Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3D printing in microfluidics: experimental optimization of droplet size and generation time through flow focusing, phase, and geometry variation
Adam Britel *a,
Giulia Tomagrab,
Pietro Aprà
*a,
Giulia Tomagrab,
Pietro Aprà a,
Veronica Varzi
a,
Veronica Varzi a,
Sofia Sturaria,
Nour-Hanne Aminea,
Paolo Olivero
a,
Sofia Sturaria,
Nour-Hanne Aminea,
Paolo Olivero a and
Federico Picollo
a and
Federico Picollo a
a
aDepartment of Physics, “NIS” Inter-departmental Centre, University of Torino, National Institute of Nuclear Physics, Sect. Torino, Via Pietro Giuria 1, 10125 Torino, Italy. E-mail: a.britel@unito.it
bDepartment of Drug and Science Technology, NIS Interdepartmental Centre, University of Torino, Corso Raffaello 30, 10125 Torino, Italy
First published on 5th March 2024
Abstract
Droplet-based microfluidics systems have become widely used in recent years thanks to their advantages, varying from the possibility of handling small fluid volumes to directly synthesizing and encapsulating various living forms for biological-related applications. The effectiveness of such systems mainly depends on the ability to control some of these systems' parameters, such as produced droplet size and formation time, which represents a challenging task. This work reports an experimental study on tuning droplet size and generation time in a flow-focusing geometry fabricated with stereolithography 3D printing by exploring the interplay of phase and geometrical parameters. We produced droplets at different low flow rates of continuous and dispersed phases to assess the effect of each of these phases on the droplets' size and formation time. We observed that smaller droplets were produced for high viscosity oil and water phase, along with high flow rates. In addition, changing the microfluidics channels' width, and morphology of the orifice has shown a similar effect on droplet size, as shown in the case of high-viscosity solutions. The variation of the bifurcation angle shows a noticeable variation in terms of the achieved droplet size and formation time. We further investigated the impact of modifying the width ratio of the continuous and dispersed phases on droplet formation.
1 Introduction
Droplet-based microfluidic systems have risen as important tools in recent years, offering various advantages ranging from the encapsulation of single living forms for biological-related applications1 to chemical syntheses2,3 and many other applications.4,5 Droplet microfluidics allow the manipulation of small volumes of fluids in immiscible phases, usually water and oil, with low Reynolds numbers. In these systems, mainly two key components can be identified, namely: the dispersed phase which refers to the solution to be encapsulated, and the continuous phase, which refers to the medium on which the droplets flow. The primary benefit of using droplet microfluidics is represented by the ability to control the encapsulation of small volumes of fluids, in the order of femto to nanolitres. In this context, the capability of controlling the generated droplet size emerges as a key requirement. Droplets in droplet-based microfluidics can be generated either through active methods or passive methods. Active methods involve the use of external sources, such as electric, optical, and thermal control to rupture the liquid–liquid interface into droplets.6–8 In contrast, passive methods take advantage of the surface properties of both the continuous and dispersed phases along with the channel geometry to produce droplets.9 When it comes to passive droplet microfluidics, there are several designs available, but the most frequently employed ones are the use of T-junctions and flow focusing, each with its specific droplet formation processes.10,11 Flow-focusing geometries stand out from other geometries due to their adaptability in terms of creating droplets of varied sizes, generation rates, and a lower coefficient of variation in droplet size.9–13 However, the efficiency of these systems depends mainly on the ability to control