Open Access Article
Open Access ArticleInvestigating the efficacy of green solvents and solvent-free conditions in hydrogen-bonding mediated organocatalyzed model reactions†
Lloyd C. Chetty a,
Hendrik G. Kruger
a,
Hendrik G. Kruger a,
Per I. Arvidsson
a,
Per I. Arvidsson ab,
Tricia Naicker
ab,
Tricia Naicker *a and
Thavendran Govender*c
*a and
Thavendran Govender*c
aCatalysis and Peptide Research Unit, University of KwaZulu Natal, Durban, 4001, South Africa
bScience for Life Laboratory, Drug Discovery & Development Platform & Division of Translational Medicine and Chemical Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden
cDepartment of Chemistry, University of Zululand, Private Bag X1001, KwaDlangezwa 3886, South Africa. E-mail: govenderthav@icloud.com; naickert1@ukzn.ac.za
First published on 7th March 2024
Abstract
In this study, we have delved into various reactions conducted using green solvents or under solvent-free conditions, employing hydrogen bonding organocatalysis to advance more sustainable practices in chemical synthesis. The outcomes suggest that cyclopentyl methyl ether could potentially replace non-polar organic solvents such as hexane and toluene with comparable enantioselectivity and yields. The non-polar nature of liquefied or supercritical CO2 restricts its application to reactions that require non-polar solvents. Furthermore, pursuing solvent-free conditions, even without liquid substrates, might result in similar conversion rates with reduced catalyst loading. These findings highlight the potential of exploring solvent-free conditions when enantioselectivity is not of concern. Based on the results, solvent-free conditions and bio-based solvents can serve as viable alternatives to conventional organic solvents without compromising performance. This is expected to influence the way chemists approach reaction optimisation within method development in the field, fostering a broader adoption of environmentally friendly approaches.
Introduction
Organocatalysis is one of the most flourishing research areas in organic synthesis that covers a series of classic catalytic modes.1–8 Among the different organocatalytic activation mechanisms, hydrogen bonding is a powerful method to activate Lewis basic substrates.9 This activation mode has laid the groundwork for a vast and dynamic field of research, resulting in the development of over 30 new asymmetric reactions.10 In addition, organocatalysis is especially appealing for constructing bioactive molecules that typically cannot withstand metal impurities.11 Consequently, in 2019, IUPAC designated enantioselective organocatalysis as one of the ten emerging technologies in the field of chemistry with the potential to make our planet more sustainable.12 However, the greenness of organocatalysis is often debated due to catalyst recycling and high catalyst loading.13 Additionally, organocatalysts often employ hazardous solvents such as DMSO, DMF, acetonitrile, dichloromethane, or toluene.14–17 Applying greener solvent alternatives or solvent-free conditions is a straightforward approach to improving the sustainability of any chemical reaction. Several factors influence the choice of a greener solvent. The solvent should be relatively non-toxic, non-hazardous and not be released into the environment.18 Furthermore, there is increasing scrutiny on solvent use, and environmental legislation is becoming increasingly stringent to ensure sustainability in chemical processes.19An examination of solvent utilisation between 1997 and 2012 indicated significant potential for improvement across the pharmaceutical industry.20 A recent trend in asymmetric chemical synthesis is the use of biosolvents, which are derived from biomass and offer properties like low toxicity and high biodegradability.21 A recent review by Corrêa and co-workers reports that chiral organocatalysts in greener solvents have been limited to ethanol, ethyl acetate, 2-methyl tetrahydrofuran, and ethyl lactate, despite the growing availability of the commercial portfolio of other green solvents.22
Among greener solvents, carbon dioxide (CO2) has attracted significant interest in green chemistry due to its availability, non-toxicity, non-flammability, and stability.23,24 Liquid and supercritical CO2 have been extensively studied in chemical reactions, including asymmetric synthesis using organometallics25–29 or biocatalysts.30–33 Currently, there are limited studies on applying CO2 as a reaction medium in asymmetric organocatalysis.23,34–37
Further research is necessary to comprehensively grasp the scope and limitations of environmentally friendly solvents. Herein, we have investigated several reactions in solvent-free conditions and green solvents that utilise hydrogen bonding organocatalysis to promote more sustainable methods within chemical synthesis.
