small and consists of most mesopores.
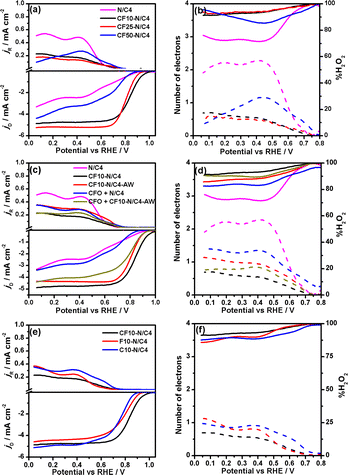
The hydrodynamic rotating ring-disk electrode voltammograms of CoFe2O4 carbon composites are shown in Fig. 4a. The pyrolysis time was optimised to 4 hours for the best ORR activity. The CF10–N/C4 shows the highest positive onset (Eonset = 1.00 V) and half-wave potential (E1/2 = 0.84 V), which is highest among all the composites synthesised with different loadings of CoFe2O4 and pyrolysis times. The Eonset and E1/2 values are decreased while increasing the CoFe2O4 content, and it may be attributed to the decrease in the electrical conductivity of the catalysts, as shown in Table S2.† Among the materials studied in this work, CF10–N/C4 and CF25–N/C4 show better E1/2 values (0.84 V and 0.81 V, respectively). The number of electrons was calculated from the ring and disk currents as 3.8 for CF10–N/C4 and CF25–N/C4, demonstrating the complete 4-electron reduction of the ORR (Fig. 4b). The CoFe2O4-free N/C4 catalysts show the lowest activity despite having a larger surface area, indicating the role of metals (Co and Fe) towards the ORR activity.31 Table S3† shows the ORR activities of the similar materials studied in the literature.
A few control experiments were performed to understand the active sites of the materials (Fig. 4c and d). The role of metals was analysed by removing them using acid-washing. The CF10–N/C4 was washed with 0.1 M HCl overnight to remove the metal and metal oxides in the material. The acid-washed catalyst was referred to as CF10–N/C4–AW. The XRD confirms the removal of the CoFe alloy and CoFe2O4 particles, and only the 002 peak corresponding to the graphitic carbon is observed (Fig. S8†). Further, the compound was analysed using the TEM and EELS mapping (Fig. S7†). Although small metal traces are present in the acid-washed samples, these metallic species should be wrapped with the graphitic carbon layers. The nanotube morphology was retained after the acid washing (Fig. S12b†). The CF10–N/C4–AW shows the Eonset and n values of 0.95 V and 3.5, respectively, slightly lower than the CF10–N/C4. The decrease in the activity can be rationalised by removing the CoFe2O4 or CoFe alloy, indicating the vital role of the metal species (in the form of alloy). The accelerated durability test (ADT) was performed using the protocol proposed by DoE. The CF10–N/C4 shows only a 10 mV negative shift after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, indicating excellent stability during the ORR (Fig. S9†).
000 cycles, indicating excellent stability during the ORR (Fig. S9†).
Further, the origin of the ORR activity can be studied to discern the roles of CoFe2O4 and CoFe alloy species. The ORR activity of metal-free N/C4 exhibits lower Eonset and E1/2 values (0.90 and >0.7 V) with a current density of 3 mA cm−2. The physical mixture of CoFe2O4 with N/C4 (CFO + N/C4) can reveal the role of the CoFe alloy and CoFe2O4. One can effectively exclude the CoFe alloy contribution in the physical mixture, which forms during the heat treatment. The ORR activity of CFO + N/C4 is closely similar to that of the N/C4, demonstrating that the CoFe2O4 did not act as ORR active sites. Furthermore, the physical mixture of CoFe2O4 with CF10–N/C4–AW (CFO + CF10–N/C4–AW) does not regain the original activity of CF10–N/C4, indicating that the acid-washed step removed the CoFe2O4, which did not involve in the ORR. Based on these studies, it was confirmed that the graphitic carbon encapsulated CoFe alloy should be the active site for the ORR.