key properties such as droplet size and formation time, which as previous studies have shown can be tuned by controlling device parameters.14–18 For example, in biological applications, the precise control over droplet size is an integral aspect of optimizing encapsulation efficiency and consistency.19 Small droplets are preferred for single-cell-related analysis while larger ones offer ease of manipulation which is essential for droplet-sorting-related applications and multiple reagents mixing.4 Correspondingly, the droplet formation time directly affects the overall efficiency of droplet production for the desired use.The methods used for device fabrication impose constraints on the geometric features of the microfluidic channels, which, as discussed above, determine the performance of droplet generation. Traditional fabrication methods to develop microfluidic devices, namely soft lithography, are often labor-intensive and time-consuming. With the use of polydimethylsiloxane (PDMS) in soft lithography, good control over channel dimensions is achieved with cost-effective approach, but the fabrication method is two-dimensional, and thus the production of three-dimensional structures requires multiple steps. For such a reason, 3D printing emerged as a solution for accelerating microfluidics prototypes development and devices production. In the present work, it allowed the introduction of various features in the same device allowing the design of customized geometries for specific applications. Moreover, the ease of use, low cost of fabrication, and the possibility of multiple applications of the same device make it an attractive option on the industrial and the research scale. The resolution of the produced devices depends on the used 3D printer and technique for the fabrication that usually associates high quality with the cost of equipment. In this work, we focus our study on the flow-focusing configuration, more specifically, we use parallel dispersed phase channels with a contraction in the continuous phase channel. In this study, we used traditional quasi 2D-flow focusing design to validate our findings, to assess the practicality of using desktop 3D printers, and to enhance the accessibility of our findings to the broader research community. We present an experimental investigation on the tuning of droplet size and formation time produced within the stereolithography (SLA) 3D-printed flow-focusing geometry, aiming to explain the interplay between the solution-related and geometrical parameters. In order to present results of interest to a wider audience within the microfluidics community, we limited our research to microfluidic channels of a few hundred microns in diameter, which can be achieved with standard desktop 3D printers. This work aims to shed light on the possibility of obtaining small droplets with a controllable formation time by adjusting different geometric and solution-related parameters. By understanding these factors, our work not only can open new possibilities to precisely controlling and optimizing droplet formation processes but also contribute to the fundamental knowledge base, enhancing the understanding and accessibility of this field for a broader scientific audience. To facilitate the observation of individual droplets and more easily understand the impact of the parameters under study, we worked at low flow rates, both for continuous and dispersed phases.
2 Materials and methods
2.1 Experimental setup
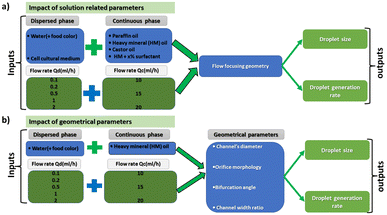
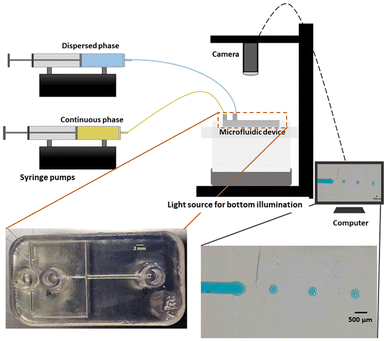
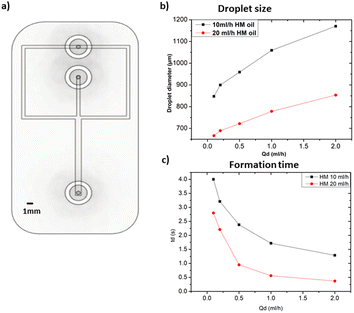
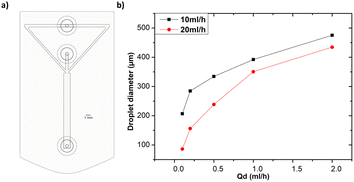