Results and discussion
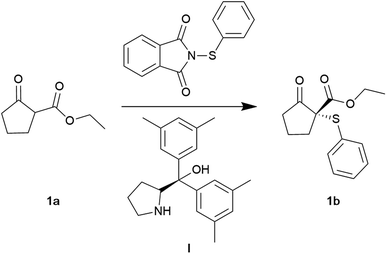
The synthesis of optically active organosulphur compounds is highly desirable as chiral sulphur-containing compounds are common in natural products, pharmaceuticals, and bioactive molecules.38–40 In particular, thioether structural fragments are important bioisosteric replacements in rational drug design.41 Functionalised β-ketoesters are versatile building blocks and synthons in organic chemistry, widely employed in efficiently synthesising various complex natural products.42 Considering their significance in medicine, there has been substantial interest in developing new methods for optically active derivatives containing a carbon–heteroatom bond.43,44 We began our investigation with the effect of greener solvents on Zhu and co-worker's asymmetric sulfenylation of β-ketoesters initially reported in hexane.45 However, European Directives and Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) now strictly regulate solvents derived from petrochemical sources such as hexane.46 In addition, hexane poses significant health risks (neurotoxin) and is at risk of being prohibited in Europe within the coming years. Accordingly, substituting hexane with environmentally sustainable alternatives is of significant interest to academic and industrial sectors.47At the reported conditions of 20 mol% of catalyst (I), RT (room temperature) in hexane, the reaction had a conversion of 99% and an ee of 82% (Table 1, entry 1). The excellent conversion was maintained even at 5 mol% catalyst loading (Table 1, entry 2). However, a further decrease in catalyst loading inhibited the reaction (Table 1, entry 3). Applying cyclopentylmethyl ether (CPME) as a solvent led to a conversion and ee identical to hexane (Table 1, entry 4). The application of N-butyl-2-pyrrolidone (NBP) led to a conversion of 90% but a poor ee of 30% (Table 1, entry 5). Applying γ-valerolactone (GVL) or 2-methyl tetrahydrofuran (2-MeTHF) had moderate conversions of 40 and 52%, respectively (Table 1, entry 6 and 7). Performing the reaction under liquid CO2 conditions (100 bars, RT) led to an excellent conversion of 96% and an ee of 72% (Table 1, entry 8). Under solvent-free conditions, a conversion of 91% was obtained with a slight decrease in ee (70%, Table 1, entry 9). As noted earlier, the increase in concentration allowed the reaction to be performed even at 1 mol% catalyst with a good conversion of 75% (Table 1, entry 11), which was impossible in hexane (Table 1, entry 3). The results demonstrate that the hydrophobic green ether CPME can replace hexane in this organocatalysed reaction. In addition, liquid CO2 and solvent-free conditions can also serve as benign alternatives, albeit with a decrease in enantioselectivity.
| Entry | Catalyst loading [mol%] | Solvent | Conversionb [%] | ee [%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.19 mmol), N-(phenylthio)phthalimide (1.2 equiv.), (S)-α,α-bis(3,5-dimethylphenyl)-2-pyrrolidinemethanol, solvent (0.1 M), for 3 hours at RT.b Conversion was determined by GC-MS analysis of the reaction mixture; entries were performed in duplicate.c Reaction conditions: stirred for 3 hours under 100 bars of CO2 at RT.d Isolated yield. | ||||
| 1 | 20 | Hexane | 99 | 82 |
| 2 | 5 | Hexane | 94 | 82 |
| 3 | 1 | Hexane | NR | — |
| 4 | 5 | CPME | 99 (99)d | 83 |
| 5 | 5 | NBP | 90 | 30 |
| 6 | 5 | GVL | 40 | — |
| 7 | 5 | 2-MeTHF | 52 | — |
| 8c | 5 | Liquid CO2 | 96 | 72 |
| 9 | 5 | Neat | 91 | 70 |
| 10 | 1 | Neat | 75 | 68 |
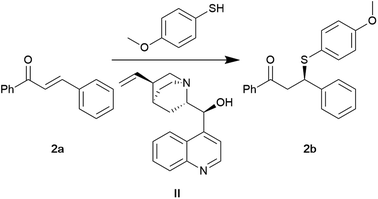
Among the strategies for synthesising chiral organosulphur compounds is the asymmetric sulfa-Michael addition.48 Turowska-Tyrk and associates investigated the asymmetric Michael addition of thiophenols to chalcones to prepare chiral building blocks, which are useful for synthesising biologically active compounds.49 We initiated our investigation with the asymmetric Michael addition of 4-methoxybenzenethiol to chalcone initially reported in anhydrous toluene. It is suspected that prolonged exposure to toluene could potentially harm an unborn child and cause organ damage. Although commonly used as a substitute for carcinogenic benzene, toluene is a non-renewable solvent under regulatory scrutiny.50 Therefore, alternatives which are safe and bio-based are essential.
