Open Access Article
Open Access ArticleIn vitro biological studies and computational prediction-based analyses of pyrazolo[1,5-a]pyrimidine derivatives†
Abdulrahman A. Almehizia a,
Wael M. Aboulthanab,
Ahmed M. Naglaha and
Ashraf S. Hassan
a,
Wael M. Aboulthanab,
Ahmed M. Naglaha and
Ashraf S. Hassan *c
*c
aDrug Exploration & Development Chair (DEDC), Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
bBiochemistry Department, Biotechnology Research Institute, National Research Centre, Dokki, 12662, Cairo, Egypt
cOrganometallic and Organometalloid Chemistry Department, National Research Centre, Dokki, 12622, Cairo, Egypt. E-mail: ashraf_salmoon@yahoo.com; as.el-salmoon@nrc.sci.eg
First published on 12th March 2024
Abstract
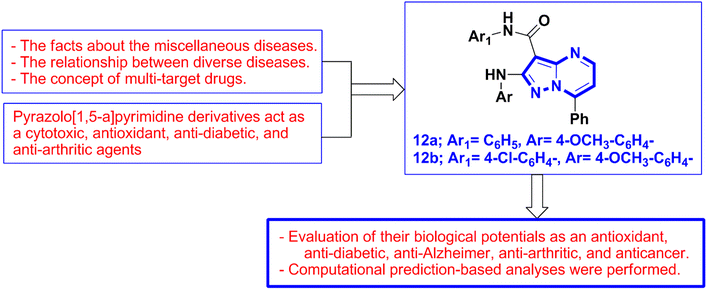
There is a need for new pharmaceutical discoveries from bioactive nitrogenous derivatives due to the emergence of scourges, numerous pandemics, and diverse health problems. In this context, pyrazolo[1,5-a]pyrimidine derivatives 12a and 12b were synthesized and screened to evaluate their biological potentials in vitro as antioxidants, anti-diabetics, anti-Alzheimer's, anti-arthritics, and anti-cancer agents. Additionally, the computational pharmacokinetic and toxicity properties of the two pyrazolo[1,5-a]pyrimidines 12a and 12b were calculated and analyzed. The preliminary studies and results of this work represent the initial steps toward more advanced studies and define the bioactive chemical structure of pyrazolo[1,5-a]pyrimidine derivatives with the goal of exploring new drugs to address numerous health problems.
1. Introduction
Antioxidant agents are a category of synthetic or natural chemical substances that can scavenge, block, or reduce oxidative stress (high free radical concentration). The free radicals are produced by the body due to the normal use of oxygen. The free radicals cause cell damage and can lead to human diseases such as cardiovascular diseases, aging, diabetes, and cancer.1,2 Examples of antioxidant agents include vitamin C (ascorbic acid), vitamin E (α-tocopherol), tripeptide glutathione (GSH), carotenoids, and flavonoids.3Diabetes is a 21st-century challenge.4 It is a deficiency in pancreatic function and secretion of the hormone insulin, which regulates glucose levels.5 Contemporary studies refer to the relationship between obesity, diabetes, and its development.6 The Arab world comprises 22 countries with 350 million humans. Six nations in the Arab world are on the top-ten list worldwide in the rate of diabetes and obesity majority. In general, almost 20% of the people in some Arab countries are diabetic.7
Pharmacological therapy for diabetes mellitus depends on the patient's case, in general, such as the influence of lifestyle modifications, during pregnancy, breastfeeding, or infection with other diseases.8 The common class examples of anti-diabetic drugs are as follows: (I) sulfonylureas, there are two generations: tolbutamide represents the first generation while glipizide and gliclazide are representatives of the second generation. The action mechanism of sulfonylureas anti-diabetic drugs is to increase insulin secretion from pancreatic β cells.9 (II) Alpha-glucosidase inhibitors (AGIs): there are various agents such as acarbose, miglitol, and voglibose, and the action mechanism is to decrease intestinal glucose absorption.10 (III) Amylin analogs: pramlintide is an injectable amylin analog, and its action mechanism is to decrease glucagon release and slow gastric emptying.11 Finally, (IV) biguanides: metformin is classified as a biguanides antidiabetic agent, and its action mechanism is to activate AMP-kinase and hepatic glucose production.12
Alzheimer's disease (AD) is a neurodegenerative disease that causes dementia.13 In 2019, the studies conducted by El-Metwally and co-workers14 demonstrated the following points: (I) Alzheimer's disease and dementia risk increased by various factors such as obesity, diabetes mellitus, and cardiovascular. (II) In Arab nations, dementia is a prevalent disease. (III) The statistical reports illustrated that between the age class of 50 and 80 years, dementia disease ranges from 1.1% to 2.3%, while in the age group of 80 years and older, dementia disease ranges from 13.5% to 18.5%. In the drug markets, there are various Alzheimer's agent therapies through acetylcholinesterase inhibition mechanism action; for example, galantamine is a type of phenanthrene alkaloid class, and donepezil is a piperidine class.15 Additionally, Alzheimer's disease therapy through acetylcholinesterase and butyrylcholinesterase inhibition mechanism action, such as rivastigmine drug, is classified as a phenylcarbamate category.16
Cancer is a genetic disease caused (I) by errors that occur in cell division, (II) by DNA damage due to harmful substances, and (III) by inherited from parents. Therefore, the body's cells grow uncontrollably and sometimes spread to other parts; this is a cancer disease.17 In 2020, the most prevalent cancers in Egypt are breast cancer and then liver cancer, as estimated by cancer statistics in Egypt. In addition, cancer mortality statistics refer to 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 cases for all cancer types (high mortality number).18 In Saudi Arabia, the most common malignancies are breast and colorectal, and cancer mortality statistics refer to 12% cases for all types in 2016 but were approximately 5% in 1990.19 As estimated by cancer statistics in the Arab World, in 2018, cancer was responsible for 16.1% of deaths in Tunisia,20 while in 2016, it was responsible for 16.2% of deaths in Jordan.21 Cancer treatment and care match the global trend, and cancer research is increasing in the Arab world.22,23
042 cases for all cancer types (high mortality number).18 In Saudi Arabia, the most common malignancies are breast and colorectal, and cancer mortality statistics refer to 12% cases for all types in 2016 but were approximately 5% in 1990.19 As estimated by cancer statistics in the Arab World, in 2018, cancer was responsible for 16.1% of deaths in Tunisia,20 while in 2016, it was responsible for 16.2% of deaths in Jordan.21 Cancer treatment and care match the global trend, and cancer research is increasing in the Arab world.22,23
The therapy strategy for cancer depends on the organ affected, the rate of spread, and the patient's health state.24 Cancer drug therapy is divided into classes according to its action mechanism as follows: (I) alkylating agents include temozolomide, cisplatin, and melphalan. These drugs add an alkyl group to the DNA of cells, damaging it; this mechanism functions at all phases of the cell cycle.25 (II) Nitrosoureas include streptozocin and lomustine. These drugs possess the same action mechanism as alkylating agents but have the distinctive property that they can reach the brain.26 (III) Antimetabolites include fluorouracil, decitabine, and thioguanine. These drugs interfere in DNA biosynthesis inducing in turn DNA replication inhibition.27 (IV) Antitumor antibiotics include bleomycin, dactinomycin, doxorubicin, and epirubicin. These drugs modify the DNA in cells, inhibiting cancer's spread.28 (V) Plant alkaloid topoisomerase inhibitors include mitoxantrone and teniposide; these drugs inhibit the separation of the two DNA strands through interaction with topoisomerase enzymes.29 (VI) Mitotic inhibitors include paclitaxel and docetaxel; these drugs prevent cell mitosis.30
The literature has revealed, in the last decade, that pyrazolo[1,5-a]pyrimidine structure 1 has promising bioactivity in numerous pharmacological applications, such as anticancer,31 antibacterial,32 anti-COVID-19,33 anti-HIV,34 TNF-α inhibitor,35 and anti-Alzheimer.36 Additionally, Vahedi et al. synthesized 8-(cyclohexylamino)-2,5-dimethyl-6H-pyrano[3,2-e]pyrazolo[1,5-a]pyrimidine derivative 2, which showed potent cytotoxicity against MCF-7 breast cells with IC50 = 19.70 ± 0.89 μM; they also prepared another 5-(2-ethoxy-2-oxoethyl)-6H-pyrano[3,2-e]pyrazolo[1,5-a]pyrimidinederivative 3 as an antioxidant agent, which exhibited free radical scavenging activity with IC50 = 12.12 ± 0.40 μM.37 Peytam and co-workers prepared substituted 6-amino-pyrazolo[1,5-a]pyrimidine 4 as an anti-diabetic agent through the inhibition of α-glucosidase enzyme with an activity IC50 of 15.2 ± 0.4 μM, which is more potent than acarbose (IC50 = 750.0 ± 1.5 μM) by around 50-fold.38 From our previous cooperation, Hassan et al. designed and prepared some 5-aryl-pyrazolo[1,5-a]pyrimidine derivatives as multi-target candidates and concluded that the 7-amino-6-cyano-pyrazolo[1,5-a]pyrimidine-3-carboxamide derivative 5 showed powerful activities as an antioxidant and anti-diabetic agent. They also recommended the 5-(2-methoxyphenyl)pyrazolo[1,5-a]pyrimidine derivative 6, which presented anti-arthritic activity toward protein denaturation and proteinase with % = 20.66 ± 0.00 and 26.42 ± 0.06, respectively.39 Furthermore, some marketed drugs possess pyrazolo[1,5-a]pyrimidine scaffolds in their structures for the treatment of miscellaneous diseases, such as anagliptin (7), which is a drug for type 2 diabetes mellitus treatment; indiplon (8), which is a sedative-hypnotic; and dinaciclib (9), a drug for cancer treatment through the inhibition of a cyclin-dependent kinase (CDK) enzyme40,41 (Fig. 1).