All the microfluidic devices developed in this work were manufactured with an SLA 3D printer from Formlabs (Form 3B) using Formlabs clear resin. We used only flow-focusing geometry as it allows to precisely adjust the size of the generated droplets by controlling the flow rates. The fabrication process starts by designing the device using any computer-aided design (CAD) program: in this work, we use Autodesk Fusion 360. All devices in this study were printed at a 50 μm resolution directly on the printer build platform. The devices were printed with clear resin because its transparency made it simpler to monitor the droplet formation inside the microfluidic channels. Once the device was ready, it was cleaned with isopropyl alcohol (IPA) of a concentration equal or higher than 99% to remove the uncured resin on the surface and inner channels of the microfluidic devices. The printed devices were agitated for 5 minutes in IPA, followed using pressurised air to force the removal of stuck resin from the channels. Then, IPA was pumped inside the channels to ensure the absence of any remaining stuck resin. The last step is the most important as poor cleaning can lead to blocked channels. Once the cleaning was performed, the device was post-cured in a UV chamber to ensure complete polymerization and to optimize material properties to their full potential. The size of the microfluidic channels has been kept in the order of 500 μm to 600 μm in order to keep our fabrication results achievable with a standard desktop 3D printer. Each printed device was rinsed with the oil that will be employed a few hours before use, which helped to reduce clogging caused by droplets sticking to the walls of the microfluidic channels. This operation allowed for the rinsed device to be used continuously for 4 to 5 hours without showing signs of cohesion, for the fluids employed in this work. In the section of this work dedicated to the influence of the solution-related parameters, we utilized distilled water and cell cultural medium (RPMI-1640) for the dispersed phase. The composition of RPMI-1640 medium is the following 10% horse serum (Invitrogen), 5% fetal bovine serum (Invitrogen) and 2% antibiotic/antimitotic (pen/strep Invitrogen), that is usually used for the culture of PC 12 cells line at a temperature of 37 °C in a 5% CO2 atmosphere. For the continuous phase, we used paraffin oil, heavy mineral oil, castor oil, and a combination of heavy mineral oil with a surfactant (Span 80, Sigma-Aldrich). In the section of this work related to the influence of the geometrical parameters, we kept the distilled water as a dispersed phase and heavy mineral oil as a continuous phase. Fig. 1 shows flow diagrams of the various experimental conditions considered for the evaluation of the impact of the solution-related and geometric parameters.Dual syringe pump (Harvard Apparatus Model 33 Twin Syringe Pump) was employed to infuse/withdraw two liquids in the microfluidic devices. The flow rates of the dispersed phase (Qd) varied between 0.1 ml h−1 to 2 ml h−1 while for the continuous phase (Qc) they were set between 10 ml h−1 and 20 ml h−1. Values of Qd and Qc limited to these ranges allow the formation of single droplets, which is the main interest of the present work. The experimental setup is composed of a syringe pump, a light source, and a camera capable of capturing 30 fps (SVPRO 4K), as shown in Fig. 2. The tubes were directly inserted into the microfluidic devices without the need of additional connectors which helps reducing the risk of leakage. Images of the produced droplets were extracted from the recorded video and analyzed by the open-source image processing software ImageJ.20 Due to the slow generation rate of droplets, we measured three distinct droplets at different timing during the experiment. This approach was used to assess the repeatability and precision of single droplet measurements. In all of the presented graphs, the error bars are not visible because their size coincides with the smallest possible size of the graph points. These error bars represent the variability observed in these repeated measurements of droplets rather than polydispersity over a large population, providing insights into the consistency of our droplet formation process. However, statistical analysis was conducted to ensure the reliability of the presented data. All the experiments were performed in the same environmental conditions to avoid any undesired effect that may arise from the change of temperature, for example.