At the reported conditions of 1.5 mol% cinchonine (II) in anhydrous toluene, a conversion of 91% and an ee of 40% were obtained (Table 2, entry 1). Applying CPME as a solvent led to an excellent conversion (87%) and equivalent ee (Table 2, entry 2). The application of other bio-based solvents had good to excellent conversions (80–99%) but were not enantioselective (Table 3, entries 2–5). Performing the reaction under liquid CO2 conditions (100 bars, −20 °C) led to a moderate conversion of 67% with poor ee (18%, Table 2, entry 6). In the absence of a solvent, the reaction maintained an excellent conversion of 88%. However, enantioselectivity was lost (14%, Table 2, entry 7). Following the previous trends, solvent-free conditions allowed for a 300× reduction in catalyst loading (Table 2, entries 8 and 9). In addition, under solvent-free conditions, the reaction could proceed uncatalysed with a moderate conversion. This was impossible in anhydrous toluene (Table 2, entries 10 and 11). The results indicate that CPME can be a green alternative to toluene. CPME demonstrates minimal acute or subchronic toxicity, coupled with moderate irritation and a lack of genotoxic and mutagenic effects.51 It is important to note that when handling CPME, care should be taken not to swallow or expose the skin and eyes.52 It should also be noted that anhydrous CPME was not required. As observed earlier, liquid CO2 and solvent-free conditions offer similar conversions and enantioselectivity. This may be due to the low solubilising power of CO2.
| Entry | Catalyst loading [mol%] | Solvent | Conversionb [%] | ee [%] |
|---|---|---|---|---|
| a Reaction conditions: 2a (0.24 mmol), methoxybenzenethiol (1.1 equiv.), cinchonine, solvent (0.33 M), 4 hours at −20 °C.b Conversion was determined by LC-MS analysis of the reaction mixture; entries were performed in duplicate.c Reaction conditions: stirred for 4 hours under 100 bars of CO2 at −20 °C.d Isolated yield. | ||||
| 1 | 1.5 | Toluene | 91 | 40 |
| 2 | 1.5 | CPME | 87 (85)d | 40 |
| 3 | 1.5 | NBP | 86 | 0 |
| 4 | 1.5 | GVL | 80 | 0 |
| 5 | 1.5 | 2-MeTHF | 99 | 0 |
| 6c | 1.5 | Liquid CO2 | 67 | 18 |
| 7 | 1.5 | Neat | 88 | 14 |
| 8 | 0.1 | Neat | 86 | — |
| 9 | 0.005 | Neat | 88 | — |
| 10 | 0 | Neat | 58 | — |
| 11 | 0 | Toluene | 6 | — |
| Entry | Catalyst loading [mol%] | Solvent | Conversionb [%] | ee [%] |
|---|---|---|---|---|
| a Reaction conditions: 3a (0.03 mmol), thiourea (1.2 equiv.), ethyl acetoacetate (3 equiv.), (S)-3,3′-bis(triphenylsilyl)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate, solvent (0.1 M), 60 hours at 50 °C.b Conversion was determined by LC-MS analysis of the reaction mixture; entries were performed in duplicate.c Reaction conditions: stirred for 60 hours under 80 bars of CO2 at 50 °C.d Isolated yield. | ||||
| 1 | 10 | Toluene | 99 | 92 |
| 2 | 10 | CPME | 99 (98)d | 92 |
| 3 | 10 | NBP | NR | — |
| 4 | 10 | GVL | NR | — |
| 5 | 10 | 2-MeTHF | 40 | — |
| 6c | 10 | scCO2 | NR | — |
| 7 | 10 | Neat | 91 | 46 |
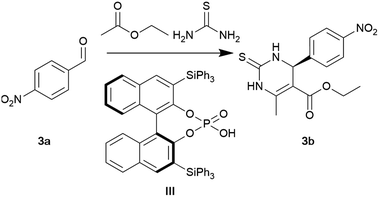
One of the classical methods for synthesising heterocycles is the Biginelli three-component condensation, which involves the combination of an aldehyde, a β-keto ester, and thiourea, resulting in the formation of dihydropyrimidinethiones (DHPMs).53 In medicinal chemistry, DHPMs are regarded as privileged structures owing to the presence of the DHPM component in numerous drug candidates.54 Enantioselective synthesis holds a crucial position in the pharmaceutical industry, as individual enantiomers may exhibit varying behaviours under physiological conditions.55,56
Accordingly, we next focused on investigating the effect of greener solvents on the asymmetric Biginelli reaction catalysed by the MacMillan TiPSY catalyst (III), as initially reported by Gong and co-workers.57 At the reported conditions of 10 mol% chiral phosphoric acid in toluene, a conversion of 99% and an ee of 92% were obtained (Table 3, entry 1). As seen in Tables 1 and 2, applying CPME as an alternative to toluene led to equivalent conversion (99%) and ee (Table 3, entry 2). Application of the polar aprotic solvents NBP and GVL inhibited the reaction (Table 3, entries 4 and 5). Performing the reaction in 2-MeTHF decreased the conversion to 40% (Table 3, entry 6), while performing the reaction under supercritical CO2 (scCO2) conditions (80 bars, 50 °C) again inhibited the reaction. In the absence of a solvent, the reaction maintained excellent conversions. However, under these conditions, the reaction possessed poor selectivity towards the desired Biginelli product (Table 3, entries 7). In alignment with prior trends under neat conditions, there was a decrease in enantioselectivity.
Reductive amination represents one of the most powerful and commonly employed techniques for producing amines.58 This versatile coupling reaction provides rapid and general access to forming C–N bonds, a crucial structural feature in natural products and pharmaceuticals.59 In metal hydride reductive processes, eliminating toxic metal impurities is challenging, yet crucial, particularly in pharmaceutical manufacturing. The pursuit of metal-free reductive methods is an important research objective. Consequently, the use of Hantzsch ester as a biomimetic, cost-effective, stable, and safe reducing agent is of great interest.60
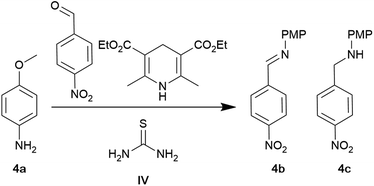
We investigated the thiourea-catalysed (IV) reductive amination of aldehydes using Hantzsch ester as the reducing agent in anhydrous toluene initially reported by Menche and Arikan.61 At the reported conditions of 10 mol% thiourea in anhydrous toluene, the conversion of p-anisidine was 99%, and the ratio between the imine and secondary amine was 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 (Table 4, entry 1). Hantzsch ester was consumed entirely, indicating an equivalence higher than 1.2 is required to drive the reaction. Replacing toluene with CPME leads to an increase in imine formation (Table 4, entry 2). The polar aprotic solvent NBP allowed an almost complete reduction of the imine (Table 4, entry 3). The use of GVL as a solvent led to a complete reduction (Table 4, entry 4). When 2-MeTHF was applied as a solvent, a ratio of 20
91 (Table 4, entry 1). Hantzsch ester was consumed entirely, indicating an equivalence higher than 1.2 is required to drive the reaction. Replacing toluene with CPME leads to an increase in imine formation (Table 4, entry 2). The polar aprotic solvent NBP allowed an almost complete reduction of the imine (Table 4, entry 3). The use of GVL as a solvent led to a complete reduction (Table 4, entry 4). When 2-MeTHF was applied as a solvent, a ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 of imine to secondary amine was obtained (Table 4, entry 5). The reaction was incompatible with scCO2, most likely due to solubility issues. Conducting the reaction under solvent-free conditions allowed for a drop in catalyst loading to 1 mol% while maintaining reactivity (Table 4, entries 7–9). As noted earlier, the uncatalysed reaction was more active under solvent-free conditions than in anhydrous toluene (Table 4, entries 10 and 11). The results illustrate that the polar aprotic bio-based GVL or NBP can be green alternatives to toluene. While NBP is not inherently derived from biological sources, it can be manufactured using renewable resources.62 It should also be noted that anhydrous solvents were not required, making this protocol more convenient and benign, as the solvent drying step is avoided. The non-polar nature of scCO2 prevented its application, but it should be kept in mind that a bio-based modifier could be added to adjust solubility. Solvent-free conditions offer similar conversion to the bio-based solvents with a decreased catalyst loading.
80 of imine to secondary amine was obtained (Table 4, entry 5). The reaction was incompatible with scCO2, most likely due to solubility issues. Conducting the reaction under solvent-free conditions allowed for a drop in catalyst loading to 1 mol% while maintaining reactivity (Table 4, entries 7–9). As noted earlier, the uncatalysed reaction was more active under solvent-free conditions than in anhydrous toluene (Table 4, entries 10 and 11). The results illustrate that the polar aprotic bio-based GVL or NBP can be green alternatives to toluene. While NBP is not inherently derived from biological sources, it can be manufactured using renewable resources.62 It should also be noted that anhydrous solvents were not required, making this protocol more convenient and benign, as the solvent drying step is avoided. The non-polar nature of scCO2 prevented its application, but it should be kept in mind that a bio-based modifier could be added to adjust solubility. Solvent-free conditions offer similar conversion to the bio-based solvents with a decreased catalyst loading.
| Entry | Catalyst loading [mol%] | Solvent | Conversionb [%] | 4bb | 4cb |
|---|---|---|---|---|---|
| a Reaction conditions: 4a (0.24 mmol), 4-nitrobenzaldehyde (1.2 equiv.), Hantzsch ester (1.2 equiv.), thiourea, solvent (0.4 M) stirred for 16 hours at 70 °C.b Conversion was determined by GC-MS analysis of the reaction mixture; entries were performed in duplicate.c Reaction conditions: stirred for 16 hours under 80 bars of CO2 at 70 °C.d Isolated yield. | |||||
| 1 | 10 | Toluene | 99 | 9 | 91 |
| 2 | 10 | CPME | 99 | 31 | 69 |
| 3 | 10 | NBP | 99 | 4 | 96 |
| 4 | 10 | GVL | 99 | — | 100 (90)d |
| 5 | 10 | 2-MeTHF | 99 | 20 | 80 |
| 6c | 10 | scCO2 | NR | — | — |
| 7 | 10 | Neat | 99 | 17 | 83 |
| 8 | 5 | Neat | 99 | 20 | 80 |
| 9 | 1 | Neat | 99 | 9 | 91 |
| 10 | 0 | Neat | 99 | 34 | 66 |
| 11 | 0 | Toluene | 99 | 66 | 34 |
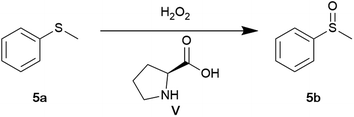
Sulfoxides are valuable synthetic intermediates in synthesising a wide range of biologically active compounds.63,64 The most direct approach to synthesising sulfoxides is through the oxidation of sulfides. While numerous reagents can be used for this purpose, many tend to over-oxidise to sulfones. Consequently, careful control of reaction conditions is necessary to prevent the formation of sulfones.65 In contrast to other oxidising agents, hydrogen peroxide (H2O2) is highly appealing from an environmental perspective. It is an ideal, waste-minimising oxidant, as its only theoretical by-product is water.66
Lastly, the investigation focused on the L-proline (V) catalysed oxidation of thioanisole. The oxidation of sulfides initially reported by Ravindra and co-workers utilised a hydrogen bonding L-proline H2O2 system.67 Ravindra reported a conversion of 98% using 10 mol% L-proline in acetonitrile (0.33 M). However, in our hands, using the exact conditions, a conversion of 4% was obtained (Table 5, entry 1). In addition, using a new bottle of H2O2 did not improve conversions. Interestingly, decreasing the solvent volume increased the conversion to 89% (Table 5, entry 2). A range of bio-based solvents were evaluated (Table 5, entries 3–6). However, poor conversions were obtained (4–28%). Under scCO2 conditions (80 bars, 40 °C), low conversion was obtained with a mixture of sulfoxide and sulfone products (Table 5, entry 7). This is most likely attributed to the low solubility of proline in scCO2 at milder pressures.36,37 It has been previously reported by Liu et al. that higher pressures increase conversions for the L-proline-catalysed aldol reaction under scCO2 conditions.36 Inspired by previous reports on the solvent-free oxidation of sulfides using H2O2, the reaction was conducted under neat conditions.68,69 Remarkably, a conversion of 99% was obtained (Table 5, entry 8). In addition, the catalyst loading could be dropped to 1 mol% while maintaining excellent conversion (Table 5, entry 9). Any further drop in catalyst loading displayed similar conversions to the uncatalysed reaction (Table 5, entries 10–12). The decrease in catalyst loading is arguably a result of an increase in concentration due to solvent-free conditions. Although bio-based solvents were unsuccessful in replacing acetonitrile, the results highlight the use of solvent-free conditions and the need to explore these conditions during method development. It is important to note that no enantioselectivity with L-proline was observed.
| Entry | Catalyst loading [mol%] | Solvent | Conversionb [%] |
|---|---|---|---|
| a Reaction conditions: 5a (0.24 mmol), H2O2 (2.5 equiv.), L-proline, solvent (3.8 M) for 2 hours at 40 °C.b Conversion was determined by LC-MS analysis of the reaction mixture; entries were performed in duplicate.c Reaction conditions: acetonitrile (0.33 M).d Reaction conditions: stirred for 2 hours under 80 bars of CO2 at 40 °C.e Isolated yield. | |||
| 1 | 10c | Acetonitrile | 4 |
| 2 | 10 | Acetonitrile | 89 |
| 3 | 10 | γ-Valerolactone (GVL) | 28 |
| 4 | 10 | N-butyl-2-pyrrolidone (NBP) | 6 |
| 5 | 10 | Cyclopentylmethyl ether (CPME) | 4 |
| 6 | 10 | 2-MeTHF | 9 |
| 7d | 10 | scCO2 | 45 |
| 8 | 10 | Neat | 99 (99)e |
| 9 | 1 | Neat | 87 |
| 10 | 0.1 | Neat | 79 |
| 11 | — | Neat | 81 |
While studying the impact of solvents on reactivity often involves considering intrinsic properties such as polarity or dielectric constants, they may not always provide a complete explanation, and the effects can be more complex.70 Solvation encompasses all types of inter-molecular interactions. Solvents employed as media for chemical reactions have a profound effect on both the thermodynamics and kinetics of chemical processes.71 Although there may be instances where reactivity and polarity do not correlate, the polarity can serve as an initial reference point during solvent selection in method development.
In academia, where small-scale chemistry predominates, there's often a perception that organic waste is primarily an industry-related concern. However, this perspective overlooks that academic research groups often contribute significantly to organic waste generation. When the total volume and weight of solvents and contaminated water used in these processes are calculated and compared to the final product's weight, the E factor can reach the hundreds.72 Hence, developing methodologies for organocatalysis in green solvents or under solvent-free conditions can substantially contribute to advancing the sustainability of organic synthesis. Additionally, the availability of greener methods from academic laboratories could support industrial pharmaceutical laboratories in adopting greener routes, even at small-scale synthesis.73
Experimental
For experimental procedures refer to the ESI.†Conclusions
Within this work, we have preliminarily assessed hydrogen bonding organocatalysts on model reactions in green solvents or under solvent-free conditions.While CPME can be considered a greener alternative to more problematic ethereal solvents, the results indicate that CPME is a potential green replacement for reactions in non-polar organic solvents such as hexane and toluene with equivalent conversion and ee (Tables 1–3).21 However, it is essential to note that while CPME may be deemed more environmentally friendly than some other solvents, responsible handling and disposal practices are still important to minimise its environmental impact.74
The polar aprotic solvents NBP and GVL can serve as green alternatives in reactions requiring heating due to their high boiling points of 240.6 °C and 207–208 °C, respectively (Table 4). However, this property also serves as a disadvantage, as these solvents are difficult to remove and recover efficiently. Notably, the workup of reaction products still required conventional organic solvent extraction or water precipitation. Both of which may impact the greenness of the protocol. Solvent-free conditions, even in the absence of liquid substrates (Table 4), may lead to comparable conversions with reduced catalyst loading; however, mostly with a drastic impact on enantioselectivity. As process chemistry laboratories are looking for more sustainable approaches, these results warrant exploring solvent-free conditions during method development when ee is not of concern. It should be highlighted that “The best solvent is no solvent”.18 Nonetheless, solvents are essential for several chemical processes, as solvation offers several key advantages, such as improved selectivity, safety (for example, as a heat sink or containing toxic reagents), and enhanced ease of handling.75
The non-polar nature of liquefied or supercritical CO2 restricts its application to reactions that require non-polar solvents (Table 1). However, the polarity of liquefied or supercritical CO2 can be adjusted with an appropriate modifier, which is worthwhile to explore further. In addition, liquefied or supercritical CO2 may be more suited to reactions requiring CO2 or gaseous reactants (such as O2 and H2) due to the miscibility of gases.76
The future for organic synthesis as a whole will most likely involve a gradual shift away from petrochemical-based solvents and unsustainable practices. It is worth mentioning that there is no absolute green solvent. It always depends on the context of its application, and it is only a relative term.
Based on the results of several model reactions presented in this study, solvent-free conditions and bio-based solvents can be applied as alternatives to conventional organic solvents without performance loss. Hence, it is advisable to include green solvents in the solvent selection process during method development. While preserving enantioselectivity under greener conditions remains a challenge, this study serves as a catalyst for future investigations in the realm of genuinely green asymmetric organocatalysis, paving the way for further advancements in this direction.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the South African National Research Foundation, the Nuclear Medicine Research Infrastructure, and the College of Health Sciences, UKZN for the financial support. The South African National Research Foundation grant numbers 137961, 145774, 120419.Notes and references
- S. Shirakawa and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 4312–4348 CrossRef CAS PubMed.
- E. A. C. Davie, S. M. Mennen, Y. Xu and S. J. Miller, Chem. Rev., 2007, 107, 5759–5812 CrossRef.
- S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471–5569 CrossRef CAS PubMed.
- K. L. Jensen, G. Dickmeiss, H. Jiang, Ł. Albrecht and K. A. Jørgensen, Acc. Chem. Res., 2012, 45, 248–264 CrossRef CAS.
- D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS.
- T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed.
- D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS.
- M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520–1543 CrossRef CAS.
- A. Franconetti and G. de Gonzalo, ChemCatChem, 2018, 10, 5554–5572 CrossRef CAS.
- A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713–5743 CrossRef CAS PubMed.
- B. Han, X.-H. He, Y.-Q. Liu, G. He, C. Peng and J.-L. Li, Chem. Soc. Rev., 2021, 50, 1522–1586 RSC.
- F. Gomollón-Bel, Chem. Int., 2019, 41, 12–17 Search PubMed.