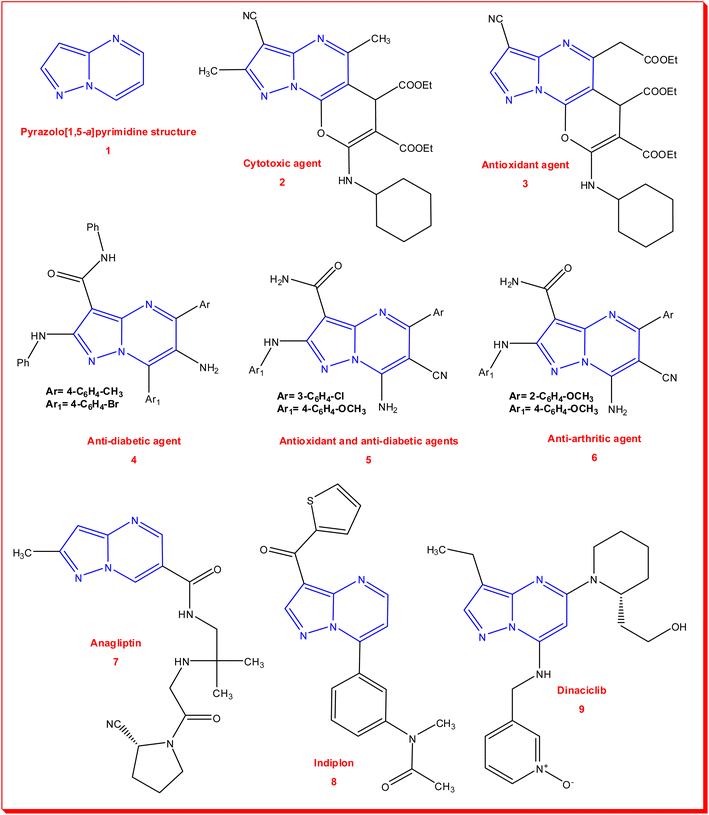 | ||
| Fig. 1 Promising bioactivity of pyrazolo[1,5-a]pyrimidine derivatives 1–6 and its drug skeletons 7–9. | ||
Based on the scientific truths mentioned above about antioxidant agents, diabetes mellitus, Alzheimer's disease, cancer, disease statistics in the Arab world, and therapeutic strategies, the bioactive pyrazolo[1,5-a]pyrimidine and its drug derivatives as well as the continuation of our target of synthesizing bioactive nitrogenous derivatives,42–44 in addition to the concept of multi-target drugs,45–51 and the relationship between diverse diseases.52–54 Accordingly, in this work, we selected the two pyrazolo[1,5-a]pyrimidines 12a and 12b, which possess pharmacology activities against MCF-7 and HepG-2 cell lines, respectively,55 and possess promising antimicrobial and immunomodulatory activities56 for evaluating of their biological potentials as antioxidant, anti-diabetic, anti-Alzheimer, anti-arthritic, and anticancer agents. Finally, computational prediction-based analyses were performed (Fig. 2).
2. Results and discussion
2.1. Chemistry
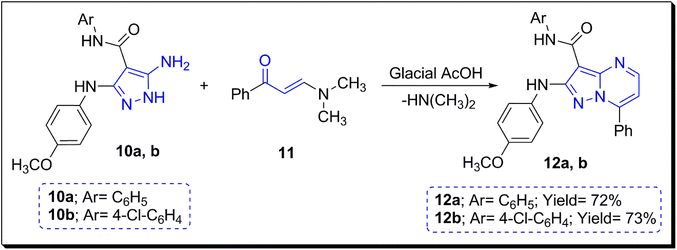
In this section, 5-amino-pyrazoles 10a and 10b benefited as starting materials for the preparation of the two selected pyrazolo[1,5-a]pyrimidines 12a and 12b via the direct condensation reaction with 3-(dimethylamino)-1-phenylprop-2-en-1-one (11) in AcOH as solvent55 (Scheme 1).The spectral data (1H and 13C NMR analyses) of pyrazolo[1,5-a]pyrimidines 12a and 12b are provided in the ESI.†
2.2. In vitro biological activities
| Derivatives | Antioxidant activity | Scavenging activity | ||
|---|---|---|---|---|
| TAC (mg gallic acid per g) | IRP (μg mL−1) | DPPH (IC50 μg ml−1) | ABTS (%) | |
| a Values were calculated from three replicates and expressed as mean ± SE. | ||||
| 12a | 30.58 ± 0.07 | 17.29 ± 0.04 | 19.63 ± 0.04 | 25.28 ± 0.06 |
| 12b | 31.27 ± 0.07 | 17.97 ± 0.04 | 18.33 ± 0.04 | 28.23 ± 0.06 |
| STD | — | — | Ascorbic acid | |
| 4.05 ± 0.01 | 39.09 ± 0.09 | |||
Cancer, atherosclerosis, rheumatoid arthritis, and aging-related degenerative processes are prevalent diseases believed to involve excessive lipid oxidation and inflammation. The primary approach to preventing and treating these conditions may be to reduce these oxidation processes through the consumption of exogenous antioxidants.61
As shown in Table 1, it was observed that both the two pyrazolo[1,5-a]pyrimidines, 12a and 12b, had approximately the same total antioxidant capacity (TAC) and inhibitory radical potential (IRP). Numerically, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b exhibited slightly higher TAC (31.27 ± 0.07 mg gallic acid per g) and IRP (17.97 ± 0.04 μg mL−1) compared to N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a (30.58 ± 0.07 mg gallic acid per g and 17.29 ± 0.04 μg mL−1, respectively).
The antioxidant activity, as assessed by scavenging activities against DPPH and ABTS radicals, showed that both the two pyrazolo[1,5-a]pyrimidines, 12a and 12b, had nearly identical scavenging activities, but the numerical data supported the antioxidant activities. However, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b showed a lower IC50 value against DPPH (18.33 ± 0.04 μg mL−1) and higher inhibitory activity against ABTS (28.23 ± 0.06%) at equal concentrations. The activity against the DPPH radical was expressed as IC50 values, with a low IC50 value indicating strong antioxidant activity. At the same concentration, the scavenging activity of the standard ascorbic acid against DPPH and ABTS was 4.05 ± 0.01 μg mL−1 and 39.09 ± 0.09%, respectively.
Similar redox properties enable the two derivatives of pyrazolo[1,5-a]pyrimidines, 12a and 12b, to act as hydrogen donors and reducing agents, which may be related to the similarity in their molecular structures.62
2.2.2.1. Enzyme assay. We measured the anti-diabetic activities of the two pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, using the procedures from ref. 63–65. The results are shown in Table 2.
| Derivatives | α-Amylase | α-Glucosidase | β-Glucosidase | |||
|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (mg ml−1) | Inhibition (%) | IC50 (mg ml−1) | Inhibition (%) | IC50 (mg ml−1) | |
| a Values were calculated from three replicates and expressed as mean ± SE. | ||||||
| 12a | 25.92 ± 0.01 | 1.92 ± 0.01 | 15.42 ± 0.01 | 3.13 ± 0.01 | 7.67 ± 0.01 | 6.47 ± 0.01 |
| 12b | 27.91 ± 0.02 | 1.80 ± 0.01 | 17.41 ± 0.02 | 2.80 ± 0.01 | 9.66 ± 0.02 | 5.18 ± 0.01 |
| Derivatives | Acarbose | |||||
|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (mg ml−1) | Inhibition (%) | IC50 (mg ml−1) | Inhibition (%) | IC50 (mg ml−1) | |
| STD | 65.95 ± 0.01 | 0.76 ± 0.01 | 55.45 ± 0.01 | 0.90 ± 0.01 | 47.70 ± 0.01 | 1.07 ± 0.01 |
Diabetes mellitus (DM) is a chronic metabolic disease characterized by elevated glucose levels.66 The enzymes α-amylase and α-glucosidase play a crucial role in regulating blood glucose levels, with α-amylase breaking down carbohydrates into disaccharides and α-glucosidase converting disaccharides into monosaccharides. Inhibiting these enzymes is a therapeutic strategy for controlling hyperglycemia.67
In the current study, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b exhibited the highest inhibitory effect on α-amylase (27.91 ± 0.02%), α-glucosidase (17.41 ± 0.02%), and β-glucosidase (9.66 ± 0.02%) compared to the standard acarbose, which had inhibitory effects at the same concentration on α-amylase (65.95 ± 0.01%), α-glucosidase (55.45 ± 0.01%), and β-glucosidase (47.70 ± 0.01%) (Table 2).
The inhibitory activity is inversely proportional to the values of the IC50, with lower IC50 values indicating higher inhibition. Compound 12b, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative, had lower IC50 values against the activities of α-amylase (1.80 ± 0.01 mg mL−1), α-glucosidase (2.80 ± 0.01 mg mL−1), and β-glucosidase (5.18 ± 0.01 mg mL−1) compared to the other tested N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a.
This may be attributed to the phenolic structure of the tested compounds, which are responsible for their inhibitory effect on these enzymes.68 Hassan and Aboulthana suggested that these synthetic derivatives may belong to hypoglycemic substances, which could be caused by two different mechanisms: either by stimulating sugar-induced insulin secretion or by improving peripheral glucose intake.50
2.2.2.2. Native electrophoretic patterns. Electrophoresis is a widely used technique for separating, identifying, and quantifying different proteins and isoenzymes expressed in various tissues. It is commonly used to analyze the stoichiometry of a specific subunit of a protein complex.69 Electrophoresis can detect mutagenic differences at a qualitative level by hiding normal bands and/or the appearance of abnormal ones. The significance index (SI) provides insight into the physiological state of the tissue and is inversely proportional to genetic variation, indicating qualitative alterations. Low SI values compared to the control group reveal differences in the number and arrangement of electrophoretically separated bands. Quantitative alterations, however, retain normal bands with their identification data, and changes occur in their quantities. Therefore, the SI value is not associated with quantitative changes.70 α-Amylase, a digestive enzyme found mainly in saliva and pancreatic juice, catalyzes the hydrolysis of α-(1,4)-D-glycosidic linkages of starch and other glucose polymers, breaking down dietary carbohydrates into oligosaccharides and disaccharides. It is considered a potential target for diabetes.71
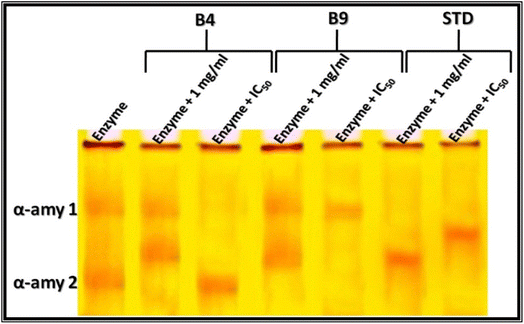
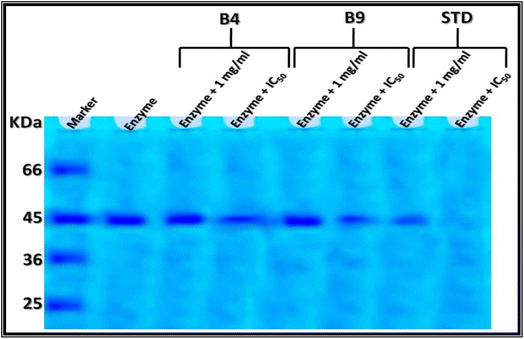
The electrophoretic α-amylase isoenzyme and α-glucosidase patterns of the two pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, were assayed using the method suggested.72,73 The results are illustrated in Fig. 3 and 4. Additionally, the main results of Native Electrophoretic Patterns are listed in Table S1 and S2 (ESI).†
The crude α-amylase enzyme was analyzed using electrophoresis, and two types were identified at Rfs 0.38 and 0.83 (Qty 10.33 and 7.32; B% 58.54 and 41.46, respectively) (Fig. 3 and Table S1†). This suggests its role in the metabolic pathway, as demonstrated by Aboulthana and co-workers.74 When treated with pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, at equal concentrations (1 mg mL−1), the enzyme showed alterations, with one isoenzyme type (α-amy2) being hidden and an abnormal band appearing at Rf 0.68 with Qty 25.52 (B% 68.53) after treatment with N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a and Qty 9.27 (B% 50.50) after treatment with N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b. Treatment with standard acarbose at the same concentration (1 mg mL−1) caused severe abnormalities, hiding both types of the enzyme (α-amy1 and α-amy2) with an abnormal band identified at Rf 0.69 (Qty 9.94 and B% 100.00). Both pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, caused physiological alterations in the α-amylase isoenzyme patterns when treated at equal concentrations (1 mg mL−1), with the patterns being similar to the electrophoretic α-amylase isoenzyme pattern by the same percent (SI = 50.00%). When treated with concentrations equivalent to the IC50 values, N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a caused alterations by hiding one type of the enzyme (α-amy1) without changing the second one (α-amy2) identified at Rf 0.84 (Qty 23.87 and B% 100.00), while N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b caused changes by hiding one type of the enzyme (α-amy2) without altering the second one (α-amy1) identified at Rf 0.37 (Qty 9.62 and B% 100.00). Both pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, caused physiological alterations in the α-amylase isoenzyme patterns when treated at concentrations equivalent to values of the IC50, with the patterns being similar to the electrophoretic α-amylase isoenzyme pattern by the same percent (SI = 33.33%). This study showed that pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, caused qualitative alterations by changing the number and arrangement of the bands, hiding normal α-Amy type with or without an abnormal band, leading to lower values of the SI% compared to the crude enzyme. This is in agreement with Aboulthana et al.75 who reported that alterations in the electrophoretic α-amylase isoenzyme pattern might be attributed to changing the fractional activity caused oxidatively by ROS. Abdel-Halim et al.76 added that the structural changes induced in the protein portion of native enzymes by oxidative stress are responsible for changing the enzymatic activities of the α-amylase isoenzyme pattern.
α-Glucosidase is a crucial enzyme in carbohydrate digestion, breaking down linear and branched isomaltose oligosaccharides to release glucose and causing postprandial hyperglycemia.77 El-Shora and his team78 visualized and confirmed the α-glucosidase using SDS-PAE, showing a single band with a molecular weight of 45 kDa.
In the current study, the crude α-glucosidase enzyme was identified as a single band at Rf 0.42 (Int. 115.00 and Qty 1.48). When treated with pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, at equal concentrations (1 mg mL−1), the band quantity decreased by 40.54% (Qty 0.88; Int. 100.80) with compound N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a and by 48.65% (Qty 0.76; Int. 87.25) with N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b. Treatment with standard acarbose at the same concentration (1 mg mL−1) caused a severe decrease in the band quantity by 84.14% (Qty 0.22; Int. 25.73). When treated with pyrazolo[1,5-a]pyrimidine derivatives, 12a and 12b, at concentrations equivalent to the IC50 values, the band quantity decreased by 56.08% (Qty 0.65; Int. 74.85) with compound N-phenyl-pyrazolo[1,5-a]pyrimidine 12a and by 68.92% (Qty 0.46; Int. 52.89) with compound N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine 12b (Fig. 4 and Table S2†). In contrast, treatment with standard acarbose caused severe alterations, completely decreasing the protein band. The electrophoretically detected alterations in the α-glucosidase pattern may be related to the disruption and denaturation of the α-glucosidase protein potentially due to the degree of ionization of certain amino acid side chains and/or the formation of reaction products or side-products that inhibit enzyme activity.79
In the present study, we assessed the inhibition percentage of the acetylcholinesterase (AChE) enzyme using Ellman's method81 and donepezil as the standard drug. It was observed that compound N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine 12b exhibited the highest inhibitory effect on AChE activity (16.00 ± 0.04%), followed by compound N-phenyl-pyrazolo[1,5-a]pyrimidine 12a (14.92 ± 0.02%) (Table 3). Compound N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine 12b also had a lower IC50 value against AChE activity (3.15 ± 0.01 mg mL−1) compared to compound N-phenyl-pyrazolo[1,5-a]pyrimidine 12a (3.34 ± 0.01 mg mL−1).
| AChE | Anti-arthritic activity | |||
|---|---|---|---|---|
| Inhibition (%) | IC50 (mg mL−1) | Proteinase denaturation (%) | Inhibition of proteinase (%) | |
| a Values were calculated from three replicates and expressed as mean ± SE. | ||||
| 12a | 14.92 ± 0.02 | 3.34 ± 0.01 | 16.24 ± 0.04 | 14.91 ± 0.03 |
| 12b | 16.00 ± 0.04 | 3.15 ± 0.01 | 17.55 ± 0.04 | 16.25 ± 0.04 |
| Donepezil | Diclofenac sodium | |||
|---|---|---|---|---|
| Inhibition (%) | IC50 (mg mL−1) | Proteinase denaturation (%) | Inhibition of proteinase (%) | |
| STD | 71.14 ± 0.01 | 0.71 ± 0.00 | 49.33 ± 0.11 | 41.88 ± 0.09 |
This finding is supported by Russo et al.82 who suggested that compounds with antioxidant activities may also exhibit anti-diabetic and anti-Alzheimer properties. Therefore, derivatives with antioxidant activities could potentially be more effective in treating and managing diabetes and Alzheimer's disease. The standard drug donepezil exhibited an inhibitory activity of 71.14 ± 0.01% against AChE at the same concentration, with an IC50 value of 0.71 ± 0.00 mg mL−1.
Arthritis is characterized by inflammation, which is one of its most important symptoms.83 The ability of the tested compounds to inhibit proteinase denaturation and proteinase enzymes, which are key features and indices for the occurrence of inflammatory diseases, including arthritis, refers to the apparent potential for anti-inflammatory activity.84 An important aspect of protein denaturation is the modification of forces that stabilize proteins essential for their structure and function, such as disulfide bridges, ionic interactions, electrostatic forces, and hydrogen bonds. Additionally, anti-inflammatory drugs inhibit protein denaturation in dose-dependent ways.85
The anti-arthritic activity was determined by quantifying the effectiveness of the pyrazolo[1,5-a]pyrimidines derivatives, 12a and 12b, in inhibiting protein denaturation and the activity of the proteinase enzyme using the procedures reported in previous works86–88 and the results in Table 3.
The current study found that compound N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine 12b had the highest inhibitory effect on proteinase denaturation (17.55 ± 0.04%) and proteinase activity (16.25 ± 0.04%), followed by compound N-phenyl-pyrazolo[1,5-a]pyrimidine 12a (16.24 ± 0.04 and 14.91 ± 0.03%, respectively). The standard drug diclofenac sodium showed inhibitory activity of 49.33 ± 0.11% and 41.88 ± 0.09% against proteinase denaturation and proteinase activity, respectively, at the same concentration. The data on the antioxidant activities, anti-diabetic, anti-Alzheimer's and anti-arthritic activities of the tested derivative were positively correlated.39,51 Additionally, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine 12b exhibited higher anti-arthritic activity due to the presence of 4-chlorophenyl rings, as suggested by Mezgebe and Mulugeta.89
| Compounds | IC50 (μg mL−1) | Therapeutic index (TI) | |||
|---|---|---|---|---|---|
| Lung cancer (A549) | Colon cancer (Caco-2) | Normal lung (WI-38) | Lung cancer (A549) | Colon cancer (Caco-2) | |
| 12a | 47.83 | 38.15 | 134.24 | 2.8 | 3.5 |
| 12b | 40.54 | 29.77 | 304.88 | 7.52 | 10.24 |
| Doxorubicin | 31.32 | 28.45 | 75.98 | 2.42 | 2.67 |
In the case of the A549 cell line, it was observed that compound 12b exhibited slightly higher cytotoxic activity with a lower IC50 = 40.54 μg mL−1 compared to compound 12a (IC50 = 47.83 μg mL−1). However, both compounds showed lower cytotoxic activity compared to doxorubicin (IC50 = 31.32 μg mL−1).
However, for the Caco-2 cell line, compound 12b demonstrated higher cytotoxic activity with an IC50 of 29.77 μg mL−1, which was relatively similar to the standard drug (IC50 = 28.45 μg mL−1). Compound 12b exhibits cytotoxic activity potentially due to its ability to induce G2/M arrest in cancer cells and increase the expression of tumor suppressor genes. Additionally, it may bind not only with DNA but also with proteins in targeted cancer cells.93 The cytotoxic activity may be linked to DNA interaction and cleavage, as well as the direct targeting of nucleic acids through the cleavage of DNA and RNA.94 The newly synthesized compound induces oxidative stress, leading to cell death in cancer cells by increasing the total oxidant status and decreasing total antioxidant levels, thereby increasing oxidative stress levels.95 The results suggest that compound 12b demonstrates excellent cancer inhibition performance and could be considered a candidate drug for human lung and colon cancer types.
In the case of the normal lung (WI-38) cell line, it was observed that compound 12b exhibited the lowest cytotoxicity, as evidenced by its highest IC50 value (304.88 μg mL−1) compared to compound 12a (134.24 μg mL−1). Doxorubicin showed higher cytotoxicity on normal cells, with the lowest IC50 value of 75.98 μg mL−1. These results suggest that compound 12b is safer for normal cells than compound 12a.
From this equation:
| Therapeutic index (TI) = IC50 on the normal cells/IC50 on the cancer cells. |
We can calculate the therapeutic index (TI) of the two pyrazolo[1,5-a]pyrimidines, 12a and 12b, to study their safety and efficacy.96
From Table 4, we can observe that the N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b possesses a therapeutic index (TI = 7.52 and 10.24) higher than doxorubicin (TI = 2.42 and 2.67) in both cases of the A549 and Caco-2 lines, respectively.
3. Computational prediction
3.1. Pharmacokinetic prediction
The computational pharmacokinetic properties of the two pyrazolo[1,5-a]pyrimidines 12a and 12b were calculated using the free pkCSM website (https://biosig.lab.uq.edu.au/pkcsm/).97 The results of the pharmacokinetic prediction properties are summarized in Table 5.| Parameter | 12a | 12b |
|---|---|---|
| CYP1A2 inhibitor | Yes | Yes |
| CYP2C19 inhibitor | Yes | Yes |
| CYP2C9 inhibitor | Yes | Yes |
| CYP2D6 inhibitor | No | No |
| CYP3A4 inhibitior | Yes | Yes |
Cytochrome P450 (CYP) is a group of enzymes that metabolize drugs and other compounds. Five CYPs (CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4) are responsible for metabolizing most approved drugs, and drug interactions involving CYPs can lead to premature termination of drug development and withdrawal from the market.98 From Table 5, we found that the two pyrazolo[1,5-a]pyrimidines, 12a and 12b, are non-inhibitors of the CYP2D6 enzyme but inhibitors of other enzymes CYP1A2, CYP2C19, CYP2C9, and CYP3A4.
3.2. Toxicity prediction
Computational toxicity estimations of the two pyrazolo[1,5-a]pyrimidines 12a and 12b were calculated using the free ProTox-II website (https://tox-new.charite.de/protox_II/index.php?site=home).99 The four toxicity endpoints of the two pyrazolo[1,5-a]pyrimidines 12a and 12b, such as carcinogenicity, cytotoxicity, mutagenicity, and immunotoxicity, were predicted with a probability of more than 70%, which refers to the prediction's confidence estimate (confidence score). Additionally, the median lethal dose (LD50, mg kg−1) and toxicity class were predicted. The toxicity endpoint properties and the median lethal dose (LD50) are summarized in Table 6.| Parameter | 12a | 12b |
|---|---|---|
| Toxicity endpoint properties | ||
| Carcinogenicity | Active | Inactive |
| Cytotoxicity | Inactive | Inactive |
| Mutagenicity | Active | Inactive |
| Immunotoxicity | Inactive | Inactive |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Median lethal dose (LD50, mg kg−1) | ||
| Predicted LD50 | 1000 | 1000 |
| Predicted toxicity class | 4 | 4 |
Carcinogenicity is the ability to induce cancer. The substance may be active and cause cancer or is inactive and safe.100 Based on computational estimation, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b is inactive, safe, and does not cause cancer, while N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a is active and causes cancer.
Cytotoxicity is the capacity of a substance to impact a cell, causing damage or death.101 According to cytotoxicity prediction, the two pyrazolo[1,5-a]pyrimidines 12a and 12b are non-toxic to cells.
Mutagenicity is the ability to cause genetic mutations in DNA. The substance may be active, mutagenic, and cause genetic mutations, or inactive, non-mutagenic, and genetically safe.102 Based on mutagenicity prediction, N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b is inactive, non-mutagenic, genetically safe, and does not cause genetic mutations in DNA, while N-phenyl-pyrazolo[1,5-a]pyrimidine derivative 12a is active, mutagenic, and causes genetic mutations in DNA.
Immunotoxicity refers to the adverse effects of substances on the immune system.103 Based on this definition, the prediction table demonstrates that the two pyrazolo[1,5-a]pyrimidines 12a and 12b are safe for the immune system and do not cause adverse effects.
The median lethal dose (LD50, mg kg−1) is the dose that causes death in 50% of the animals tested. The toxicity classification of substances according to the Globally Harmonized System (GHS) is divided into six classes: class I (LD50 ≤ 5) fatal if swallowed; class II (5 < LD50 ≤ 50) fatal if swallowed; class III (50 < LD50 ≤ 300) toxic if swallowed; class IV (300 < LD50 ≤ 2000) harmful if swallowed; class V (2000 < LD50 ≤ 5000) may be harmful if swallowed; and Class VI (LD50 > 5000) non-toxic.104 According to the prediction study, the two pyrazolo[1,5-a]pyrimidines 12a and 12b possess a median lethal dose of LD50 that is equal to 1000 mg kg−1. In this context, the two derivatives were classified as class IV.
4. Conclusions
In summary, we synthesized pyrazolo[1,5-a]pyrimidine derivatives 12a and 12b. Additionally, pyrazolo[1,5-a]pyrimidine derivatives 12a and 12b were screened to evaluate their biological potentials in vitro. The antioxidant results demonstrated that the N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b exhibited slightly higher TAC (31.27 ± 0.07 mg gallic acid per g) and IRP (17.97 ± 0.04 μg mL−1) compared to derivative 12a (30.58 ± 0.07 mg gallic acid per g and 17.29 ± 0.04 μg mL−1, respectively). Additionally, pyrazolo[1,5-a]pyrimidine derivative 12b showed a lower IC50 value against DPPH (18.33 ± 0.04 μg mL−1) and higher inhibitory activity against ABTS (28.23 ± 0.06%).Anti-diabetic results refer to compound 12b with lower IC50 values against the activities of α-amylase (1.80 ± 0.01 mg mL−1), α-glucosidase (2.80 ± 0.01 mg mL−1), and β-glucosidase (5.18 ± 0.01 mg mL−1) compared to the other tested derivative 12a. The electrophoretic isoenzyme pattern showed that the N-(4-chlorophenyl)-pyrazolo[1,5-a]pyrimidine derivative 12b exhibited the highest anti-diabetic activity by altering the physiological state of α-amylase enzyme and denaturation of the protein portion in α-glucosidase enzyme. Pyrazolo[1,5-a]pyrimidine derivative 12b exhibited the highest inhibitory effect on AChE activity (16.00 ± 0.04%) as an anti-Alzheimer's agent, and 12b also had the highest inhibitory effect on proteinase denaturation (17.55 ± 0.04%) and proteinase activity (16.25 ± 0.04%) as an anti-arthritics agent.
In vitro cytotoxicity results indicate that pyrazolo[1,5-a]pyrimidine derivative 12b possesses a lower IC50 of 40.54 and 29.77 μg mL−1 towards lung (A549) and colon (Caco-2), respectively. The safety and efficacy studies refer to derivative 12b possessing a therapeutic index (TI) of 7.52 and 10.24, higher than doxorubicin (TI = 2.42 and 2.67) in both cases of the A549 and Caco-2 lines, respectively. According to the prediction study, the two pyrazolo[1,5-a]pyrimidines 12a and 12b are non-inhibitors of the CYP2D6 enzyme but inhibitors of other enzymes CYP1A2, CYP2C19, CYP2C9, and CYP3A4. In addition, the two derivatives 12a and 12b possess a median lethal dose of LD50, which is equal to 1000 mg kg−1. In this context, the two derivatives were classified as class IV. Pyrazolo[1,5-a]pyrimidine derivative 12b is inactive and safe for carcinogenicity, cytotoxicity, mutagenicity, and immunotoxicity.
We are currently conducting additional mechanistic studies in our laboratories and will report our findings in the future.
5. Materials and methods
5.1. Chemistry
Pyrazolo[1,5-a]pyrimidines12a and 12b were prepared according to the approach illustrated in our previous work, and their spectral data (1H and 13C NMR analyses) are presented in ESI†.555.2. In vitro biological activities
The iron-reducing power was determined in μg mL−1 using the method proposed by Oyaizu, with ascorbic acid as the standard58 (ESI†).
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activities were assessed using the method described by Rahman and Co-work59 (ESI†).
For the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, the procedure followed the method suggested by Arnao et al. with some modifications60 (ESI†).
5.2.2.1. Enzyme assay. This assay involved calculating the inhibition percentage (%) of an α-amylase enzyme using the method based on the technique demonstrated by Wickramaratne (2016) with acarbose as the standard drug63 (ESI†).
The inhibition percentage (%) of the α-glucosidase enzyme was determined using the method proposed by Pistia-Brueggeman and Hollingsworth with acarbose as the standard drug64 (ESI†).
The β-glucosidase enzyme inhibition percentage (%) was measured using the pNPG method suggested by Han et al. with acarbose as the standard drug65 (ESI†).
5.2.2.2 Native electrophoretic patterns.
5.2.2.2.1 Electrophoretic α-amylase isoenzyme pattern. This assay used polyacrylamide gel electrophoresis (PAGE) following the method suggested by Rammesmayer and Praznik72 (ESI†).
5.2.2.2.2 Electrophoretic α-glucosidase pattern. The vertical slab polyacrylamide gel electrophoresis (PAGE) was conducted following the method suggested by Laemmli using mini-gel electrophoresis (BioRad, USA) to determine the activity of the α-glucosidase enzyme73 (ESI†).
In the anti-arthritic activity study, this assay involved determining the percentage of protein denaturation86 and proteinase inhibition87 using diclofenac sodium as the standard non-steroidal anti-inflammatory drug. The diclofenac sodium was prepared according to Meera et al.88 (ESI†).
Ethical statement
The experimental design involving human cancer cell lines was conducted in accordance with the protocol approved by the Medical Research Ethics Committee of the National Research Centre, located in Dokki, Cairo, Egypt (no: EX-11441223).Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs; (Drug Exploration and Development Chair).References
- V. Lobo, A. Patil, A. Phatak and N. Chandra, Pharmacogn. Rev., 2010, 4, 118–126 CrossRef CAS PubMed.
- K. Chand, A. Hiremathad, M. Singh, M. A. Santos and R. S. Keri, Pharmacol. Rep., 2017, 69, 281–295 CrossRef CAS PubMed.
- K. Jomova, R. Raptova, S. Y. Alomar, S. H. Alwasel, E. Nepovimova, K. Kuca and M. Valko, Arch. Toxicol., 2023, 97, 2499–2574 CrossRef CAS PubMed.
- P. Z. Zimmet, D. J. Magliano, W. H. Herman and J. E. Shaw, Lancet Diabetes Endocrinol., 2014, 2, 56–64 CrossRef PubMed.
- M. S. Rahman, K. S. Hossain, S. Das, S. Kundu, E. O. Adegoke, M. A. Rahman, M. A. Hannan, M. J. Uddin and M. G. Pang, Int. J. Mol. Sci., 2021, 22, 6403 CrossRef CAS PubMed.
- L. Szczerbinski and J. C. Florez, Lancet Diabetes Endocrinol., 2023, 11, 861–878 CrossRef PubMed.
- N. Alzaman and A. Ali, J. Taibah Univ. Med. Sci., 2016, 11, 301–309 Search PubMed.
- C. L. I. Rph and R. T. Tinong, Cureus, 2023, 15, e44468 Search PubMed.
- A. Babiker and M. Al Dubayee, Sudan. J. Paediatr., 2017, 17, 11–20 CrossRef PubMed.
- N. U. Khwaja and G. Arunagirinathan, Curr. Drug Saf., 2021, 16, 122–128 Search PubMed.
- M. Samsom, L. A. Szarka, M. Camilleri, A. Vella, A. R. Zinsmeister and R. A. Rizza, Am. J. Physiol.: Gastrointest. Liver Physiol., 2000, 278, G946–G951 CrossRef CAS PubMed.
- M. Foretz, B. Guigas, L. Bertrand, M. Pollak and B. Viollet, Cell Metab., 2014, 20, 953–966 CrossRef CAS PubMed.
- K.-Y. Yoo and S.-Y. Park, Molecules, 2012, 17, 3524–3538 CrossRef CAS PubMed.
- A. El-Metwally, P. Toivola, M. Al-Rashidi, S. Nooruddin, M. Jawed, R. AlKanhal, H. A. Razzak and N. Albawardi, Behav. Neurol., 2019, 2019, 3935943 Search PubMed.
- G. T. Grossberg, Curr. Ther. Res., 2003, 64, 216–235 CrossRef CAS PubMed.
- S. Anwar, W. Rehman, R. Hussain, S. Khan, M. M. Alanazi, N. A. Alsaif, Y. Khan, S. Iqbal, A. Naz and M. A. Hashmi, Pharmaceuticals, 2023, 16, 909 CrossRef CAS PubMed.
- H. S. Salem, J. Cancer Res. Clin. Oncol., 2023, 149, 5139–5163 CrossRef PubMed.
- A. H. Ibrahim and E. Shash, General Oncology Care in Egypt, in Cancer in the Arab World, ed. H. O. Al-Shamsi, I. H. Abu-Gheida, F. Iqbal and A. Al-Awadhi, Springer, Singapore, 2022, 4, pp. 41–61, DOI:10.1007/978-981-16-7945-2_4.
- M. A. Althubiti and M. M. NourEldein, Saudi Med. J., 2018, 39, 1259–1262 CrossRef PubMed.
- N. Mejri, H. Rachdi, L. Kochbati and H. Boussen, General Oncology Care in Tunisia, in Cancer in the Arab World, ed H. O. Al-Shamsi, I. H. Abu-Gheida, F. Iqbal and A. Al-Awadhi, Springer, Singapore, 2022, 18, pp. 285–299, DOI:10.1007/978-981-16-7945-2_18.
- S. Khatib and O. Nimri, General Oncology Care in Jordan, in Cancer in the Arab World, ed. H. O. Al-Shamsi, I. H. Abu-Gheida, F. Iqbal and A. Al-Awadhi, Springer, Singapore, 2022, 6, pp. 83–98, DOI:10.1007/978-981-16-7945-2_6.
- A. A. Siddiqui, J. Amin, F. Alshammary, E. Afroze, S. Shaikh, H. A. Rathore and R. Khan, Burden of cancer in the Arab world, in Handbook of Healthcare in the Arab World, ed. I. Laher, Springer, Cham. 2021, 23, pp. 495–519. DOI:10.1007/978-3-030-36811-1_182.
- R. R. Hamadeh, S. M. Borgan and A. M. Sibai, Sultan Qaboos Univ. Med. J., 2017, 17, e147–e154 CrossRef PubMed.
- T. Schepis, S. S. De Lucia, A. Pellegrino, A. del Gaudio, R. Maresca, G. Coppola, M. F. Chiappetta, A. Gasbarrini, F. Franceschi, M. Candelli and E. C. Nista, Cancers, 2023, 15, 3423 CrossRef CAS PubMed.
- R. Ralhan and J. Kaur, Expert Opin. Ther. Pat., 2007, 17, 1061–1075 CrossRef CAS.
- J. Fahrer and M. Christmann, Int. J. Mol. Sci., 2023, 24, 4684 CrossRef CAS PubMed.
- A. Lansiaux, Bull. Cancer, 2011, 98, 1263–1274 CrossRef CAS PubMed.
- L. Xie, L. Cheng and Y. Wei, Pathol., Res. Pract., 2023, 243, 154350 CrossRef CAS PubMed.
- P. A. Yakkala, N. R. Penumallu, S. Shafi and A. Kamal, Pharmaceuticals, 2023, 16, 1456 CrossRef CAS PubMed.
- M. Hashemi, M. A. Zandieh, Y. Talebi, P. Rahmanian, S. S. Shafiee, M. M. Nejad, R. Babaei, F. H. Sadi, R. Rajabi, Z. O. Abkenar and S. Rezaei, Biomed. Pharmacother., 2023, 160, 114392 CrossRef CAS PubMed.
- H. R. Elgiushy, S. H. Mohamed, H. Taha, H. Sawaf, Z. Hassan, N. A. Abou-Taleb, E. M. El-labbad, A. S. Hassan, K. A. M. Abouzid and S. F. Hammad, Bioorg. Chem., 2022, 120, 105646 CrossRef CAS PubMed.
- A. S. Hassan, G. O. Moustafa, A. A. Askar, A. M. Naglah and M. A. Al-Omar, Synth. Commun., 2018, 48, 2761–2772 CrossRef CAS.
- A. M. Abdelghany, T. K. Khatab and A. S. Hassan, Bull. Chem. Soc. Ethiop., 2021, 35, 185–196 CrossRef CAS.
- Y. Tian, D. Du, D. Rai, L. Wang, H. Liu, P. Zhan, E. De Clercq, C. Pannecouque and X. Liu, Bioorg. Med. Chem., 2014, 22, 2052–2059 CrossRef CAS PubMed.
- D. E. P. Rao, M. D. Raju, N. R. K. Reddy, C. Rajendiran, M. S. Praneeth, M. B. Tej, M. V. B. Rao, R. Kapavarapu and M. Pal, Polycyclic Aromat. Compd., 2023, 43, 1451–1468 CrossRef.
- S. Kumari, K. Maddeboina, R. D. Bachu, S. H. Boddu, P. C. Trippier and A. K. Tiwari, Drug Discovery Today, 2022, 27, 103322 CrossRef CAS PubMed.
- M. M. Vahedi, S. Asghari, M. Tajbakhsh, M. Mohseni and A. Khalilpour, J. Mol. Struct., 2023, 1284, 135446 CrossRef CAS.
- F. Peytam, M. Adib, R. Shourgeshty, L. Firoozpour, M. Rahmanian-Jazi, M. Jahani, S. Moghimi, K. Divsalar, M. A. Faramarzi, S. Mojtabavi and F. Safari, Sci. Rep., 2020, 10, 2595 CrossRef CAS PubMed.
- A. S. Hassan, N. M. Morsy, W. M. Aboulthana and A. Ragab, Drug Dev. Res., 2023, 84, 3–24 CrossRef CAS PubMed.
- S. Cherukupalli, R. Karpoormath, B. Chandrasekaran, G. A. Hampannavar, N. Thapliyal and V. N. Palakollu, Eur. J. Med. Chem., 2017, 126, 298–352 CrossRef CAS PubMed.
- C. Bagul, G. K. Rao, I. Veena, R. Kulkarni, J. R. Tamboli, R. Akunuri, S. P. Shaik, M. Pal-Bhadra and A. Kamal, Mol. Diversity, 2023, 27, 1185–1202 CrossRef CAS PubMed.
- A. S. Hassan, S. A. Osman and T. S. Hafez, Egypt. J. Chem., 2015, 58, 113–139 CrossRef.
- N. M. Morsy, A. S. Hassan, T. S. Hafez, M. R. H. Mahran, I. A. Sadawe and A. M. Gbaj, J. Iran. Chem. Soc., 2021, 18, 47–59 CrossRef CAS.
- S. S. Mukhtar, A. S. Hassan, N. M. Morsy, T. S. Hafez, F. M. Saleh and H. M. Hassaneen, Synth. Commun., 2021, 51, 1564–1580 CAS.
- X. Chen and M. Decker, Curr. Med. Chem., 2013, 20, 1673–1685 CrossRef CAS PubMed.
- Q. Jiang, M. Li, H. Li and L. Chen, Biomed. Pharmacother., 2022, 150, 112974 CrossRef CAS PubMed.
- W. A. Badawi, M. Rashed, A. Nocentini, A. Bonardi, M. M. Abd-Alhaseeb, S. T. Al-Rashood, G. B. Veerakanellore, T. A. Majrashi, E. B. Elkaeed, B. Elgendy and P. Gratteri, J. Enzyme Inhib. Med. Chem., 2023, 38, 2201407 CrossRef PubMed.
- H. N. Khedkar, L. C. Chen, Y. C. Kuo, A. T. Wu and H. S. Huang, Int. J. Mol. Sci., 2023, 24, 10247 CrossRef CAS PubMed.
- H. M. Alkahtani, A. A. Almehizia, M. A. Al-Omar, A. J. Obaidullah, A. A. Zen, A. S. Hassan and W. M. Aboulthana, Molecules, 2023, 28, 7125 CrossRef CAS PubMed.
- A. S. Hassan and W. M. Aboulthana, Egypt. J. Chem., 2023, 66, 441–455 Search PubMed.
- A. S. Hassan, N. M. Morsy, W. M. Aboulthana and A. Ragab, RSC Adv., 2023, 13, 9281–9303 RSC.
- I. Z. Sadiq, Curr. Mol. Med., 2023, 23, 13–35 CrossRef CAS PubMed.
- A. B. Jena, R. R. Samal, N. K. Bhol and A. K. Duttaroy, Biomed. Pharmacother., 2023, 162, 114606 CrossRef CAS PubMed.
- D. C. Shippy and T. K. Ulland, Sci. Rep., 2023, 13, 14800 CrossRef CAS PubMed.
- M. El-Naggar, A. S. Hassan, H. M. Awad and M. F. Mady, Molecules, 2018, 23, 1249 CrossRef PubMed.
- A. M. Naglah, A. A. Askar, A. S. Hassan, T. K. Khatab, M. A. Al-Omar and M. A. Bhat, Molecules, 2020, 25, 1431 CrossRef CAS PubMed.
- P. Prieto, M. Pineda and M. Aguilar, Anal. Biochem., 1999, 269, 337–341 CrossRef CAS PubMed.
- M. Oyaizu, Jpn. J. Nutr. Diet., 1986, 44, 307–315 CrossRef CAS.
- M. M. Rahman, M. B. Islam, M. Biswas and A. H. M. Khurshid Alam, BMC Res. Notes, 2015, 8, 621 CrossRef PubMed.
- M. B. Arnao, A. Cano and M. Acosta, Food Chem., 2001, 73, 239–244 CrossRef CAS.
- Z. Kermanshah, H. Samadanifard, O. M. Moghaddam and A. Hejrati, Pak. J. Med. Health Sci., 2020, 14, 1301–1312 Search PubMed.
- W. M. K. Aboulthana, E. Refaat, S. E. Khaled, N. E. Ibrahim and A. M. Youssef, Asian Pac. J. Cancer Prev., 2022, 23, 3457–3471 CrossRef CAS PubMed.
- M. N. Wickramaratne, J. Punchihewa and D. Wickramaratne, BMC Complementary Altern. Med., 2016, 16, 466 CrossRef PubMed.
- G. Pistia-Brueggeman and R. I. Hollingsworth, Tetrahedron, 2001, 57, 8773–8778 CrossRef CAS.
- S. J. Han, Y. J. Yoo and H. S. Kang, J. Biol. Chem., 1995, 270, 26012–26019 CrossRef CAS PubMed.
- K. Kozieł and E. M. Urbanska, Cells, 2023, 12, 460 CrossRef PubMed.
- A. S. Alqahtani, S. Hidayathulla, M. T. Rehman, A. A. ElGamal, S. Al-Massarani, V. Razmovski-Naumovski, M. S. Alqahtani, R. A. El Dib and M. F. AlAjmi, Biomolecules, 2020, 10, 61 CrossRef CAS PubMed.
- Z. D. Kifle, J. S. Yesuf and S. A. Atnafie, J. Exp. Pharmacol., 2020, 12, 151–167 CrossRef CAS PubMed.
- W. M. Aboulthana, M. Ismael and H. S. Farghaly, Int. J. Curr. Pharm. Res., 2016, 7, 347–359 Search PubMed.
- W. M. Aboulthana, W. G. Shousha, E. A.-R. Essawy, M. H. Saleh and A. H. Salama, Asian Pac. J. Cancer Prev., 2021, 22, 3267–3286 CrossRef CAS PubMed.
- M. Najafian, A. Ebrahim-Habibi, P. Yaghmaei, K. Parivar and B. Larijani, Acta Biochim. Pol., 2010, 57, 553–560 CrossRef CAS.
- G. Rammesmayer and W. Praznik, J. Chromatogr. A, 1992, 623, 399–402 CrossRef CAS.
- U. K. Laemmli, Nature, 1970, 227, 680–685 CrossRef CAS PubMed.
- W. M. Aboulthana, A. M. El-Feky, N. E. Ibrahim, R. K. Sahu and A. B. El-Sayed, J. Appl. Pharm. Sci., 2018, 8, 046–058 CrossRef CAS.
- W. M. Aboulthana, N. E. Ibrahim, A. K. Hassan, W. K. Bassaly, H. Abdel-Gawad, H. A. Taha and K. A. Ahmed, J. Genet. Eng. Biotechnol., 2023, 21, 158 CrossRef PubMed.
- A. H. Abdel-Halim, A. A. Fyiad, W. M. Aboulthana, N. M. El-Sammad, A. M. Youssef and M. M. Ali, J. Appl. Pharm. Sci., 2020, 10, 077–091 CAS.
- H. M. El-Shora, M. E. Ibrahim and M. W. Alfakharany, Annu. Res. Rev. Biol., 2018, 24, 1–9 CrossRef.
- H. M. El-Shora, A. M. Metwally and A. K. Salwa, Ann. Microbiol., 2009, 59, 285–291 CrossRef CAS.
- H. El-Shora, S. M. Messgo, M. E. Ibrahim and M. W. Alfakharany, Int. J. Phytomed., 2018, 10, 175–180 CAS.
- N. Suganthy, V. S. Ramkumar, A. Pugazhendhi, G. Benelli and G. Archunan, Environ. Sci. Pollut. Res., 2018, 25, 10418–10433 CrossRef CAS PubMed.
- G. L. Ellman, K. D. Courtney, V. J. Andres and R. M. Featherstone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- D. Russo, P. Valentão, P. Andrade, E. Fernandez and L. Milella, Int. J. Mol. Sci., 2015, 16, 17696–17718 CrossRef CAS PubMed.
- B. Balraj, N. Senthilkumar, I. V. Potheher and M. Arulmozhi, Mater. Sci. Eng., B, 2018, 231, 121–127 CrossRef CAS.
- R. Ayman, A. M. Radwan, A. M. Elmetwally, Y. A. Ammar and A. Ragab, Arch. Pharm., 2023, 356, e2200395 CrossRef PubMed.
- R. Thirumalaisamy, F. Ameen, A. Subramanian, T. Selvankumar, S. S. Alwakeel and M. Govarthanan, Int. J. Pept. Res. Ther., 2010, 26, 2179–2189 CrossRef.
- S. Das and P. Sureshkumar, Res. J. Biotechnol., 2016, 11, 65–74 Search PubMed.
- O. O. Oyedapo and A. J. Famurewa, Int. J. Pharmacogn., 1995, 33, 65–69 CrossRef.
- S. Meera, N. Ramaiah and N. Kalidindi, Saudi Pharm. J., 2011, 19, 279–284 CrossRef PubMed.
- K. Mezgebe and E. Mulugeta, RSC Adv., 2022, 12, 25932–25946 RSC.
- A. S. Hassan, H. M. Awad, A. A. Magd-El-Din and T. S. Hafez, Med. Chem. Res., 2018, 27, 915–927 CrossRef CAS.
- V. Vichai and K. Kirtikara, Nat. Protoc., 2006, 1, 1112–1116 CrossRef CAS PubMed.
- A. M. Hassan, A. O. Said, B. H. Heakal, A. Younis, W. M. Aboulthana and M. F. Mady, ACS Omega, 2022, 7, 32418–32431 CrossRef CAS PubMed.
- J.-D. Hsu, S.-H. Kao, T.-T. Ou, Y.-J. Chen, Y.-J. Li and C.-J. Wang, J. Agric. Food Chem., 2011, 59, 1996–2003 CrossRef CAS PubMed.
- A. Barilli, C. Atzeri, I. Bassanetti, F. Ingoglia, V. Dall'Asta, O. Bussolati, M. Maffini, C. Mucchino and L. Marchiò, Mol. Pharmaceutics, 2014, 11, 1151–1163 CrossRef CAS PubMed.
- Y. Wang, X. Zhang, Q. Zhang and Z. Yang, BioMetals, 2010, 23, 265–273 CrossRef CAS PubMed.
- Y. Gorshkova, M.-E. Barbinta-Patrascu, G. Bokuchava, N. Badea, C. Ungureanu, A. Lazea-Stoyanova, M. Răileanu, M. Bacalum, V. Turchenko, A. Zhigunov and E. Juszynska-Galazka, Nanomaterials, 2021, 11, 1811 CrossRef CAS PubMed.
- D. E. V. Pires, T. L. Blundell and D. B. Ascher, J. Med. Chem., 2015, 58, 4066–4072 CrossRef CAS PubMed.
- D. Ai, H. Cai, J. Wei, D. Zhao, Y. Chen and L. Wang, Front. Pharmacol, 2023, 14, 1099093 CrossRef CAS PubMed.
- N. D. Rajaselvi, M. D. Jida, D. B. Nair, S. Sujith, N. Beegum and A. R. Nisha, In Silico Pharmacol., 2023, 11, 34 CrossRef PubMed.
- D. C. Wolf, S. M. Cohen, A. R. Boobis, V. L. Dellarco, P. A. Fenner-Crisp, A. Moretto, T. P. Pastoor, R. S. Schoeny, J. G. Seed and J. E. Doe, Regul. Toxicol. Pharmacol., 2019, 103, 86–92 CrossRef CAS PubMed.
- O. A. Peters, Int. Endod. J., 2013, 46, 195–197 CrossRef CAS PubMed.
- K. Słoczyńska, B. Powroźnik, E. Pękala and A. M. Waszkielewicz, J. Appl. Genet., 2014, 55, 273–285 CrossRef PubMed.
- M. B. Zerdan, S. Moussa, A. Atoui and H. I. Assi, Int. J. Mol. Sci., 2021, 22, 8242 CrossRef PubMed.
- T. T. V. Tran, A. S. Wibowo, H. Tayara and K. T. Chong, J. Chem. Inf. Model., 2023, 63, 2628–2643 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: All data that support the finding of this study are available in the ESI File. See DOI: https://doi.org/10.1039/d4ra00423j |
| This journal is © The Royal Society of Chemistry 2024 |