3 Results and discussions
3.1 Investigation of solution related parameters on droplet size and formation time
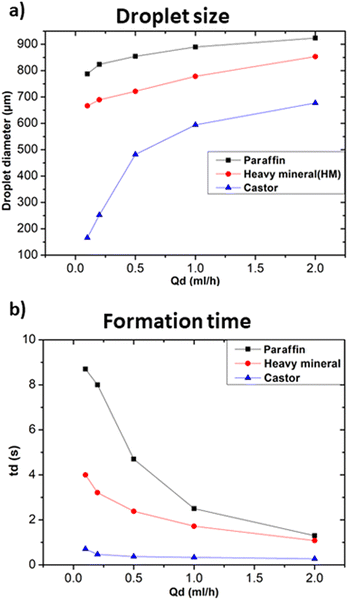
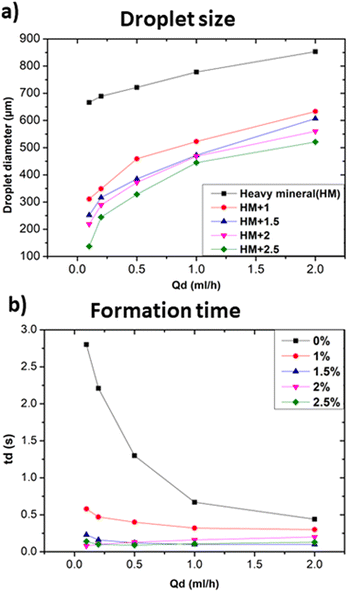
In particular, Fig. 5a shows that the addition of 1% wt Span 80 to heavy mineral oil drastically reduced the droplet size with respect to the case of only heavy mineral oil, while increasing the surfactant concentration resulted in a relatively smaller decrease of the droplet size. These experiments proved the impact of the interfacial tension on the droplet's formation process, i.e. at lower interfacial tension smaller droplets were observed.28 All curves characterised by a different surfactant concentration versus the variation of the dispersed flow approximately follow the same trend. At 1% wt, the surfactant molecules reduce the interfacial tensions effects resulting in an easier the breakup of water-in-droplets which significantly reduces the sizes of the droplets. At higher concentrations, i.e., 2.5% wt, the size of the droplets keeps slowly decreasing since is approaching the limit at which the interfacial tension can be lowered. The evolution of the droplet formation time at different surfactant concentration follows the same trend as the one of droplets size as described above. However, these results cannot be generalized to all kinds of surfactants, as this effect depends on their compatibility with the used phase.29 A comparison of the droplet size obtained using continuous phases of different viscosities with respect to heavy mineral oil, including various concentrations of surfactant, is shown in Fig. 6. A flow rate ratio of 100 was selected, corresponding to Qd = 0.2 ml h−1 and Qc = 20 ml h−1. Notably, using castor oil (i.e. high viscosity regime) as the continuous phase or supplementing heavy mineral oil with 2.5 wt% surfactants yielded to comparably sized droplets. This confirms that the reduction of droplet size can be achieved through two distinct mechanisms, namely either by modulating the interfacial tension via surfactant addition or by increasing the viscosity of the continuous phase.
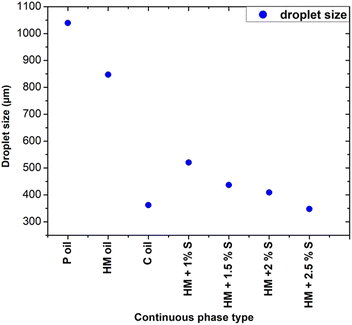 | ||
| Fig. 6 Influence of continuous phase viscosity and concentration of surfactant on the droplet size at a flow ratio of 100. P refers to paraffin, HM to heavy mineral, S to surfactant, C to castor. | ||
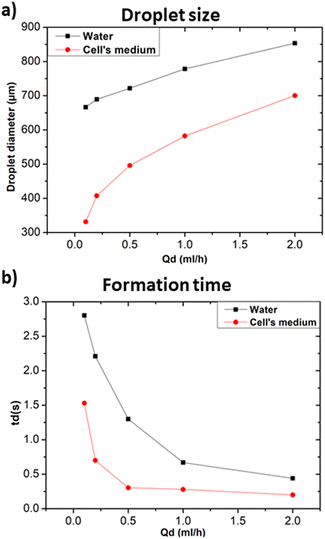
In the following the effect of changing the composition of the dispersed phase while keeping heavy mineral oil as the continuous phase was investigated. Given the general interest in the development of droplet microfluidic systems for cell encapsulation, a standard medium for cell culture (RPMI-1640, Invitrogen) was selected as dispersed phase and compared with distilled water. Fig. 7a and b show that cellular medium improved the performance of the microfluidic systems resulting in the production of smaller droplets and faster formation time in comparison to the case of distilled water. Different factors such as changes in viscosity, surface tension, or interfacial properties affect the droplet formation. Distilled water and cell medium are likely to have distinct surface tensions with the used continuous phase resulting in a modification of the droplet formation mechanism. As we experienced a decrease in the droplet size, we assumed that the modification of the dispersed phase caused a decrease in the surface tension.30 In terms of interfacial properties, it is most likely that the composition of the used cell medium is more compatible with heavy mineral oil, contrarily to what obtained with distilled water. Hence, the observed change concerning the size and formation time of the produced droplets.