- D. W. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS PubMed.
- S. Schenker, A. Zamfir, M. Freund and S. B. Tsogoeva, Eur. J. Org Chem., 2011, 2011, 2209–2222 CrossRef.
- P. Chauhan, S. Mahajan, U. Kaya, D. Hack and D. Enders, Adv. Synth. Catal., 2015, 357, 253–281 CrossRef CAS.
- X. Fang and C.-J. Wang, Chem. Commun., 2015, 51, 1185–1197 RSC.
- Š. Toma, M. Mečiarová and R. Šebesta, Eur. J. Org Chem., 2009, 2009, 321–327 CrossRef.
- R. A. Sheldon, Green Chem., 2005, 7, 267–278 RSC.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- C. P. Ashcroft, P. J. Dunn, J. D. Hayler and A. S. Wells, Org. Process Res. Dev., 2015, 19, 740–747 CrossRef CAS.
- M. Miele, V. Pillari, V. Pace, A. R. Alcántara and G. de Gonzalo, Molecules, 2022, 27, 6701 CrossRef CAS PubMed.
- L. S. R. Martelli, I. V. Machado, J. R. N. dos Santos and A. G. Corrêa, Catalysts, 2023, 13, 553 CrossRef CAS.
- A. G. Nigmatov, I. V. Kuchurov, D. E. Siyutkin and S. G. Zlotin, Tetrahedron Lett., 2012, 53, 3502–3505 CrossRef CAS.
- L. C. Chetty, H. G. Kruger, P. I. Arvidsson and T. Naicker, Synthesis, 2022, 54, 4827–4833 CrossRef CAS.
- J. Xiao, S. C. A. Nefkens, P. G. Jessop, T. Ikariya and R. Noyori, Tetrahedron Lett., 1996, 37, 2813–2816 CrossRef CAS.
- S. Kainz and W. Leitner, Catal. Lett., 1998, 55, 223–225 CrossRef CAS.
- G. Franciò and W. Leitner, Chem. Commun., 1999, 1663–1664, 10.1039/A904281D.
- G. Franciò, K. Wittmann and W. Leitner, J. Organomet. Chem., 2001, 621, 130–142 CrossRef.
- W. Leitner, Acc. Chem. Res., 2002, 35, 746–756 CrossRef CAS.
- M. T. Reetz, W. Wiesenhöfer, G. Franciò and W. Leitner, Chem. Commun., 2002, 992–993, 10.1039/B202322A.
- R. A. Sheldon, Chem.–Eur. J., 2016, 22, 12984–12999 CrossRef CAS PubMed.
- T. Matsuda, J. Biosci. Bioeng., 2013, 115, 233–241 CrossRef CAS PubMed.
- P. Lozano, Green Chem., 2010, 12, 555–569 RSC.
- E. V. Filatova, O. V. Turova, I. V. Kuchurov, A. A. Kostenko, A. G. Nigmatov and S. G. Zlotin, J. Supercrit. Fluids, 2016, 109, 35–42 CrossRef CAS.
- I. V. Kuchurov, A. G. Nigmatov, E. V. Kryuchkova, A. A. Kostenko, A. S. Kucherenko and S. G. Zlotin, Green Chem., 2014, 16, 1521–1526 RSC.
- L. Liu, Z.-T. Liu, Z.-W. Liu and D. Xue, Sci. China: Chem., 2010, 53, 1586–1591 CrossRef CAS.
- Z. Li, J.-G. Chen, L.-P. Song, Z. W. Liu, D. Xue and Z.-T. Liu, Russ. J. Org. Chem., 2013, 49, 1854 CrossRef CAS.
- J.-S. Yu, H.-M. Huang, P.-G. Ding, X.-S. Hu, F. Zhou and J. Zhou, ACS Catal., 2016, 6, 5319–5344 CrossRef CAS.
- Y. Liang and X. Zhao, ACS Catal., 2019, 9, 6896–6902 CrossRef CAS.
- D. Zhu, T. Mu, Z.-L. Li, H.-Y. Luo, R.-F. Cao, X.-S. Xue and Z.-M. Chen, Angew. Chem., Int. Ed., 2024, e202318625 CAS.
- Q. Tan, Q. Chen, Z. Zhu and X. Liu, Chem. Commun., 2022, 58, 9686–9689 RSC.
- M. Jereb and A. Togni, Chem.–Eur. J., 2007, 13, 9384–9392 CrossRef CAS PubMed.
- T. Govender, P. I. Arvidsson, G. E. Maguire, H. G. Kruger and T. Naicker, Chem. Rev., 2016, 116, 9375–9437 CrossRef CAS PubMed.
- C. Wiśniewska, D. Koszelewski, M. Zysk, S. Kłossowski, A. Żądło, A. Brodzka and R. Ostaszewski, Eur. J. Org Chem., 2015, 2015, 5432–5437 CrossRef.
- L. Fang, A. Lin, H. Hu and C. Zhu, Chem.–Eur. J., 2009, 15, 7039–7043 CrossRef CAS PubMed.
- E. Yara-Varon, A.-S. Fabiano-Tixier, M. Balcells, R. Canela-Garayoa, A. Bily and F. Chemat, RSC Adv., 2016, 6, 27750–27759 RSC.
- K. Häckl and W. Kunz, C. R. Chim., 2018, 21, 572–580 CrossRef.
- C. Niu and D.-M. Du, Chem. Rec., 2023, 23, e202200258 CrossRef CAS PubMed.
- J. Skarżewski, M. Zielińska-Błajet and I. Turowska-Tyrk, Tetrahedron: Asymmetry, 2001, 12, 1923–1928 CrossRef.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- V. Antonucci, J. Coleman, J. B. Ferry, N. Johnson, M. Mathe, J. P. Scott and J. Xu, Org. Process Res. Dev., 2011, 15, 939–941 CrossRef CAS.
- K. Watanabe, Molecules, 2013, 18, 3183–3194 CrossRef CAS PubMed.
- J.-P. Wan and Y. Liu, Synthesis, 2010, 2010, 3943–3953 CrossRef.
- F. Sánchez-Sancho, M. Escolano, D. Gaviña, A. G. Csáky, M. Sánchez-Roselló, S. Díaz-Oltra and C. Del Pozo, Pharmaceuticals, 2022, 15, 948 CrossRef PubMed.
- S. B. Ötvös and C. O. Kappe, Green Chem., 2021, 23, 6117–6138 RSC.
- Z. Zeng, C. Liao and L. Yu, Chin. Chem. Lett., 2023, 109349, DOI:10.1016/j.cclet.2023.109349.
- N. Li, X.-H. Chen, J. Song, S.-W. Luo, W. Fan and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 15301–15310 CrossRef CAS PubMed.
- O. I. Afanasyev, E. Kuchuk, D. L. Usanov and D. Chusov, Chem. Rev., 2019, 119, 11857–11911 CrossRef CAS PubMed.
- W. Shang, H. Sun, W. Chen and J. Liu, Green Synth. Catal., 2023, 4, 104–123 CrossRef.
- Q. P. B. Nguyen and T. H. Kim, Tetrahedron, 2013, 69, 4938–4943 CrossRef CAS.
- D. Menche and F. Arikan, Synlett, 2006, 2006, 841–844 CrossRef.
- J. Sherwood, H. L. Parker, K. Moonen, T. J. Farmer and A. J. Hunt, Green Chem., 2016, 18, 3990–3996 RSC.
- X. Zhang, F. Wang and C.-H. Tan, JACS Au, 2023, 3, 700–714 CrossRef CAS PubMed.
- E. Wojaczyńska and J. Wojaczyński, Curr. Opin. Chem. Biol., 2023, 76, 102340 CrossRef PubMed.
- K. Kaczorowska, Z. Kolarska, K. Mitka and P. Kowalski, Tetrahedron, 2005, 61, 8315–8327 CrossRef CAS.
- E. Skolia, P. L. Gkizis and C. G. Kokotos, ChemPlusChem, 2022, 87, e202200008 CrossRef CAS PubMed.
- K. Rajender Reddy, C. V. RajaSekhar and A. Ravindra, Synth. Commun., 2006, 36, 3761–3766 CrossRef.
- F. Shi, M. K. Tse, H. M. Kaiser and M. Beller, Adv. Synth. Catal., 2007, 349, 2425–2430 CrossRef CAS.
- M. Jereb, Green Chem., 2012, 14, 3047–3052 RSC.
- J. J. Varghese and S. H. Mushrif, React. Chem. Eng., 2019, 4, 165–206 RSC.
- N. Winterton, Clean Technol. Environ. Policy, 2021, 23, 2499–2522 CrossRef PubMed.
- B. H. Lipshutz, F. Gallou and S. Handa, ACS Sustainable Chem. Eng., 2016, 4, 5838–5849 CrossRef CAS.
- S. Sharma, J. Das, W. M. Braje, A. K. Dash and S. Handa, ChemSusChem, 2020, 13, 2859–2875 CrossRef CAS PubMed.
- ECHA, Substance Information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.104.006 Search PubMed.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Brohl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- G. Musie, M. Wei, B. Subramaniam and D. H. Busch, Coord. Chem. Rev., 2001, 219–221, 789–820 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00679h |
| This journal is © The Royal Society of Chemistry 2024 |