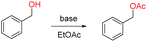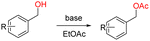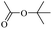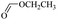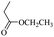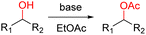Open Access Article
Open Access ArticleEthyl acetate as an acetyl source and solvent for acetylation of alcohols by KOH†
Xiaodan Wang‡
ab,
Huilin Sun‡ab,
Yao Cheng‡b,
Wenfei Yangb,
Xinmei Wangb,
Liting Xiongb,
Yiying Lic,
Jinhui Wangab,
Chunli Gan*a,
Chunguang Lin*b and
Huanjun Xu *b
*b
aDepartment of Medicinal Chemistry and Natural Medicine Chemistry, College of Pharmacy, Harbin Medical University, Harbin, 150081, China. E-mail: chunligan@126.com
bSchool of Science, Qiongtai Normal University, Haikou 571127, China. E-mail: 15798946232@163.com; 3895477@qq.com
cCollege of Basic Medicine and Life Sciences, Hainan Medical University, Haikou, 571127, China
First published on 18th April 2024
Abstract
A KOH mediated mild, efficient, convenient and gram-scalable protocol for the acetylation of alcohols with EtOAc as acetyl source and solvent. Various types of alcohols were successfully transformed into according acetylated products. Good to excellent yields were offered by primary alcohols and low to moderate yields were offered by secondary alcohols.
In organic synthesis, aromatic esters are highly popular synthetic intermediates for chemicals and pharmaceuticals.1 Meanwhile, acetylation could be the method of choice for protecting an alcoholic group in a complex synthetic sequence for its easy introduction, stability towards acidic reaction conditions, and mild removal by alkaline hydrolysis.2 In which, acetic anhydride and acetyl chloride are the most common sources for acetylation for alcohols.3 But acetic anhydride and acetyl chloride are very unstable especially because of their high sensitivity to environmental moisture. And those acetylation reagents are usually activated in the presence of a stoichiometric quantity basic4 or acidic catalyst.5 Recently, metal triflates6 and other metal salts7 have also been reported as the catalysts for this transformation.
To comply with the principles of green synthesis, ethyl acetate (EtOAc) as acetyl source has attracted more attention, which is more environmental friendly and less-cost, ideally only producing ethanol as the by-product. Meanwhile, EtOAc with low electrophilicity. There are few reports for the esterification of alcohols by EtOAc in presence of different transition metal catalysts. The metal-based catalysts including dilithium tetra-tert-butylzincate (TBZL),8 LiClO4/In(OTf)3,9 Y5(Oi Pr)13O,9 ZrCl4-Mg(ClO4)2,10 CoCl2·6H2O,10 heterogeneous zinc/imidazole,11 Zn4(OCOCF3)6O,12 InI3/I2,13 Zn reagent.14 And some transesterification of alcohols under metal-free conditions were reported, such as Cs2CO3,15 I2,16 KOtBu,17 K2CO3,18 Novozyme-435,19 TsOH,20 (Table 1).
| Entry | Catalyst | Catalyst loading | Temperature/°C | Time | Solvent | Yield/% | Reference |
|---|---|---|---|---|---|---|---|
| 1 | TBZL | 10 mol% | 0 | 1 h | EtOAc | 41 | 8 |
| 2 | Zinc/imidazole | 5 mol% | 120 | 1 h | EtOAc | 99 | 11 |
| 3 | Zn4(OCOCF3)6O | 1.25 mol% | 77–78 | 18 h | EtOAc | 99 | 12 |
| 4 | InI3/I2 | 10 mol%/15 mol% | 77–78 | 14 h | EtOAc | 82 | 13 |
| 5 | Zn reagent | 500 mol% | rt | 24 h | EtOAc | 89 | 14 |
| 6 | Cs2CO3 | 15 mol% | 125 | 1.5 h | EtOAc | 81 | 15 |
| 7 | I2 | 10 mol% | 85–90 | 2 h | EtOAc | 98 | 16 |
| 8 | KOtBu | 200 mol% | rt | 20 min | DMSO | 84 | 17 |
| 9 | K2CO3 | 100 mol% | 77–78 | 1 h | EtOAc | 90 | 18 |
| 10 | Novozyme-435 | 5 wt% | rt | 24 h | EtOAc | 93 | 19 |
| 12 | TsOH | 100 mol% | rt | 3 h | EtOAc | 82 | 20 |
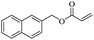
| 13 | KOH | 75 mol% | rt | 10 min | EtOAc | 99 | This work |
Although, the yield of these acetylation was satisfied, but they have obvious disadvantages such as toxicity, long reaction time, complex operation, strict reaction condition and so on. Like the procedure mediated by KOtBu which need KOtBu (1.0 mmol), DMSO (2 mL) and stirred at room temperature under argon atmosphere for 30 min. Then 1.0 mL of EtOAc was added and stirred for an additional 30 min.