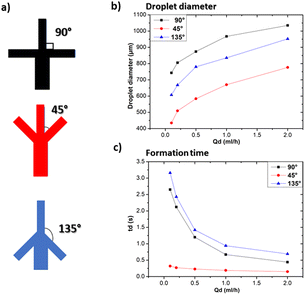
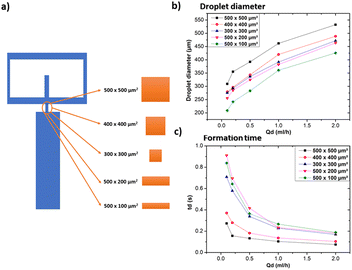
3.2 Investigation of geometrical parameters on droplet size and formation time
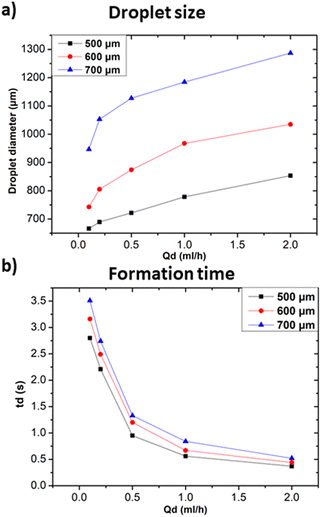
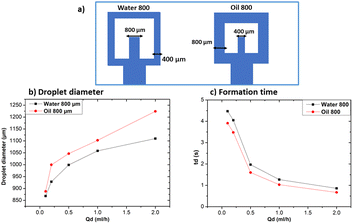
The oil 800 units produced larger droplets compared to water 800. In the case of oil 800, the large width of the continuous phase channel allows a greater volume of continuous phase to interact with the dispersed phase at the droplet formation site. As a result, a greater volume of the dispersed phase can be encapsulated, resulting in larger droplets. Similar considerations can be made for the difference in formation times reported in Fig. 11c.
4 Optimized geometrical combination for minimized droplet production
After examining how various geometric and solution related parameters impact droplet size, this section aims to shed light on the combined effect of these geometric parameters to achieve the smallest droplet size. We maintained the microfluidic channel width in the 500–600 μm range and a height of 1 mm to ensure that it can be reproduced by using any standard desktop 3D printer. Distilled water and heavy mineral oil were respectively used as the dispersed and continuous phases to easily compare these results with the ones shown in Fig. 3. The design of this device that integrates all the geometric features discussed in the previous section (i.e. orifice morphology, bifurcation angle, and channel width) is depicted in Fig. 12a. The observed droplet size is presented in Fig. 12b. As anticipated, a noticeable reduction in droplet size is observed as compared to the previous results. In fact, for Qd = 0.1 ml h−1 and Qc = 20 ml h−1, the droplet size becomes approximately 90% smaller with respect to the same parameters in Fig. 3. This shows the effectiveness of systematic understanding of the effect of device geometry on droplet size, thus facilitating the production of smaller droplets in a quick, simple, and cost-effective way.5 Conclusion
This work offers a systematic experimental investigation of the different parameters influencing droplet size and formation time in 3D printed microfluidic devices, reporting on the interplay between the flow rates of the used phases, viscosity and composition of the used fluids, the addition of surfactants, and geometric parameters of the microfluidic device. 3D printing was selected as a tool for rapid prototyping allowing the investigation of multiple geometric parameters in the droplet formation process. Through this work, we have demonstrated the effective use of desktop 3D printer in the field of droplet microfluidics, thus highlighting their capability to achieve an accurate control over droplet characteristics under accessible conditions. In our experiments we systematically investigated various parameters, leading to the generation of droplets as small as 80 μm. This represents a remarkable result, considering the simplicity and affordability of the used equipment. The key findings of our study reveal that careful manipulation of flow rates, fluid viscosities, and geometric parameters of the