To understand the role of individual atomic species in the CoFe alloy, the iron oxide + ZIF-8 and cobalt oxide + ZIF-8 composites were synthesised and pyrolysed separately, referred to as C10–N/C and F10–N/C materials (Fig. 4e and f). The XRD pattern indicates the combination of Fe3O4 and Fe metallic particles in the F10–N/C catalysts, whereas the C10–N/C catalyst shows only Co metallic nanoparticles (Fig. S10†). The SEM images of C10–N/C4 and F10–N/C4 catalysts reveal a highly porous morphology wherein the formation of the nanotubes (or core–corona structural motif) is not observed (Fig. S11†). The RRDE voltammograms of the C10–N/C4 and F10–N/C4 materials demonstrate that the ORR activities of both these materials are lower than that of CF10–N/C4 (Eonset = 0.95 and 0.91 for F10–N/C4 and C10–N/C4 catalysts, respectively), indicating that the encapsulated CoFe alloy, as a whole, acts as an active site for the ORR.
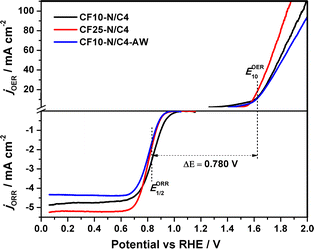
Bimetallic materials could be the best choice due to their bifunctional activity in the ZAB application. The OER activity of these materials is evaluated in the 1 M KOH electrolyte, as shown in Fig. 5. The E10 values (potential at which the current reaches 10 mA cm−2) indicate that the CF10–N/C4 (1.607 V), CF25–N/C4 (1.592 V) and CF10–N/C4–AW (1.616 V) materials are good OER catalysts. The acid-washed compound (CF10–N/C4–AW) shows a slight anodic shift. The potential difference between the ORR (E1/2) and OER (E10) is a measure of bifunctional catalyst activity, referred to as ΔE. The ΔE value of CF10–N/C4 and CF25–N/C4 catalysts was estimated to be 0.76 and 0.78 V, respectively, which are excellent values for a material to be a bifunctional catalyst. Hence, these materials can be used as oxygen electrocatalysts in rechargeable zinc–air battery applications.
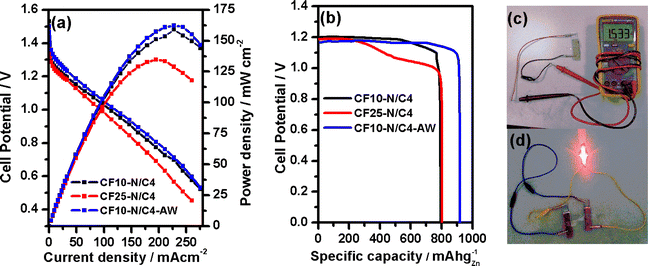
An aqueous Zn–air battery was constructed using CF10–N/C4, CF25–N/C4 and CF10–N/C4–AW, as explained in the methodology section. The SEM images of the CF10–N/C4 coated carbon paper air electrode indicate the uniform distribution of the particles (Fig. S15a†). The open-circuit potential (EOCP) was measured as 1.53 V for CF10–N/C4, followed by CF25–N/C4 (1.50 V) and CF10–N/C4–AW (1.50 V). The polarization curves demonstrate that the power density of CF10–N/C4 and CF10–N/C4–AW is 159 mW cm−2 (at 211 mA cm−2) and 162 mW cm−2 (at 227 mA cm−2), respectively, as shown in Fig. 6a. A similar trend was observed with gravimetric energy density values (Fig. S13†). Almost similar power density values were observed for CF10–N/C4 and CF10–N/C4–AW, indicating that the metal content outside the graphitic layer is not primarily involved in the activity. In contrast, the activity is enhanced by the encapsulated CoFe alloy. The increasing CoFe2O4 creates a negative impact on the overall efficiency. The specific capacity at the constant discharge current density of 20 mA cm−2 was estimated as 900 mA h gZn−1 for CF10–N/C4–AW, whereas the other two compounds exhibit lower specific capacities (Fig. 6b). These values demonstrate that a small portion could be the active site in the composite, where the CoFe plays an essential role in enhancing the activity. The galvanostatic charge–discharge experiment was performed with a charging density of 5 mA cm−2 and 20 minutes per cycle. The catalysts are stable until 45 hours of operation (120 cycles), wherein no change was observed in the charge and discharge potential (Fig. S14†). The longer the number of cycles, the lower the cell performance, which may be attributed to the carbon corrosion at higher positive potentials. This was further confirmed by the SEM analysis of the carbon air electrode, indicating the formation of porous carbon bulky particles with fewer carbon nanotubes on the surface of the particles (Fig. S15b†). Further, the catalyst was analysed using the XPS technique after the galvanostatic charge–discharge tests. Besides the typical elements present in the CF10–N/C4, potassium (K-2p, 295.9 eV), fluorine (F-1s, 689.0 eV), sulphur (S-2p, 170.5 eV) and zinc (Zn-2p3/2, 1023.5 eV) were found in larger portions in the survey spectrum. The presence of these elements (K, F, S and Zn) may be attributed to the KOH electrolyte, Nafion (tetrafluoroethylene sulphonic acid polymer) binder and zinc oxide (from anode). Traces of Co and Fe were found and deconvoluted to study the oxidation states. The Co0 and Fe0 (encapsulated with thinner carbon layers) peaks indicates the presence of the CoFe alloy in the catalyst layer (Fig. S16†), which is lower than the amount of CoFe estimated before the zinc–air battery testing. A comparative study of the zinc–air battery performances using similar materials is given in Table S4.†
The solid-state zinc–air battery experiment was performed using the gel electrolyte prepared from polyvinyl alcohol (PVA) and KOH,49 as shown in Fig. 6c and d. The EOCP was measured as 1.53 V for CF10–N/C4, close to that of the benchmark Pt/C + RuO2 catalysts (1.56 V). The power density value was calculated as 22.4 mW cm−2 (Fig. S17†), and the galvanostatic charge–discharge test (for 1 mA cm−2 current density) exhibits cycling stability comparable with that of the Pt/C + RuO2 catalysts (Fig. S18†).
Conclusions
Porous CoFe2O4-containing nitrogen-doped carbon was synthesised from ZIF-8 and CoFe2O4 for use as oxygen electrocatalysts in the zinc–air battery electrode. The CoFe2O4 incorporation increases the graphitisation and produces carbon nanotubes on the surface of porous N-doped carbon particles (core–corona structure). Despite having the higher surface area of metal-free porous nitrogen-doped carbon (from ZIF-8), the metal-containing (Co and Fe) CF10–N/C4 exhibits much higher ORR and OER activity, which is comparable to those of the benchmark Pt/C (ORR) and RuO2 (OER) catalysts. The formation of the CoFe alloy was confirmed using the spectroscopic techniques, and the control experiments indicate that CoFe alloy particles are the main active sites for the ORR activity along with N-doped carbon. Besides, the OER activity is also improved due to the CoFe alloy, indicating the excellent bifunctional activity of the CoFe alloy. The liquid electrolyte zinc–air battery exhibits excellent power density and specific capacity, and the solid-state zinc–air battery was demonstrated to show the real-time application.Author contributions
SB performed synthesis and electrochemical analysis and drafted the manuscript. EAA performed the liquid and solid-state battery experiments. AM conceptualised, supervised the work and finalised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the IISER Thiruvananthapuram for the facilities and the financial support.Notes and references
- J.-S. Lee, S. Tai Kim, R. Cao, N.-S. Choi, M. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 2011, 1, 34–50 CrossRef CAS.
- J. Fu, Z. P. Cano, M. G. Park, A. Yu, M. Fowler and Z. Chen, Adv. Mater., 2017, 29, 1604685 CrossRef.
- A. Iqbal, O. M. El-Kadri and N. M. Hamdan, J. Energy Storage, 2023, 62, 106926 CrossRef.
- R. Zhao, Q. Li, X. Jiang, S. Huang, G. Fu and J.-M. Lee, Mater. Chem. Front., 2021, 5, 1033–1059 RSC.
- W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Energy Environ. Sci., 2015, 8, 1404–1427 RSC.
- S. Dresp, F. Luo, R. Schmack, S. Kühl, M. Gliech and P. Strasser, Energy Environ. Sci., 2016, 9, 2020–2024 RSC.
- K. Tang, H. Hu, Y. Xiong, L. Chen, J. Zhang, C. Yuan and M. Wu, Angew. Chem., Int. Ed., 2022, 61, e202202671 CrossRef CAS.
- K. Tang, C. Yuan, Y. Xiong, H. Hu and M. Wu, Appl. Catal., B, 2020, 260, 118209 CrossRef CAS.
- R. Na, K. Min, H. Kim, Y. Son, S. E. Shim and S.-H. Baeck, J. Energy Chem., 2023, 84, 140–152 CrossRef CAS.
- R. S. Kumar, S. Prabhakaran, S. Ramakrishnan, S. C. Karthikeyan, A. R. Kim, D. H. Kim and D. J. Yoo, Small, 2023, 19, 2207096 CrossRef CAS.
- J. Wang, C.-X. Zhao, J.-N. Liu, D. Ren, X. Ma, B.-Q. Li, J.-Q. Huang and Q. Zhang, Particuology, 2023, 77, 146–152 CrossRef CAS.
- B. Ricciardi, W. da Silva Freitas, B. Mecheri, K. U. Nisa, J. Montero, V. C. A. Ficca, E. Placidi, C. Alegre and A. D'Epifanio, Carbon, 2024, 219, 118781 CrossRef CAS.
- S. Ren, X. Duan, S. Liang, M. Zhang and H. Zheng, J. Mater. Chem. A, 2020, 8, 6144–6182 RSC.
- Y.-j. Wu, X.-h. Wu, T.-x. Tu, P.-f. Zhang, J.-t. Li, Y. Zhou, L. Huang and S.-g. Sun, Appl. Catal., B, 2020, 278, 119259 CrossRef CAS.
- D. Xie, D. Yu, Y. Hao, S. Han, G. Li, X. Wu, F. Hu, L. Li, H.-Y. Chen, Y.-F. Liao and S. Peng, Small, 2021, 17, 2007239 CrossRef CAS PubMed.
- M. Mechili, C. Vaitsis, N. Argirusis, P. K. Pandis, G. Sourkouni and C. Argirusis, Renewable Sustainable Energy Rev., 2022, 156, 111970 CrossRef CAS.
- Y. Kumar, M. Mooste and K. Tammeveski, Curr. Opin. Electrochem., 2023, 38, 101229 CrossRef CAS.
- E. Davari and D. G. Ivey, Sustainable Energy Fuels, 2018, 2, 39–67 RSC.
- G. Janani, Y. Chae, S. Surendran, Y. Sim, W. Park, J. K. Kim and U. Sim, Appl. Sci., 2020, 10, 3165 CrossRef CAS.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed.
- S. Jha, P. Jain, R. Palkovits and P. Popinand Ingole, J. Mater. Chem. A, 2023, 11, 23034–23047 RSC.
- L.-L. Liu, D.-H. Wu, L. Zhang, J.-J. Feng and A.-J. Wang, J. Colloid Interface Sci., 2023, 639, 424–433 CrossRef CAS PubMed.
- Y. Sun, J. Wang, Q. Liu, M. Xia, Y. Tang, F. Gao, Y. Hou, J. Tse and Y. Zhao, J. Mater. Chem. A, 2019, 7, 27175–27185 RSC.
- L. Liu, Z. Xu, T. Lu, Z. Wang, X. Shi, C. Li, Y. Wei and Z. Zi, J. Alloys Compd., 2022, 906, 164296 CrossRef CAS.
- A. Samanta and C. R. Raj, J. Phys. Chem. C, 2018, 122, 15843–15852 CrossRef CAS.
- J. M. Gonçalves, M. I. da Silva, M. N. T. Silva, P. R. Martins, E. Nossol, H. E. Toma and L. Angnes, Energy Adv., 2022, 1, 793–841 RSC.
- Y. Song, C. Yu, D. Ma and K. Liu, Coord. Chem. Rev., 2024, 499, 215492 CrossRef CAS.
- J. Liu, Y. Xie, Y. Nan, G. Gou, X. Li, Y. Fang, X. Wang, Y. Tang, H. Yang and J. Ma, Electrochim. Acta, 2017, 257, 233–242 CrossRef CAS.
- H.-X. Zhong, J. Wang, Y.-w. Zhang, W.-l. Xu, W. Xing, D. Xu, Y.-f. Zhang and X.-b. Zhang, Angew. Chem., Int. Ed., 2014, 53, 14235–14239 CrossRef CAS PubMed.
- R. R. Salunkhe, Y. Kamachi, N. L. Torad, S. M. Hwang, Z. Sun, S. X. Dou, J. H. Kim and Y. Yamauchi, J. Mater. Chem. A, 2014, 2, 19848–19854 RSC.
- M. Jiang, X. Cao, D. Zhu, Y. Duan and J. Zhang, Electrochim. Acta, 2016, 196, 699–707 CrossRef CAS.
- M. S. Matseke, H. Zheng and Y. Wang, Appl. Surf. Sci., 2020, 516, 146105 CrossRef CAS.
- X. Li, Y. Zhao, L. Ding, D. Wang, Q. Guo, Z. Li, H. Luo, D. Zhang and Y. Yu, Nanomaterials, 2021, 11, 1088 CrossRef CAS PubMed.
- H. Wang, C. Liu, X. Yang, J. Gu, M. Niu, L. Yang and Z. Bai, Int. J. Hydrogen Energy, 2022, 47, 6059–6066 CrossRef CAS.
- Y. Go, K. Min, H. An, K. Kim, S. Eun Shim and S.-H. Baeck, Chem. Eng. J., 2022, 448, 137665 CrossRef CAS.
- L. Gan, S. Shang, C. W. M. Yuen, S.-x. Jiang and E. Hu, Appl. Surf. Sci., 2015, 351, 140–147 CrossRef CAS.
- E. L. Bustamante, J. L. Fernández and J. M. Zamaro, J. Colloid Interface Sci., 2014, 424, 37–43 CrossRef CAS PubMed.
- J. Anjana and A. Muthukrishnan, Catal. Sci. Technol., 2022, 12, 6246–6255 RSC.
- A. N. Eledath, A. Edathiparambil Poulose and A. Muthukrishnan, ACS Appl. Eng. Mater., 2023, 1, 2304–2314 CrossRef CAS.
- A. N. Eledath, A. E. Poulose and A. Muthukrishnan, ACS Appl. Nano Mater., 2022, 5, 10528–10536 CrossRef CAS.
- Y. Lu, Z. Zhu and Z. Liu, Carbon, 2005, 43, 369–374 CrossRef CAS.
- H. Ji, M. Wang, S. Liu, H. Sun, J. Liu, T. Qian and C. Yan, Electrochim. Acta, 2020, 334, 135562 CrossRef CAS.
- S. K. Singh, K. Takeyasu and J. Nakamura, Adv. Mater., 2019, 31, 1804297 CrossRef PubMed.
- S. Arya Gopal, A. Edathiparambil Poulose, C. Sudakar and A. Muthukrishnan, ACS Appl. Mater. Interfaces, 2021, 13, 44195–44206 CrossRef CAS PubMed.
- W. Chaikittisilp, M. Hu, H. Wang, H.-S. Huang, T. Fujita, K. C. W. Wu, L.-C. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259–7261 RSC.
- J. Hernández-Ferrer, M. Gracia-Martín, A. M. Benito, W. K. Maser and E. García-Bordejé, Catal. Today, 2023, 423, 113993 CrossRef.
- H. Qutaish, J. Lee, Y. Hyeon, S. A. Han, I.-H. Lee, Y.-U. Heo, D. Whang, J. Moon, M.-S. Park and J. H. Kim, Appl. Surf. Sci., 2021, 547, 149134 CrossRef CAS.
- J. Anjana, A. N. Eledath and A. Muthukrishnan, Mater. Adv., 2023, 4, 4216–4225 RSC.
- L. Wang, M. Xu, H. Li, Z. Huang, L. Wang, T. Taylor Isimjan and X. Yang, Inorg. Chem., 2023, 62, 13284–13292 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se00084f |
| This journal is © The Royal Society of Chemistry 2024 |