Thus in spite of the recent advances, the development of a simple, mild and time efficient procedure is still of critical importance. Herein, we are reporting a short, mild and efficient acetylation methodology at room temperature with excellent yields and selectivity towards various primary alcohols.
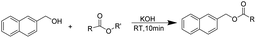
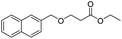
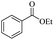
In our initial studies, taking benzyl alcohol as the represent compound, we conducted the reactions using EtOAc as solution with KOH under room temperature. To our delight, it was clearly shown that 95% yield of desired product was obtained after only for 5 min of reaction. Based on this, we prolonged the time for 10 min, 15 min and 20 min, the yield of product was up to 99%, 97% and 95%, respectively. Then we screened other inorganic bases and observed that NaOH, LiOH could offer the desired product in a relatively lower yield. For KOtBu, NaOtBu and LiOtBu as the base, KOtBu offered a yield high to 95%. For all of the inorganic bases we screened, the Li2CO3 failed to carry out the reaction. Other types of organic bases, such as Et3N and DBU, did not catalyzed this transformation. Then to find the optimum value of the amount of KOH, it was clearly shown that 97% and 95% of acetylated product was offered with 0.5 mmol and 0.25 mmol of KOH, respectively.
The reaction mechanism were similar to ref. 17 reported. The step-I is a typical acid base reaction and hence there will be an equilibrium between HO− and the alkoxide as formed. The step-II is a transesterification reaction and the equilibrium depends upon stability of the alkoxide and ethoxide. Notably, the alkali metal ions plays a key role in promoting the reaction. In this reaction, the yield was decreased as following: KOtBu > NaOtBu > LiOtBu, KOH > NaOH > LiOH, K2CO3 > Na2CO3 > Li2CO3. Moreover, no product was formed with organic base as the base. It indicated that the potassium ions was the best suitable ions. When the bases with potassium ions, the yield of product was ordered as KOH > KOtBu > K2CO3, it might be the alkalinity and alkoxide size co-effected the reaction.
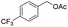
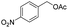
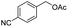
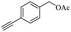
Thus, the optimized reaction conditions were: substrate (0.5 mmol), KOH (0.75 mmol) and EtOAc (3 mL) were stirred at room temperature for 10 min in open air. With the optimal reaction conditions in hands, we employed various types of substrates to evaluate versatility of the procedure. At first, we tested the substrates with different substituted groups for different positions. It was clearly shown that both of the substrates with electron-donating and electron-withdrawing group could be converted to products with high to excellent yield (Table 2, entries 1–16). And the substrates with substitute at the adjacent position on phenyl ring also gave a satisfactory yield (Table 3, entries 2, 6 and 12). The substrates containing halogen substituent also gave excellent yield of according products while no dehalogened compounds were produced (Table 3, entries 8–12). For the substrates with various electron-withdraw group (–CF3 or –NO2 or –CN or –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) also could be smoothly reacted under the standard condition, giving 87%, 83% and 84% yield of the desired products, respectively (Table 3, entries 13–16). In which, the –CN and –C
C) also could be smoothly reacted under the standard condition, giving 87%, 83% and 84% yield of the desired products, respectively (Table 3, entries 13–16). In which, the –CN and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C groups could be tolerated under the reaction condition. Meanwhile, primary saturated alcohols with a long chain offered high yield of products (Table 3, entries 17–20), and it noteworthy that the length of chain did not effect the acetyl procedure. For the allylic alcohols, those were converted to their corresponding products with yield high to 96%, 86% and 92%, and the C
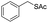
C groups could be tolerated under the reaction condition. Meanwhile, primary saturated alcohols with a long chain offered high yield of products (Table 3, entries 17–20), and it noteworthy that the length of chain did not effect the acetyl procedure. For the allylic alcohols, those were converted to their corresponding products with yield high to 96%, 86% and 92%, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond were not affected during the reaction (Table 3, entries 22–24). More important, when farnesol was scaled up to 0.5 g, the yield was still up to 85%, which indicated that this protocol has great potential for industrial applications. We also tested benzylamine and 2-benzene-methanethiol under the standard condition, unfortunately, those type of substrates cannot be reacted (Table 3, entries 25–26). And for other types of primary alcohols with heterocyclic ring, such as imidazole and thiazole cycle, which also could offer a satisficated yield (Table 3, entries 27–28). Moreover, for primary polyols, all of the OH groups were acetylated and offered a moderate yield of product (Table 3, entry 29). From the above results, it shown that this protocol could be successfully transformed various types of primary alcohols to according acetylated products in good to excellent yield.
C bond were not affected during the reaction (Table 3, entries 22–24). More important, when farnesol was scaled up to 0.5 g, the yield was still up to 85%, which indicated that this protocol has great potential for industrial applications. We also tested benzylamine and 2-benzene-methanethiol under the standard condition, unfortunately, those type of substrates cannot be reacted (Table 3, entries 25–26). And for other types of primary alcohols with heterocyclic ring, such as imidazole and thiazole cycle, which also could offer a satisficated yield (Table 3, entries 27–28). Moreover, for primary polyols, all of the OH groups were acetylated and offered a moderate yield of product (Table 3, entry 29). From the above results, it shown that this protocol could be successfully transformed various types of primary alcohols to according acetylated products in good to excellent yield.
| Entry | Base/mmol | T/min | Yield (%) |
|---|---|---|---|
| a Substrate (0.5 mmol), base, EtOAc (3 mL), room temperature, GC yield.b Isolated yield. | |||
| 1 | KOH/0.75 | 5 | 95 |
| 2 | KOH/0.75 | 10 | 99/94b |
| 3 | KOH/0.75 | 15 | 97 |
| 4 | KOH/0.75 | 20 | 95 |
| 5 | NaOH/0.75 | 10 | 75 |
| 6 | LiOH/0.75 | 10 | 15 |
| 7 | KOtBu/0.75 | 10 | 95 |
| 8 | NaOtBu/0.75 | 10 | 84 |
| 9 | LiOtBu/0.75 | 10 | 13 |
| 10 | K2CO3/0.75 | 10 | 88 |
| 11 | Na2CO3/0.75 | 10 | 84 |
| 12 | Li2CO3/0.75 | 10 | 0 |
| 13 | Et3N/0.75 | 10 | 0 |
| 14 | DBU/0.75 | 10 | 0 |
| 15 | KOH/0.25 | 10 | 95 |
| 16 | KOH/0.5 | 10 | 97 |
| 17 | KOH/1.0 | 10 | 84 |
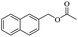
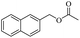
| Entry | Substrate | Product/yieldb | |
|---|---|---|---|
| a Substrate (0.5 mmol), KOH (0.75 mmol), EtOAc (3 mL), room temperature, 10 min, open air, isolated yield.b GC yield.c Substrate (2.25 mmol), KOH (1.69 mmol), EtOAc (15 mL). | |||
| 1 | 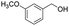 |
 |
90% |
| 2 |  |
 |
92% |
| 3 |  |
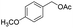 |
99% |
| 4 | 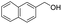 |
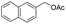 |
92% |
| 5 | 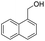 |
 |
90% |
| 6 |  |
 |
93% |
| 7 |  |
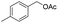 |
96% |
| 8 | 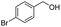 |
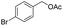 |
96% |
| 9 | 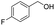 |
 |
90% |
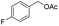
| 10 | 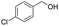 |
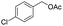 |
93% |
| 11 | 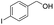 |
 |
94% |
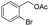
| 12 | 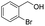 |
 |
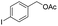
87% |
| 13 | 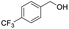 |
 |
87% |
| 14 | 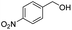 |
 |
83% |
| 15 | 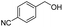 |
 |
84% |
| 16 | 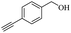 |
 |
91% |
| 17 | 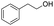 |
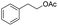 |
80% |
| 18 | 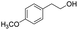 |
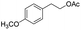 |
85% |
| 19 | 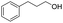 |
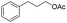 |
83% |
| 20 | 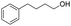 |
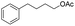 |
86% |
| 21 | 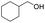 |
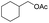 |
92%b |
| 22 | 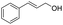 |
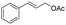 |
96% |
| 23 | 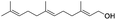 |
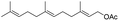 |
86%/85%c |
| 24 | 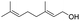 |
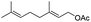 |
92% |
| 25 | 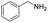 |
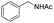 |
0% |
| 26 | 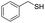 |
 |
0% |
| 27 | 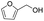 |
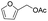 |
92% |
| 28 | 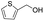 |
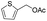 |
95% |
| 29 | 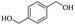 |
 |
62% |
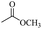
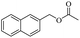
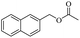
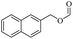
Based on this, we initiated our studies by the catalytic acetylation of a representative substrate, 2-naphthalene-methanol, using various ester as acylating agent. Further investigation of the functionalized esters revealed that the reactivity was affected by the nature of the substituted group. These results suggest that this protocol can be applied for the acylation of alcohols with different ester.
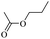
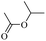
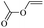
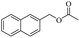
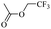
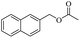
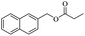
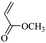
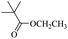
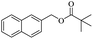
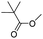
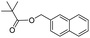
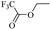
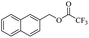
When 2-naphthalenemethanol and methyl acetate reacted under standard condition, acetylated product was obtained in greater than 92% yield (Table 4, entry 1). The reaction using n-propyl acetate (Table 4, entry 2, 94% yield), isopropyl acetate (Table 4, entry 3, 81% yield), tert-butyl acetate (Table 4, entry 4, 19% yield), vinyl acetate (Table 4, entry 5, 53% yield) and 2,2,2-trifluoroethyl acetate (Table 4, entry 6, 93% yield) also proceeded smoothly, in which the tert-butyl group, vinyl group and allyl group were clearly prevented the reverse reaction to some extent. Those results were indicated that the type of alcohol in ester affects the progress of the reaction, that is, the length of chain effected the reaction less, but the steric-effect could result in a lower yield (Table 4, entries 1–6). For the vinyl acetate as acetyl source, the product yield was lower than others, might be effected by the conjugated effect to prevent the departure of ethyleneoxy group. And for the 2,2,2-trifluoroethyl acetate, after the alkoxide attacked the carbonyl of ester, the trifluoroethoxy group was more easier to leave.
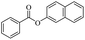
For ethyl formate as solvent, formylation product also could be obtained in high to 88% yield (Table 4, entry 7). When ethyl propionate was used, 86% yield was obtained (Table 4, entry 8). Except ethyl trifluoroacetate (0%, Table 4, entry 12), other type of esters could offer a satisfied yield of desired product. Like methyl acrylate (42% yield, Table 4, entry 9), ethyl trimethylacetate (57% yield, Table 4, entry 10), ethyl benzoate (84% yield, Table 4, entry 13) could be also as acetyl donors. From those results, it was clearly shown that the steric hindrance effect significantly affects the yield of the product (Table 4, entries 3, 4, 10 and 11). For ethyl trifluoroacetate, the ethyl trifluoroacetate offered no product, it might be due to this acetate was very easily hydrolyzed to trifluoroacetic acid, which affects the progress of the reaction. And for methyl acrylate also offered a relatively lower yield of according product, due to a by-product was produced under this condition, which was under a Michael addition of alcohols to activated alkenes promoted by KOH21 (Table 4, entry 9).
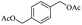
We further employed the screened reaction on different substituted secondary alcohols to expand the scope of the substrates. When we applied the standard condition to the 1-phenylethanol, the according product was only in 14% yield. We investigated the effect of the reaction time and amount of KOH on the reaction, it observed that this reaction gave 52% yield by being stirred with 1.25 mmol KOH for 1 h (Table S1†).
Then we applied this modified reaction condition to various secondary alcohols. Compared with primary alcohols with similar substituted group, the secondary alcohols with electron rich substrates or electron deficient substrates offered the corresponding acetylated products in low to moderate yield. The analogues with a different length of alkyl group on its β-position of the methyl group were successfully transformed to according acetyled products in low to moderate yield. It also showed that the length of alkyl group could affect the reaction, that is, with the increasing of the alkyl group length, the yield of according product was decreased (Table 5, entries 1–3).
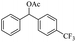
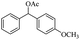
| Entry | Substrate | Product/yield | |
|---|---|---|---|
| a Substrate (0.5 mmol), KOH (1.25 mmol), EtOAc (3 mL), room temperature, 60 min, isolated yield.b GC yield. | |||
| 1 | 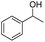 |
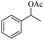 |
52%b |
| 2 | 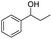 |
 |
44% |
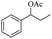
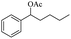
| 3 | 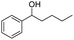 |
 |
33% |
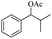
| 4 | 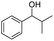 |
 |
15% |
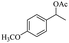
| 5 | 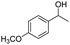 |
 |
63% |
| 6 | 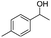 |
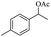 |
58% |
| 7 | 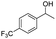 |
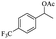 |
53% |
| 8 | 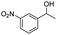 |
 |
69% |
| 9 | 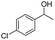 |
 |
64% |
| 10 | 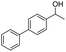 |
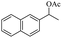
 |
58% |
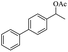
| 11 | 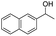 |
 |
57% |
| 12 | 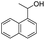 |
 |
53% |
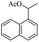
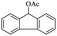
| 13 | 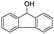 |
 |
78% |
| 14 | 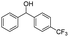 |
 |
49% |
| 15 | 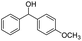 |
 |
51% |
| 16 | 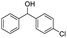 |
 |
56% |
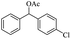
| 17 | 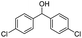 |
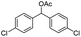 |
45% |
| 18 | 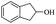 |
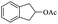 |
56% |
| 19 | 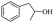 |
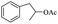 |
32% |
| 20 | 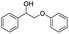 |
 |
69% |
| 21 | 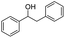 |
 |
44% |
| 22 | 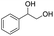 |
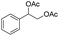 |
25% |
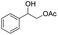 |
51% | ||
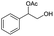 |
14% | ||
| 23 | 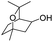 |
 |
44% |
| 24 | 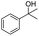 |
 |
0% |
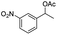
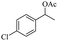
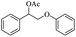
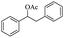
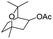
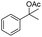
Meanwhile, 2-methyl-1-phenylpropanol with a steric hindrance group on the β-position was transformed into the desired product in relatively lower yield (15% yield), due to the steric hindrance effect. And for –CO3, –CH3, –CF3, –NO2 and –Cl substituted analogues, the products were yielded in 63%, 58%, 53%, 69% and 64%, respectively (Table 5, entries 5–9). For 1-phenylethanol derivative with a phenyl substitute group, the reaction also could be processed (Table 5, entries 10–12). For diaryl secondary alcohols, the according product was obtained at 45–78% yield (Table 5, entries 13–17). For saturated secondary alcohols, 2-indanol and 1-phenyl-2-propanol offered 56% and 32% yield (Table 5, entries 18–19). More important, the β-O-4 dimer model compound in lignin could be converted smoothly to the acetylated product with 69% yield (Table 5, entry 20). And the β-1 model compound also offered 44% yield, which indicated this protocol might have promising application in biomass fields (Table 5, entry 21). Polyols with both primary and secondary alcohol groups, both of those group were acetylated in standard condition, offered the di-acetylated product lower than monoacetylation product (Table 5, entry 22). More important, it indicated that the primary alcohol group was more easier to be acetylated than secondary alcohol group. And we also applied this protocol to nature product, it showed that the according product was yield at 44%, indicated this methodology had potential in medical synthesis (Table 5, entry 23). Meanwhile, the tertiary alcohol offered no product under the reaction condition, that is, the inertness towards tertiary alcohols may be attributed to steric repulsion between ethyl acetate and the hindered alcohol (Table 5, entry 24). We further tested phenols and anilines under the standard condition, but it offered no acetylated products (Table S2†). In all, while the substrate with the same substituted group, the order of reaction of reactivity of alcohols are 1° > 2° > 3°.
Conclusions
In conclusion, we have developed a KOH mediated mild, efficient and convenient procedure for the acetylation of alcohols with EtOAc as acetyl source and solvent in room temperature for a short time. In this process, various types of alcohols were successfully transformed into according acetylated product, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, –CN, –C
C bond, –CN, –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond and halogen bond could be not affected under reaction condition. For those substrates, alcohols with electron rich or electron deficient substrates offered the corresponding acetylated products, in which, low to moderate yields were offered by secondary alcohols, while good to excellent yields were offered by primary alcohols. Among of them, the β-O-4 lignin dimer compound and β-1 lignin model offered the acetylated product in moderate yield. The reaction was also applicable for gram-scale synthesis. It is hopeful that this methodology is very much useful in organic synthesis and lignin transform.
C bond and halogen bond could be not affected under reaction condition. For those substrates, alcohols with electron rich or electron deficient substrates offered the corresponding acetylated products, in which, low to moderate yields were offered by secondary alcohols, while good to excellent yields were offered by primary alcohols. Among of them, the β-O-4 lignin dimer compound and β-1 lignin model offered the acetylated product in moderate yield. The reaction was also applicable for gram-scale synthesis. It is hopeful that this methodology is very much useful in organic synthesis and lignin transform.
Author contributions
Conceptualization, Huanjun Xu and Chunguang Lin; methodology, Xiaodan Wang, Huilin Sun and Yao Cheng; formal analysis, Xinmei Wang and Chunli Gan; investigation, Xiaodan Wang, Huilin Sun, Yao Cheng and Wenfei Yang; resources, Huanjun Xu and Jinhui Wang; writing – original draft preparation, Xiaodan Wang and Liting Xiong; writing – review and editing, Huanjun Xu and Jinhui Wang; supervision, Huanjun Xu; project administration, Huanjun Xu; funding acquisition, Huanjun Xu, Chunli Gan, Jinhui Wang and Yiying Li. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (22165009), the Hainan Provincial Natural Science Foundation of China (821QN260, 220QN281), the Project of Hainan Association for Science and Technology of Youth Science and Talents (QCXM202023), National Science and Technology Major Project of the Ministry of Science and Technology of China (2018ZX09735005), the Scientic Research Foundation of the Higher Education Institutions of Hainan Province (Hnky2021ZD-22) and National Undergraduate Training Program for Innovation and Entrepreneurship (S202113811005, S202113811008).Notes and references
- (a) L. Mrozek, L. Dvorakova, Z. Mandelova, L. Rarova, A. Rezacova, L. Placek, R. Opatrilova, J. Dohnal, O. Paleta, V. Kral, P. Drasar and J. Jampilek, Steroids, 2011, 76, 1082 CrossRef CAS PubMed; (b) T. Verdelet, G. Mercey, N. Correa, L. Jean and P. Y. Renard, Tetrahedron, 2011, 67, 8757 CrossRef CAS.
- (a) J. Otera, Chem. Rev., 1993, 93, 1449 CrossRef CAS; (b) H. Kitano, H. Ito and K. Itami, Org. Lett., 2018, 20, 2428 CrossRef CAS PubMed.
- (a) D. B. Bajracharya and S. S. Shrestha, Synth. Commun., 2018, 48, 1688 CrossRef; (b) V. R. Choudhary and D. K. Dumbre, Catal. Commun., 2011, 12, 1351 CrossRef CAS.
- (a) A. Orita, C. Tanahashi, A. Kakuda and J. Otera, Angew. Chem., Int. Ed., 2000, 39, 2877 CrossRef CAS; (b) R. Alleti, M. Perambuduru, S. Samanha and V. P. Reddy, J. Mol. Catal. A: Chem., 2005, 226, 57 CrossRef CAS; (c) B. Karimi and J. Maleki, J. Org. Chem., 2003, 68, 4951 CrossRef CAS PubMed; (d) N. Ahmed and J. E. Van Lier, Tetrahedron Lett., 2006, 47, 5345 CrossRef CAS; (e) R. H. Tale and R. N. Adude, Tetrahedron Lett., 2006, 47, 7263 CrossRef CAS; (f) T. S. Reddy, M. Narasimhulu, N. Suryakiran, K. C. Mahesh and K. Y. Ashalatha, Tetrahedron Lett., 2006, 47, 6825 CrossRef CAS; (g) X. Fei, J. G. Wang, J. Zhu, X. Z. Wang and X. Q. Liu, ACS Sustain. Chem. Eng., 2020, 8, 8471 CrossRef CAS.
- (a) E. Vedejs and T. S. Diver, J. Am. Chem. Soc., 1993, 115, 3358 CrossRef CAS; (b) E. F. V. Scriven, Chem. Soc. Rev., 1983, 12, 129 RSC.
- (a) M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. M. Baltork, M. Babghanbari and L. Zarea, J. Iran. Chem. Soc., 2009, 6, 523 CrossRef CAS; (b) N. Iranpoor and M. Shekarrize, Bull. Chem. Soc. Jpn., 1993, 72, 455 CrossRef; (c) R. Alleti, M. Perambuduru, S. Samantha, V. Prakash and V. P. Reddy, J. Mol. Catal. A: Chem., 2005, 226, 57 CrossRef CAS.
- (a) G. Bartoli, M. Bosco, R. Dalpozzo, E. Marcantoni, M. Massaccesi, S. Rinaldi and L. Sambri, Synlett, 2003, 1, 39 Search PubMed; (b) S. Velusamy, S. Borpuzari and T. Punniyamurthy, Tetrahedron, 2005, 61, 2011 CrossRef CAS; (c) H. Firouzabadi, N. Iranpoor and S. Farahi, J. Mol. Catal. A: Chem., 2008, 289, 61 CrossRef CAS.
- M. Oshimura, Y. Oda, K. Kondoh, T. Hirano and K. Ute, Tetrahedron Lett., 2016, 57, 2070 CrossRef CAS.
- (a) C. J. Chapman, C. G. Frost, J. P. Hartley and A. J. Whittle, Tetrahedron Lett., 2001, 42, 773 CrossRef CAS; (b) M. H. Lin and T. V. RajanBabu, Org. Lett., 2000, 2, 997 CrossRef CAS PubMed.
- (a) M. M. Alam, S. T. Atkore, V. T. Kamble and R. Varala, Bull. Korean Chem. Soc., 2022, 43, 570 CrossRef CAS; (b) S. Velusamy, S. Borpuzari and T. Punniyamurthy, Tetrahedron, 2005, 61, 2011 CrossRef CAS.
- D. Nakatake, R. Yazaki, Y. Matsushima and T. Ohshima, Adv. Synth. Catal., 2016, 358, 2569 CrossRef CAS.
- T. Iwasaki, Y. Maegawa, Y. Hayashi, T. Ohshima and K. Mashima, Synlett, 2009, 10, 1659 Search PubMed.
- B. C. Ranu, P. Dutta and A. Sarkar, J. Chem. Soc., Perkin Trans. 1, 2000,(14), 2223 RSC.
- R. Fujihara and K. Nakata, ChemistrySelect, 2019, 4, 75 CrossRef CAS.
- Y. Xiong and X. Zhang, Chin. J. Chem., 2011, 29, 1143 CrossRef CAS.
- G. Basumatary and G. Bez, Tetrahedron Lett., 2017, 58, 4312 CrossRef CAS.
- R. Singha and J. K. Ray, Tetrahedron Lett., 2016, 57, 5395 CrossRef CAS.
- N. Mallesha, S. P. Rao, R. Suhas and D. C. Gowda, J. Chem. Res., 2011, 35, 536 CrossRef CAS.
- E. C. De Souza, M. Romero-Ortega and H. F. Olivo, Tetrahedron Lett., 2018, 59, 287 CrossRef CAS.
- I. Ferreira Pereira da Silva, L. Cavalcante dos Santos Giló, A. Campos Reis, J. Raimundo da Rocha, A. J. de Oliveira, H. Fonseca Goulart and A. Euzébio Goulart Santana, ChemistrySelect, 2023, 8, e202301291 CrossRef CAS.
- S. Guo, S. Xing, S. Mao, Y. Gao, W. Chen and Y. Wang, Tetrahedron Lett., 2014, 55, 6718 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08717d |
| ‡ These authors have contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2024 |