employed microfluidic device can finely tune droplet size and formation time. In particular, this work shows the role of channel width, and orifice morphology, providing a path towards optimizing droplet characteristics even with the constraints of affordable desktop 3D printers. Our results resonate with the findings of advanced research in this field which shows that complex microfluidic tasks, typically reliant on specific equipment, can be replicated to a certain extent using more accessible technology. Overall, these results provide practical strategies for manipulating droplet size and formation time within the constraints of affordable 3D printing methods. The comprehensive exploration of the diverse solution-related, and geometrical parameters offers valuable insights for the design and operation of droplet microfluidic systems within the constraints of limited facilities.Author contributions
A. B. conducted the formal analysis, investigation, methodology and drafted the manuscript. G. T. provided resources for experiments and validated the procedures. P. A. performed formal analysis. V. V., S. S., and N. A. were involved in the validation and review of the first draft. P. O. was responsible for manuscript review and editing. F. P. conceptualized the study, provided supervision, created visualizations, and contributed to manuscript drafting. All authors have read and agreed on the content of the manuscript.Conflicts of interest
The authors declare that they have no conflicts that may influence the work reported here.Acknowledgements
This research has received funding from the European Union's H2020 Marie Curie ITN project LasIonDef (GA no. 956387), and “QuantDia” project funded by the Italian Ministry for Instruction, University and Research within the “FISR 2019 ” program.References
- D. Liu, M. Sun, J. Zhang, R. Hu, W. Fu, T. Xuanyuan and W. Liu, Analyst, 2022, 147, 2294–2316 RSC.
- J. Ji, Y. Zhao, L. Guo, B. Liu, C. Ji and P. Yang, Lab Chip, 2012, 12, 1373 RSC.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550–2583 CrossRef CAS PubMed.
- A. Huebner, S. Sharma, M. Srisa-Art, F. Hollfelder, J. B. Edel and A. J. deMello, Lab Chip, 2008, 8, 1244 RSC.
- K. Schroen, C. Berton-Carabin, D. Renard, M. Marquis, A. Boire, R. Cochereau, C. Amine and S. Marze, Micromachines, 2021, 12, 863 CrossRef PubMed.
- D. R. Link, E. Grasland-Mongrain, A. Duri, F. Sarrazin, Z. Cheng, G. Cristobal, M. Marquez and D. A. Weitz, Angew. Chem., Int. Ed., 2006, 45, 2556–2560 CrossRef CAS PubMed.
- C. N. Baroud, M. R. d. S. Vincent and J.-P. Delville, Lab Chip, 2007, 7, 1029 RSC.
- S.-H. Tan, S. M. S. Murshed, N.-T. Nguyen, T. N. Wong and L. Yobas, J. Phys. D Appl. Phys., 2008, 41, 165501 CrossRef.
- P. Zhu and L. Wang, Lab Chip, 2017, 17, 34–75 RSC.
- Y. Shi, G. H. Tang and H. H. Xia, Comput. Fluids, 2014, 90, 155–163 CrossRef.
- S. L. Anna, N. Bontoux and H. A. Stone, Appl. Phys. Lett., 2003, 82, 364–366 CrossRef CAS.
- S. Xu, Z. Nie, M. Seo, P. Lewis, E. Kumacheva, H. A. Stone, P. Garstecki, D. B. Weibel, I. Gitlin and G. M. Whitesides, Angew. Chem., Int. Ed., 2005, 44, 724–728 CrossRef CAS PubMed.
- S. Wiedemeier, M. Eichler, R. Römer, A. Grodrian, K. Lemke, K. Nagel, C. Klages and G. Gastrock, Eng. Life Sci., 2017, 17, 1271–1280 CrossRef CAS PubMed.
- S. G. Sontti and A. Atta, Phys. Fluids, 2023, 35(1), 012010 CrossRef CAS.
- S. Mottaghi, M. Nazari, S. M. Fattahi, M. Nazari and S. Babamohammadi, Biomed. Microdevices, 2020, 22, 61 CrossRef PubMed.
- T. Ward, M. Faivre, M. Abkarian and H. A. Stone, Electrophoresis, 2005, 26, 3716–3724 CrossRef CAS PubMed.
- L. Peng, M. Yang, S. Guo, W. Liu and X. Zhao, Biomed. Microdevices, 2011, 13, 559–564 CrossRef PubMed.
- A. M. Ibrahim, J. I. Padovani, R. T. Howe and Y. H. Anis, Micromachines, 2021, 12, 590 CrossRef PubMed.
- S. Da Ling, Y. Geng, A. Chen, Y. Du and J. Xu, Biomicrofluidics, 2020, 14, 061508 CrossRef PubMed.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- A. S. Khorrami and P. Rezai, Biomicrofluidics, 2018, 12(3), 034113 CrossRef PubMed.
- S. Srikanth, S. Raut, S. K. Dubey, I. Ishii, A. Javed and S. Goel, Eur. Phys. J. E: Soft Matter Biol. Phys., 2021, 44, 108 CrossRef CAS PubMed.
- J. Yao, F. Lin, H. Kim and J. Park, Micromachines, 2019, 10, 808 CrossRef PubMed.
- Q. Hu, T. Jiang and H. Jiang, Micromachines, 2020, 11, 169 CrossRef PubMed.
- Y. Nakama, in Cosmetic Science and Technology, Elsevier, 2017, pp. 231–244 Search PubMed.
- J.-C. Baret, Lab Chip, 2012, 12, 422–433 RSC.
- J. Bibette, D. C. Morse, T. A. Witten and D. A. Weitz, Phys. Rev. Lett., 1992, 69, 2439–2442 CrossRef CAS PubMed.
- S. van der Graaf, C. G. P. H. Schroën, R. G. M. van der Sman and R. M. Boom, J. Colloid Interface Sci., 2004, 277, 456–463 CrossRef CAS PubMed.
- Y. Chen, S. Narayan and C. S. Dutcher, Langmuir, 2020, 36, 14904–14923 CrossRef CAS PubMed.
- T. Fu, Y. Wu, Y. Ma and H. Z. Li, Chem. Eng. Sci., 2012, 84, 207–217 CrossRef CAS.
- O. Sartipzadeh, S. M. Naghib, A. Seyfoori, M. Rahmanian and F. S. Fateminia, Mater. Sci. Eng., C, 2020, 109, 110606 CrossRef CAS PubMed.
- S. K. Jena, T. Srivastava, S. S. Bahga and S. Kondaraju, Phys. Fluids, 2023, 35(2), 022107 CrossRef CAS.
- W. Yu, X. Liu, B. Li and Y. Chen, Int. J. Multiphase Flow, 2022, 149, 103973 CrossRef CAS.
- M. Outokesh, H. A. Amiri and M. Miansari, Chem. Eng. Process., 2022, 170, 108696 CrossRef CAS.
- A. Gupta, H. S. Matharoo, D. Makkar and R. Kumar, Comput. Fluids, 2014, 100, 218–226 CrossRef CAS.
- F. Mardani, S. Falahatian and M. Taghipoor, Results Eng., 2023, 18, 101125 CrossRef CAS.
- P. Sajeesh, M. Doble and A. K. Sen, Biomicrofluidics, 2014, 8(5), 054112 CrossRef CAS PubMed.
- G. F. Christopher and S. L. Anna, J. Phys. D Appl. Phys., 2007, 40, R319–R336 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |