Open Access Article
Open Access ArticleFunctional electrospun nanofibers: fabrication, properties, and applications in wound-healing process
Qianlan Zheng a,
Yuewei Xi
a,
Yuewei Xi *ab and
Yunxuan Weng*ab
*ab and
Yunxuan Weng*ab
aCollege of Light Industry Science and Engineering, Beijing Technology and Business University, Beijing 100048, China. E-mail: zheng_qianlan@163.com; xiyuewei@btbu.edu.cn; wyxuan@th.btbu.edu.cn
bBeijing Key Laboratory of Quality Evaluation Technology for Hygiene and Safety of Plastics and Beijing Technology and Business University, Beijing 100048, China
First published on 22nd January 2024
Abstract
Electrostatic spinning as a technique for producing nanoscale fibers has recently attracted increasing attention due to its simplicity, versatility, and loadability. Nanofibers prepared by electrostatic spinning have been widely studied, especially in biomedical applications, because of their high specific surface area, high porosity, easy size control, and easy surface functionalization. Wound healing is a highly complex and dynamic process that is a crucial step in the body's healing process to recover from tissue injury or other forms of damage. Single-component nanofibers are more or less limited in terms of structural properties and do not fully satisfy various needs of the materials. This review aims to provide an in-depth analysis of the literature on the use of electrostatically spun nanofibers to promote wound healing, to overview the infinite possibilities for researchers to tap into their biomedical applications through functional composite modification of nanofibers for advanced and multifunctional materials, and to propose directions and perspectives for future research.
1. Introduction
In recent years, there has been a rapid development of research on nanomaterials. Nanofibers are prepared via stretching, deposition, self-assembly, phase separation, etc., but there are problems such as complicated processes, low efficiency, and high environmental requirements, which limit the application of nanofibers.1 Electrostatic spinning technology has emerged to keep pace with the times.2Electrostatic spinning was initially used to produce nanofibers for textiles3 and filtration applications.4 With the continuous development of science and technology, electrostatic spinning has important applications in many fields. For instance, noteworthy applications exist in the realm of food packaging pertaining to the preservation of food through antimicrobial and antioxidant properties,5–7 enhancement in the encapsulation efficiency (EE) of actives,5,8 enzyme immobilization,9,10 edible food coatings,11 and smart packaging,6,12,13 and thermal management composites for phase-change materials also have significant applications. For example, they are used in water filtration treatment,14 photocatalytic treatment of water pollution,15 electrocatalysis,16 and filtration in the field of environmental protection. According to reports, there are already dozens of polymers that have been made into nanofiber structures through electrospinning technology,17,18 such as poly-L-lactic acid (PLA),19,20 polyvinyl alcohol (PVA),21,22 polycaprolactone (PCL),22–25 polyurethane (PU),26 polyvinylidene fluoride (PVDF),27 and polyhydroxybutyric acid ester hydroxy valerate (PHBV),28,29 which are also used in the development of electrospinning materials.
Functional electrostatically spun nanofibers are a kind of fibrous material prepared by the electrostatic spinning technology with multiple functions. Due to their simplicity, multifunctionality, and loadability; their high specific surface area and porosity; biocompatibility and biodegradability; easy control over size; and easy surface functionalization (e.g., surface coating and surface modification), they have recently attracted increasing interest, and they have a promising future for a wide range of applications in the field of biomedicine, especially in the field of wound healing.30 In the field of biomedicine as well as tissue engineering,31 Hu et al.32 prepared composite nanofibers with polydopamine (PDA)-modified hydroxyapatite nanoparticles and PLA by the ES method, which exhibit better hydrophilicity, mechanical properties, and cellular activity, and can be used for tissue engineering scaffolds. Hwang et al.33 coated the surface of PLA fibers with tantalum (Ta), which has high biocompatibility, to improve the bone conduction of PLA. This can be used as a guiding membrane for bone regeneration. Liu et al.34 encapsulated cinnamaldehyde (CA) in cyclodextrin (CD) and introduced CA/CD into PLA composite fibers by electrospinning, thereby improving the mechanical properties, hydrophilicity, and antibacterial activity, as well as lowering the cytotoxicity, of the nanofibers. They can be used as wound dressings. Electrospinning has certain advantages in the preparation of fiber membranes. For example, electrospun fibers with randomly oriented network structures can simulate the three-dimensional structure of the extracellular matrix (ECM), with high porosity and permeability. They are nontoxic, do not cause rejection when implanted in the body, and can be completely metabolized and excreted from the body.25,35
Currently, numerous studies have reported the application of functional electrospun nanofibers in promoting wound healing of various types. For instance, they can be used in acute skin wounds,36,37 chronic wound healing,38–40 and other aspects. At the same time, there are many studies devoted to the development of new functional electrospun nanofiber materials and exploring different application areas and mechanisms41 (Fig. 1).
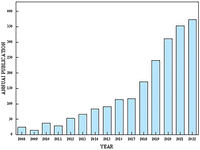 | ||
| Fig. 1 Annual issues (2008–2022); search the science web for terms “electrospinning” and “wound healing”. | ||
The popularization of electrostatically spun nanotechnology has expanded the use of nanomaterials in biomedical applications. This paper reviews the applications of electrostatically spun nanofibers in the field of promoting wound healing. An introduction to the ES technology is given, followed by a collation of the structure and healing mechanisms of skin and wound healing. In this review, we focus on the preparation and properties of functionalized nanofiber dressings such as antimicrobial, antiinflammatory, hemostatic, antioxidant, controlled drug release, responsiveness, exudate management, and wound monitoring, as well as their applications in the wound-healing process. Recent advances in research on the healing of chronic wounds, such as burns and diabetes-related wounds, are also briefly described. Functional electrostatically spun nanofiber dressings provide a suitable microenvironment at the wound site and provide a powerful aid to wound healing and consequently better addressing the critical needs in biomedicine.
1.1. ES
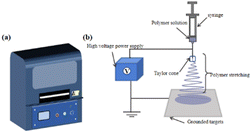
The history of ES dates back as far as 1897, when it was first observed by Riley. In 1934, a patent was filed by Formhals. At that time, L. Bocquillon, an American chemist, reported – for the first time – a method of converting polymer materials into fine filaments using electrostatic force. This technology was then called “electrospinning” and was applied to the manufacture of chemical fiber products. In the following decades, ES technology was continuously improved and developed. Taylor's work with electric jets laid the groundwork for ES, and in 1969, DuPont introduced Kevlar, a polyamide fiber based on ES technology, for the first time.42,43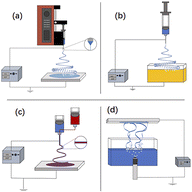 | ||
| Fig. 3 (a) Dry electrospinning (b) wet electrospinning (c) coaxial electrospinning and (d) bubble electrospinning, illustrated from the literature.22 | ||
Table 1 shows a comparison of the classification as well as advantages and disadvantages of dry, wet, coaxial, and bubble ES.
| Classification | Fiber diameter | Fiber morphology | Advantages | Disadvantages |
|---|---|---|---|---|
| Dry electrospinning | Tens of microns to over a millimeter | Single root | Fast and efficient manufacturing processes; mild process conditions; does not contain irritating solvents | Poor mechanical strength |
| Wet electrospinning | Tens of microns to over a millimeter | Single root | Rapid and efficient manufacturing process; high porosity and large pore size | Limited substance encapsulation induced by longer chemical exposure times; poor mechanical strength |
| Coaxial electrospinning | Micron to submicron/nano | Single fiber with porous, robust, hollow, core–shell structure; aligned/random pattern | Multiple polymer compatibility; multiple system solution spinning; ability to easily fabricate nanofibers; high surface area-to-volume ratio; easy handling; excellent material handling; scalable production | High voltage required; the solvent needs to be removed; selection of nuclear sheath solution |
| Bubble electrospinning | Nanoscale | Multi-jet twisting | Spinning is done without static electricity, avoiding static pollution and fire explosion caused by high voltage static electricity | The size of the bubbles is difficult to control, and the bubbles affect each other |
Depending on the number of needles, the main types of ES techniques for the preparation of nanofibers are needleless ES31 (Fig. 4(a)), single-needle ES52 (Fig. 4(b)), coaxial ES53,54 (Fig. 4(c)), and multineedle ES55,56 (Fig. 4(d)).
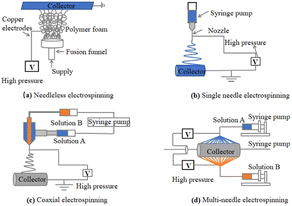 | ||
| Fig. 4 (a) Needleless electrospinning57–59 (b) single-needle electrospinning60 (c) coaxial electrospinning61–63 and (d) multineedle electrospinning.64 | ||
Table 2 lists the classification comparison as well as the advantages and disadvantages of needleless, single-needle, coaxial, and multineedle ES.
| Classification | Advantages | Disadvantages | References |
|---|---|---|---|
| Needleless electrospinning | Strong self-cleaning ability, stable multi-jet ejection, and improved productivity | High production environment requirements; requires a large amount of organic solvents | 43 and 50 |
| High fidelity, high purity, high productivity | Easy to tangle, easy to form beaded fibers | 51 and 52 | |
| Single-needle electrospinning | Long-term stable operation, also easy to handle during the lamination process | Low productivity | 49 and 53 |
| Coaxial electrospinning | The release rate can be adjusted by adjusting parameters such as fiber diameter and porosity | The selection range of materials is narrow | 54–56 |
| Multi-needle electrospinning | Containing a variety of active substances; enhancing productivity | Needle interactions are not conducive to stable spraying; film uniformity is poor; nozzles clog easily | 57 |
| Long-term stretching maintains stability, good repeatability and stabilization; sensing performance and capacitance changes are unaffected by stretching rate | The production environment for manufacturing smart wearable electronics requires high standards | 49 |
| Classification | Impact parameters | Effect on fiber morphology | References |
|---|---|---|---|
| Solution parameters | Relative molecular mass | Fiber diameters obtained from polymer solutions with too high a relative molecular mass are generally larger | 43 |
| Concentration | Fiber diameter increases with concentration, while strength and modulus decrease | 56 | |
| The higher the concentration, the smaller the chance of forming microspheres, the higher the concentration, the easier it is to clog the nozzle, the lower the concentration, the lower the splash | 65 and 66 | ||
| Viscosity | As viscosity increases, the number of droplets and stringers decreases and fiber diameter increases | 67 | |
| High conductivity forms thick and continuous nanofibers, low viscosity forms fine and shorter nanofibers | 68 | ||
| Conductivity | Fiber diameter decreases with increasing conductivity; high conductivity leads to thinner nanofibers and less chance of bead formation; high conductivity affects coaxial fiber uniformity | 69 | |
| Polarity | The greater the polarity of the solvent, the smaller the diameter of the fiber | 5 | |
| Surface tension | High surface tension leads to instability of the jet, usually low surface tension helps the solution to be electrostatically spun at a low electric field | 46 | |
| Volatile | Higher volatility of solvents is associated with higher porosity and increased surface area | 50 | |
| Process parameters | Voltage | Too low a voltage, a high number of fiber beads and larger diameter; too high a voltage cannot form a stable jet, fiber breakage and non-uniformity | 26 |
| The higher the voltage, the smaller the diameter of the fiber, but will also increase the corresponding fiber jet speed and fiber scattering problems | 70 | ||
| Within a certain range, the higher the voltage, the smaller the fiber diameter | 65 | ||
| Feed speed | Fiber diameter decreases with decreasing feed rate, and when the flow rate exceeds a critical value and continues to increase, the fiber diameter increases and the number of beaded structures increases | 42 | |
| Lower transport velocities increase the retention time of the solution between the electrodes, which facilitates the generation and alignment of nanofibers; an increase in flow rate is associated with an increase in fiber diameter | 5 | ||
| Needle diameter | Needle diameter will directly affect the fiber diameter, large diameter is easy to form beaded fibers | 71 | |
| Work distance | Both excessive proximity and excessive distance can result in the formation of bead-like structures in fibers | 47 | |
| Collector | Smooth fibers can be obtained from a metal collector, while flat collection yields randomly oriented fibers, and rotary collection can generate fibers with a certain degree of orientation, demonstrating anisotropy | 72 | |
| Environmental parameters | Humidity | In high humidity, fibers tend to form beads and pores | 58 and 73 |
| Temperature | The formation of fibers is influenced by both environmental and fluid temperatures, typically resulting in uniform nanofiber diameters at higher temperatures | 22 |
| Skin structure | Connect with the outside world | Formation | Functionality |
|---|---|---|---|
| Epidermis | Direct contact | Cuticle and hair growth layer | Blocking tissue fluid outflow, friction reduction, anti-infection |
| Dermis | Not in direct contact | Papillary and reticular layers | Provides mechanical strength to the skin |
1.2. Introduction of skin and wound healing
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 medical insurance beneficiaries, and the estimated medical insurance cost for all such wounds is projected to increase from $220 million to $8.1 billion. This not only requires more medical treatment resource, but also imposes endless economic burdens on society and families.
000 medical insurance beneficiaries, and the estimated medical insurance cost for all such wounds is projected to increase from $220 million to $8.1 billion. This not only requires more medical treatment resource, but also imposes endless economic burdens on society and families.Before discussing the process of wound healing, we first understand the structure of normal skin. Under normal human conditions, the body's autoimmunity can heal skin wounds, which itself has dynamic and complex responses. The physiological structure of the skin is usually divided into the epidermis and dermis,77,78 as shown in Table 4.
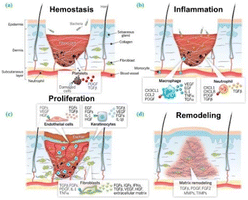 | ||
| Fig. 5 Four stages of wound healing: (a) hemostasis (b) inflammation (c) proliferation and (d) remodeling. The aforementioned references serve as the foundation for this information. The citation is derived from ref. 81. | ||
The process of wound healing is an intricate and sophisticated one, with each stage playing a crucial role in achieving favorable healing outcomes. It involves the participation of various cell types and cytokines, including platelets, lymphocytes, and fibroblasts.81,82 This paper mainly concerns the following aspects, reviewing researchers' advanced multifunctionalization of materials by functional composite modification of nanofibers to promote the process of wound healing. Future research directions and perspectives are proposed for the scientific study of ES in wound healing.
2. Functionality of electrostatically spun nanofiber materials
The original use of electrospun nanofiber wound dressings was to simply physically isolate the outside environment.14,15 However, with the advancement of basic research and the increasing demand for wound healing in clinical settings, there has been a growing requirement for enhanced fiber material properties. Consequently, there has been an emergence of electrospun nanofiber dressings with increasingly diverse dressings.2.1. Antibacterial and antiinflammatory electrostatically spun fiber wound dressing
In recent years, antimicrobial and antiinflammatory fiber dressings with excellent mechanical properties83 and superior biocompatibility prepared using nanotechnology have received increasing attention.84,85 Not only do these materials exhibit antibacterial and antiinflammatory properties (effectively alleviating wound infection and pain as well as promoting wound healing) but they can also activate the repair mechanisms of damaged tissues, playing a crucial role in the wound-healing process.86,87Bacterial infection poses the most common and inevitable challenge to wound healing. Inflammation – the second phase of wound healing88,89 – primarily serves to eradicate bacteria and clear debris. Excessive generation of free radicals at the wound site leads to tissue damage, enzyme inactivation, and lipid peroxidation, as well as significantly delays the wound-healing process.90 In the case of wound infection, bacteria may induce persistent inflammatory responses at the infection site, further prolonging the healing process of the inflammatory stage.
Severe inflammation often results in poor wound healing and can even lead to complications, including bacteremia, sepsis, and septicemia.
Commonly used antimicrobial additives include antimicrobial metal nanoparticles, such as zinc oxide nanoparticles (ZnONPs),91,92 silver nanoparticles (AgNPs),93 and silver oxide (Ag2O). A study found that the use of PLA nanofibers containing nanosilver significantly promoted the healing of skin wounds in mice,94,95 which was attributed to the fact that nanosilver could kill bacteria in the wounds, and at the same time, the high specific surface area of nanofibers could increase the contact area between the cells and the ECM, which could promote the regeneration of cells and healing.96 Furthermore, it has been reported that the long-term use of excessive Ag-containing content can considerably increase the prevalence of “Argyria”.97
ZnO nanoparticles are also widely used in wound dressings due to their excellent antimicrobial activity, low cytotoxicity, and ability to promote the proliferation of fibroblasts. Zhou et al.98 investigated a bilayer electrostatically spun fibrous membrane with an outer layer of randomly oriented ZnO/PCL fibers and an inner layer of neatly aligned nucleosheath structure of PCL@CS. The results showed that the outer layer could confer strong antibacterial activity on the bilayer membrane and inhibit bacterial growth. The inner layer could provide antiinflammatory and effective functions of guiding cellular arrangement. The bilayer CS/PCL electrostatically spun membrane loaded with 1.2 wt% ZnO showed enhanced tensile strength, significant inhibition of Escherichia coli and Staphylococcus aureus, and exhibited no cytotoxic behavior toward fibroblasts. In addition, the bilayer membrane was able to maintain the high bioavailability of ZnO nanoparticles and synchronized with the aligned structural features of CS fibers, thereby reducing inflammation and stimulating cell migration and reepithelialization in vivo.
Sun et al.99 prepared tunable core–sheath-structured polyacrylonitrile (PAN)/HBPE nanofibers using hyperbranched polyester (HBPE) loaded with ZnONPs by phase separation of the two components during centrifugal spinning and further introduced ZnONPs into the sheath layer. The results showed that the ZnONPs were dispersed by HBPE encapsulation. The PAN/HBPE/ZnONP nanofibers doped with 17 wt% ZnONPs successfully delivered 86.6 wt% of Zn to the surface of the nanofibers, which is a 90% improvement compared with nanofibers without HBPE. PAN/HBPE/ZnONP nanofibers exhibited excellent breathability and good biocompatibility, as well as antibacterial rates as high as 96%. This facile processing strategy cleverly combines material and structural advantages and demonstrates an efficient and stable method to prepare core–sheath-structured nanofibers with functional nanoparticles. The method is also expected to be used for loading small molecules, showing a variety of potential applications such as organophosphorus pesticide detection100 and food-packaging materials.101
To date, antimicrobial drugs are still the preferred strategy for the clinical treatment of infected wounds, and a large number of drugs have been reported to be encapsulated in electrostatically spun nanofibers for the preparation of antimicrobial wound dressings, including penicillins (e.g., penicillin, amoxicillin, and so on), macrolides (such as erythromycin and clarithromycin), aminoglycosides (e.g., gentamicin, kanamycin,102 streptomycin eticlopramide, and amikacin), tetracyclines (e.g., doxycycline and tetracycline), sulfonamides (e.g., metribenzylidene and complex sulfonamides), fluoroquinolone antibiotics, and ciprofloxacin hydrochloride.103 Alotaibi et al.104 prepared composite electrostatically spun nanofibers loaded with neomycin (Ne) carrageenan, polyacrylonitrile and pullulan polysaccharides20,88,105–110 (CG/PAN/PU) by ES for cutaneous wound healing, which was especially effective for burn wounds. In rabbit model experiments, wounds healed in a short period even with drug-free CG/PAN/PU/Ne nanofibers compared with conventional surgical gauze; therefore, drug-loaded CG/PAN/PU/Ne nanofibers have greater healing potential. Peng et al.111 successfully prepared hybrid poly(lactic glycolic acid) (PLGA)/silkfibroin (SF) membranes loaded with artemisinin (ART) as an antiinflammatory agent onto fiber membranes by the ES technique. The experimental results revealed that the cumulative ART release could reach 69% after three weeks, and the prepared membrane had a good slow-release effect. Meanwhile, the PLGA/SF/ART fiber membrane shortened the inflammatory period of the wound and facilitated skin regeneration by promoting the expression of inflammatory factors.
Although antibiotics provide excellent clinical control of infections, the problem of bacterial resistance is becoming increasingly serious. Therefore, the search for better antimicrobial strategies has become a topic of considerable interest.112
To address the challenges of long acute wound-healing time and post-healing scars, Jatoi et al.113 developed a three-phase sustained antimicrobial nanofiber of PVA/carbon nanotubes–AgNPs. Dai and Qin et al.114 presented a new method for the preparation of multifunctional nanomaterials with antimicrobial, antioxidant, and thermoregulatory functions. This material was prepared by the coaxial ES technique for core–sheath-structured fibers – PLA/Cur/n-octadecane (OD) phase-change thermoregulatory fibers were prepared by selecting OD as the core material. By optimizing the manufacturing conditions of the nanofibers, the researchers successfully fabricated this composite nanomaterial containing linear copolymers, natural antimicrobial substances, and nanoparticles of zinc oxide (ZnO), as well as successfully applied it to functional materials for antimicrobials, which have a wide range of applications in many fields, such as medicine, food, and pharmaceuticals.
Tang et al.11 fabricated edible gelatin fibers by incorporating peppermint oil (PO) and chamomile oil (CO). The experimental results showed that all the gelatin nanofibers containing PO, CO, or PO/CO exhibited concentration-dependent antimicrobial properties against E. coli and S. aureus, as well as some antioxidant properties. In gelatin nanofibers, the combined action of PO and CO exhibited better bioactivity than either PO or CO alone. Finally, the absence of cytotoxicity of PO/CO-composite gelatin fibers was revealed by the tetramethyl azole salt colorimetric assay. The developed gelatin/PO/CO nanofibers were shown to be biologically safe wound-healing dressings.
Dalgic et al.115 prepared a bilayer electrospun scaffold by the dry ES of hydrophilic PUL fiber membranes onto hydrophobic PHBV3D fiber mats that underwent wet electrospinning in the presence of the crosslinking agent glutaraldehyde (GTA), using pullulan polysaccharide and PHBV116 as the raw materials. The results showed that the PHBV layer of the scaffold was developed as a micron-sized (5.68 ± 2.12 μm) regenerated fiber layer (regeneration layer) with a large pore size, which promotes cell survival, proliferation, and migration, as well as O2 and nutrient delivery. Meanwhile, the PUL layer of the scaffold was produced as a nanosized (264.1 ± 38.8 nm) barrier fiber layer (protective membrane) with a smaller pore size, and the nanosized pores are expected to provide the required barrier properties to effectively block bacterial transmission while achieving optimal O2 and water vapor transport. In in vivo enzymatic degradation experiments, the PUL/PHBV scaffolds lost approximately 20% of their body weight after incubation in a lysozyme solution at 37 °C for 14 days,29 and the degradation of the PUL/PHBV scaffolds is more suitable for wound-healing-promoting applications; therefore, this new naturally sourced PUL/PHBV-type bilayered scaffolds are a promising material for modified wound repair.
In addition, Wang et al.117 prepared poly(γ-benzyl-L-glutamic acid)/poly(lactic acid) (PBLG/PLA) nanofibrous membranes as a three-dimensional culture matrix for melanoma cells in vitro and found that compared with PLA nanofibrous membranes, PBLG/PLA nanofibrous membranes could better support cell survival and proliferation, showing that PBLG/PLA nanofibrous membranes promote a tumor-like structure. Bi et al.21 prepared PLA and PLA/PVA/sodium alginate (SA) nanofibrous membranes by ES and carried out a comparative study and found that PLA/PVA/SA nanofibrous membranes showed slightly better adhesion and proliferation of rat fibroblasts than PLA fibrous membranes and reduced inflammatory response during early wound healing71 (Table 5 summarizes the main features of this subsection).
| Polymer matrix | Active ingredient | Results | References |
|---|---|---|---|
| PCL/CS | ZnO | The double membrane has strong antimicrobial activity, the inner layer is anti-inflammatory, reduces inflammation, stimulates cell migration and re-epithelialization in the body | 97 |
| HBPE | ZnO | PAN/HBPE/ZnONP nanofibers exhibit excellent air permeability and good biocompatibility, with a high antibacterial rate of 96% | 204 |
| CG/PAN/PU | Ne | It has a significant effect on burn wound healing. Drug-loaded CG/PAN/PU/NE nanofibers heal in a short time | 103 |
| PLGA/SF | ART | PLGA/SF/ART fiber membrane shortens the inflammatory phase of wounds by promoting the expression of inflammatory factors, which is conducive to skin regeneration | 110 |
| PLA | Cur | The composite film exhibits excellent bactericidal and antioxidant properties | 55 |
| Gelatin | PO and CO | Gelatin nanofibers containing PO, CO or PO/CO all exhibited antimicrobial properties with concentration and had certain antioxidant properties. PO has good antibacterial activity, while CO has good antioxidant activity | 11 |
| Pullulan and PHBV | Fiber promotes cell survival, proliferation, and migration, while promoting the transmission of O2 and nutrients. Nanoscale pores provide barrier properties that effectively stop the spread of bacteria while enabling optimal O2 oxygen and water vapor transport | 28 | |
| PLA/PVA | SA | PLA/PVA/SA nanofiber membranes promote cell adhesion and proliferation, and can reduce inflammatory responses during early wound healing | 21 |
| PLA | PBLG | PBLG/PLA nanofiber membranes can better support the survival and proliferation of cells | 116 |
2.2. Electrostatically spun nanofiber wound dressing with hemostatic, adhesive, and antioxidant properties
Hemostasis occurs at the earliest stage of wound healing. Hemostatic wound dressings include not only hydrogel dry-frozen “hemostatic sponges”18,118,119 and peptide-based hemostatic collagen scaffolds105 but also electrostatically spun nanofiber dressings with hemostatic properties that have been shown to have a positive impact on promoting wound healing. Studies have shown that hemostasis, as an essential function, is usually used in combination with other functions.120 For example, combining the adhesive properties of electrostatically spun nanofibers not only seals the wound to provide hemostasis121 but also seamlessly adheres to the wound site for a long period, avoiding the potential risk of infection caused by contact between the wound and the external environment.122,123Jiang et al.124 first used different concentrations of sodium hypochlorite (NaClO) to isolate SF to obtain multiscale silk fibers (MSFs). These MSFs are then implanted into the PLA fiber membrane by electrospinning to improve hydrophilicity. The results showed that the MSFs obtained by splitting SF by NaClO not only improved the hydrophilicity of PLA but also improved the cytocompatibility, promoted cell proliferation, and did not cause inflammation. Yao et al.24 introduced vascular endothelial growth factor (VEGF) on the surface of electrostatically spun fibrous scaffolds of polycaprolactone (ε-DA) polymers by UV photoinitiation using thiol-ene chemistry. Experimental studies showed that the tensile strength and modulus of elasticity of the 30% PCL–DA fibrous scaffolds were significantly increased compared with unfunctionalized scaffolds. Coupling with the VEGF peptide increased the surface-water wettability of the scaffolds. Experimental studies in vitro and in chicken chorioallantoic membrane (CAM) showed that scaffolds functionalized with the VEGF peptide induced the phosphorylation of VEGF receptor and promoted the survival, proliferation, and adhesion of vascular endothelial cells. It suggests that VEGF-peptide-functionalized PCL nanofiber scaffolds promote angiogenesis in vivo.
Investigations have shown that wound dressings doped with antioxidant-rich ingredients can scavenge free radicals from the wound site and increase the rate of healing.22,79,125 Antioxidants are substances that help trap and neutralize free radicals, thereby eliminating the damage caused by free radicals to the body.126 Therefore, electrostatically spun nanofibers with antioxidant properties can significantly promote wound healing. Common antioxidants include vitamin C, vitamin E, and glutathione. Polyphenolic8,74,110,127 antioxidants are one of the most widely used natural antioxidants with good stability and storage resistance. Common natural polyphenols such as tea polyphenols, resveratrol, ferulic acid,128,129 curcumin,88,130 and anthocyanins, as well as some flavonoids, have been loaded into electrostatically spun nanofibers to play an antioxidant role in promoting wound healing.131,132
Polyvinylpyrrolidone (PVP) is a nonionic, water-soluble, high-molecular-weight compound formed by the polymerization of N-vinylpyrrolidone monomers. It exhibits excellent physiological inertness; does not participate in human metabolism; and demonstrates remarkable biocompatibility and not irritating to the skin, mucous membranes, or eyes.133 Pusporini et al.134 demonstrated that blending PVP with green tea extract (GTE) and subsequent spinning can produce PVP/GTE nanofibers with antioxidant activity. Polyethylene oxide (PEO), a nontoxic and biologically safe substance, has been widely utilized in the development of composite functional materials and nanocomposites. Locilento et al.135 utilized the electrospinning technique to produce nanofiber membranes composed of PLA and PEO, with the addition of grape seed extract (GSE). These membranes exhibited antioxidant properties and demonstrated effective control over the release of GSE. Furthermore, the PLA/PEO nanofiber membranes loaded with GSE displayed excellent hydrophilicity and demonstrated good biocompatibility with cells (Table 6 summarizes the main features of this subsection).
| Polymer matrix | Active ingredient | Results | References |
|---|---|---|---|
| PLA | SF | Increased hydrophilicity for PLA, improved cytocompatibility, promoted cell adhesion and proliferation, and did not cause inflammation | 123 |
| PCL | VEGF | PCL scaffolds functionalized with VEGF peptides were able to induce phosphorylation of VEGF receptors and promote angiogenesis in vivo | 24 |
| PVP | GTE | Antioxidant active PVP/GTE nanofibers promote wound healing | 133 |
| PLA and PEO | GSE | GSE with antioxidant properties controlled release, and GSE-loaded PLA/PEO nanofiber membranes have good hydrophilicity and cytocompatibility | 134 |
2.3. Electrostatically spun nanofiber wound dressing for drug delivery and controlled release
The porous structure of electrospun nanofibers makes them naturally suitable for loading various substances and slowly releasing them at specific locations. Electrospun nanofibers are widely applied due to their high efficiency in sustained drug release136 and inherent antibacterial properties.137 However, as mentioned earlier, most of the drugs related to antibacterial and antiinflammatory delivery are antibiotics. Wound healing also requires cellular interactions between various cell types, including keratinocytes, fibroblasts, endothelial cells, neutrophils, and macrophages, which are regulated by the endogenous release of cytokines and chemokines at the site of the wound. Therefore, the local delivery of exogenous cells or cytokines has shown significant advantages in promoting wound healing.36,37 In general, biomedical materials should have the following basic requirements when used in clinical medicine: they should be nontoxic, noncarcinogenic, and nonteratogenic, and should show no sudden reaction to human cells and tissue cells; good compatibility with human tissues and show no poisoning, hemolysis, coagulation, fever, and allergic reactions; stable chemical properties and resistance to the action of antibodies, blood, and enzymes; possess physical and mechanical characteristics that are compatible with natural tissues; and specific functions for different purposes. For example, they can have applications in biomedical nanomaterials such as drug release materials and drug carriers.138,139 It is crucial to ensure the safety of the materials to the environment and the human body, and simultaneously improve the encapsulation efficiency (EE) of the contents or loaded components as well as protecting their activity and effectiveness.Fig. 6 is a simple schematic of a device with controlled drug release for ES.140 The device mainly consists of a high-voltage power supply, a nozzle, a drug liquid supply system, and a collector. In operation, the drug solution is fed into the nozzle through the drug supply system, whereas the high-voltage power supply provides a high voltage to eject the drug solution through the nozzle, and is subjected to electrostatic action as it is being ejected to form nanoscale fibers. These nanofibers have a large specific surface area and high porosity, which can increase the solubility and release rate of the drug, thereby achieving the controlled release of the drug (Fig. 7).
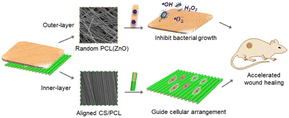 | ||
| Fig. 6 Graphical abstract from the article titled “Electrospun ZnO-loaded chitosan/PCL bilayer membranes with spatially designed structure for accelerated wound healing” is paraphrased here. | ||
 | ||
| Fig. 7 Schematic of the controlled release of electrospinning drugs, from the literature.140 | ||
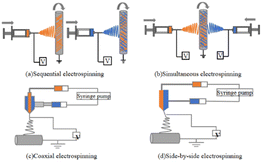
| Categorization | Sequential electrostatic spinning | Synchronized electrostatic spinning | Coaxial electrostatic spinning | Side-by-side electrostatic spinning |
|---|---|---|---|---|
| Schematic diagram of fiber morphology |  |
 |
 |
 |
| Advantages | Builds multi-layer fiber structures: adapts to a variety of materials; no equipment modifications required | Construction of hybrid fiber structures; independent of compatibility with different materials | Construction of fibers with different morphologies; making fibrous structures out of non-electrospinnable materials | Construction of Janus fibers; making fiber structures out of non-electrospinnable materials |
| Disadvantages | Poor contact surface bonding | The collector needs to avoid interactions | Small range of material options | Janus structures are of low quality |
| Applications | Multifunctional scaffolds; modulation of single drug release profiles | Multiple characterization scaffolds; multidrug delivery | Multiple characterization scaffolds; single drug release; multi-drug co-delivery | Amphiphilic applications |
| References | 115 | 116 | 117 | 118 |
Sequential ES is the ES of different material solutions or melts one at a time through a single spinneret on a case-by-case basis, which can be regarded as multiple replications of the ES of a single material.145 Therefore, sequential ES can be easily realized from a variety of materials using the same experimental setup and process parameters as conventional ES.141 A major disadvantage of sequential ES is poor interfacial bonding between the layers of different materials. The controlled release of drugs is used to regulate the release of single drugs, as well as the co-delivery of multiple drugs.
Simultaneous ES,72 sometimes referred to as co-ES, refers to the simultaneous ES of nanofibers of different materials stacked on top of each other using two or more spinnerets on a single collector to achieve an integrated fiber structure.146 When multiple jets are simultaneously ejected from different spinnerets, the interaction of external electric fields and Coulomb repulsion between the jets usually results in unstable and irregular paths of the jets, which makes it difficult to achieve the desired fiber structure.141 Synchronized ES is suitable for more selective material systems. Thus, these well-mixed fiber structures produce more biomimetic scaffolds by mimicking multiple features of the natural ECM and integrating multiple drug-carrying fibers for co-delivery.
Coaxial ES technology is the most widely studied. Coaxial ES employs a coaxial nozzle, consisting of two or more concentrically nested needles supplied by solutions or melts of different materials,147 to synthesize multiple fluids into one-dimensional nanostructures with different morphologies. It is capable of forcing materials without filament-forming properties into one-dimensional structures or producing high-quality nanofibers from poorly spinnable polymers. The main disadvantage of coaxial ES is the limited choice of material pairs that can be used to prepare high-quality core–shell fibers. Fine-tuning of the drug release profiles through shells that act as buffers,148 as well as the co-delivery of multiple drugs with independent controlled release behavior, offers greater potential for tissue engineering and drug delivery applications.51
In side-by-side ES,144 Janus fibers are fabricated by using a spinneret having two or more parallel-needle arrangements in which each side of the structure can be individually designed on the basis of the chemical composition and function. Side-by-side ES also allows for the treatment of nonspinnable fluids with ES fluids to build Janus-structured nanocomposites. However, ES solutions can easily mix, resulting in the inability to produce well-defined Janus fibers.70,149 To date, Janus fibers have been widely used in adjustable single-drug-release profiles or controlled multidrug delivery by doping drug active ingredients into both sides of the Janus structure.150
Alves et al.151 successfully prepared drug-loaded PLA fibers using the electrospinning technique, with acetic acid dexamethasone (DEX) or betamethasone 17-valerate (BET) as the corticosteroids. PLA/BET and PLA/DEX electrospun films exhibited promising drug release properties for sustained release in local applications, thus providing a potential drug delivery system.
Glycyrrhizoidoic acid (GA),138 a natural product of the genus Glycyrrhizae, is a novel mitochondrion that targets ligands and improves mitochondrial permeability and enhances mitochondrial drug uptake. GA-functionalized nanocarriers have also shown low toxicity, suggesting that it could be a useful tool for drug delivery. Cacciotti et al.152 prepared 18-beta-glycyrrhetinic acid (GA)-nanoparticle-loaded chitosan and poly(lactic acid-glycolic acid copolymer)-based nanoparticles (GA-NPs) as well as poly(lactic acid) fibers (GA-FBs) to compare the release behaviors of free and encapsulated GA in two different biopolymer delivery systems to ensure the controlled release of GA in vitro. MTT assays demonstrated that no cytotoxic effects on regular human gingival fibroblasts (HGFs) were observed. On the other hand, GA-NPs and GA-FBs increased the toxic effects on human oral squamous cell carcinoma (PE/CA-PJ15) cells in vitro. The specific effect of GA on PE/CA-PJ15 cells was mainly due to the different sensitivity of cancer cells to reactive oxygen species (ROS) overproduction; GA-NP and GA-FB preparations increased the toxic effects on oral cancer cells in vitro. Therefore, FBs can be considered for the treatment of local manifestations of systemic diseases (i.e., blistering diseases, oral lichen planus, and aphthous ulcers), as there is no need for the drug to penetrate the oral mucosa.
Locilento et al.153 prepared nanofibrous membranes based on PLA and PEO with GSE by the ES technique with antioxidant properties and well-controlled release of GSE, and PLA/PEO nanofibrous membranes loaded with GSE had good hydrophilicity and good cytocompatibility. Goreninskii et al.154 used ES to form PCL/PVP scaffolds with hexafluoroisopropanol as a solvent, which had extreme hydrophilicity and high cellular activity. The PVP-containing materials exhibited enhanced initial release. Because of the good solubility of proteins, drugs and other bioactive compounds in this solvent, the fabrication of PCL/PVP scaffolds using HFIP expands their application in drug delivery systems.
Yang et al.155 developed a detachable triaxial spinneret to direct three ES fluids for the preparation of a series of nucleosheath nanostructures. The three fluids used for ES consisted of a common solvent (outer fluid), an electrospinnable dilute cellulose acetate (CA)143 solution (intermediate fluid), and an electrospinnable ibuprofen–methanolysin solution (inner fluid), which led to the formation of an ibuprofen/methanolysin amorphous solid dispersion coated with a thin layer of CA, and the thickness of the coating could be adjusted by precisely adjusting the CA concentration of the intermediate solution. Experimental results show that single ibuprofen/myristolol protein fibers produce an initial burst release, but this release is eliminated in systems with CA coatings, and this tunable way of precisely manipulating drug release provides a new approach for the development of advanced functional nanomaterials, which can be controlled by the process nanostructure in triaxial ES property relationship to control their performance. Kyzioł et al.103 developed a novel method for the fabrication of SA nanofibers loaded with ciprofloxacin hydrochloride (CpHCl) under the presence of PEO and Pluronic F-127 surfactant. The experimental findings indicate that approximately 24% of CpHCl is released from alginate fibers within the first 20 h, exhibiting an overall loading efficiency of 51% for the investigated antibiotic.
Dual drug-carrying drug delivery scaffolds147 and multiaxial ES technology adds new dynamics by fabricating multilayered nano- and microsized fibers.158 These techniques offer the possibility of forming fibers with features such as co-bonding, reinforced cores, and porous and hollow structures. Unique fabrication processes can be used to tailor the mechanical energy, biological, and release of various factors that may be useful in various controlled drug delivery applications. Utilizing these advantages, various polymers and their combinations have been explored in many drug delivery and controlled release applications.
Ma et al.159 successfully prepared PLA/SF nucleosheath fibers containing diclofenac sodium as a steroidal antiinflammatory drug and ZnONPs by coaxial ES. Their results showed that the initial release concentration was reduced, and the cumulative drug release rates of the nucleosheath fibers were 48.8% and 72.6% at 100 and 600 min, respectively, which showed significant slow-release antimicrobial effects and could be used for the prevention of wound infections (Table 8 summarizes the main features of this subsection).
| Polymer matrix | Active ingredient | Results | References |
|---|---|---|---|
| PLA | DEX and BET | PLA/BET and PLA/DEX electrospun membranes provide sustained drug release for topical applications | 144 |
| CS | GA | GA-NPs and GA-FBs have toxic effects on PE/CA-PJ15 cells | 145 |
| CA | Ibuprofen-gliadin solution | Monoibuprofen/gliadin fibers with CA coating completely eliminate the initial burst release, precisely manipulating the drug release | 148 |
| PEO | CpHCl | CpHCl releases the studied antibiotic from alginate fibers within the first 20 hours with a final loading efficiency of 51% | 102 |
| PLA/SF | ZnO NPs | PLA/SF fibers, with reduced initial release concentration, have a sustained release effect, improved antibacterial properties, and good cytocompatibility, which can be used to prevent wound infection | 151 |
2.4. Self-healing and stimulus-responsive electrostatically spun nanofiber wound dressing
Zhu et al.161 developed intrinsic self-healing thermochromic fiber membranes (STFMs) with biomimetic-constrained protective structures. The self-healing fibers mainly consist of synthesized polydimethylsiloxane-based polyurea (PDMS-PUa), known as the original STFMs. A “closed protective layer” composed of polyacrylic acid (PAA) and branched polyethyleneimine (bPEI) is self-assembled onto the shell of the original STFM. This protective layer prevents supramolecular interactions between PDMS-PUa and ensures the stability of the inherent morphology of the STFMs. By introducing thermochromic microcapsules into the system, the resulting fiber membrane exhibits the thermochromic functionality. Furthermore, without the use of external adhesives, the surface chemical structure of the fiber membrane can be further adjusted by utilizing hydrogen bonds formed at the interfaces between different functional layers to assemble electronic skin sensors. The artificial electronic skin demonstrates self-healing capabilities without external stimuli, offering new directions for the field of biomimetic and advanced flexible electronic skin.162
As an alternative antimicrobial method, photodynamic therapy (PDT),169 which has recently attracted considerable attention, utilizes photosensitizers (PSs) to produce highly bactericidal ROS in the presence of light and oxygen.
γ-PGA,170 also known as poly-γ-glutamic acid, is a peptide molecule synthesized through the amide bond polymerization of the monomers of L-glutamic acid and D-glutamic acid.171 Its main chain consists of numerous peptide bonds and carboxyl groups. γ-PGA is a natural anionic biopolymer that exhibits excellent moisturizing and water-absorbing properties.172 Due to its outstanding biocompatibility and biodegradability, it has found wide applications in the fields of drug delivery, wound dressings, and tissue engineering. Sun et al.173 prepared an electrospun nanofiber scaffold composed of γ-PGA and a cationic photosensitizer, namely, 5,10,15,20-tetra(1-methylpyridin-4-yl)porphyrin tetra(p-toluenesulfonate), which was chemically crosslinked for stability. This scaffold demonstrates excellent cytocompatibility and antibacterial properties, as it utilizes the PS to generate highly bactericidal ROS under oxygen presence and light irradiation.
Tao et al.88 conducted a study and observed that the abundance of aromatic rings in mesoporous polydopamine nanoparticles (PDANPs) enables the loading of a substantial amount of drugs through π–π stacking and/or hydrogen-bonding interactions. These loaded drugs can be released upon exposure to near-infrared laser irradiation.
Han et al.174 developed the first self-incinerating polymer (SIP) nanofiber membrane in which self-incineration could be triggered by an external stimulus. Electrostatically spun SIP/PAN fibers provided depolymerization 25 times faster and were more responsive (i.e., incineration) than cast membranes under triggering conditions. The depolymerization of SIP in the SIP/PAN co-blended fibrous membranes resulted in a shift in surface properties from hydrophobic (∼110°) to hygroscopic (∼0°). The triggered release of encapsulated functional molecules was demonstrated using coaxial electrostatically spun fiber membranes made of SIP/PAN co-blended sheaths and PVP/dye cores. The coaxial fiber with a SIP/PAN sheath provided a minimal release of the encapsulated material in a nontriggered solution, whereas it immediately released the encapsulated material when the triggering condition was met. The versatility is enhanced compared to non-SIP coaxial fibers, which do not trigger a response due to external stimuli (Table 9 summarizes the main features of this subsection).
| Polymer matrix | Active ingredient | Results | References |
|---|---|---|---|
| γ-PGA | TMPyP | γ-PGA-TMPyP nanofiber mats show good cytocompatibility and antimicrobial properties using photosensitizers to produce highly bactericidal reactive oxygen species (ROS) in the presence of oxygen in the presence of light | 131 |
| PEGDA | Cur | Under NIR laser irradiation, the concentration of released Cur increased dramatically, showing a concentration gradient-dependent drug diffusion | 78 |
| PAN | SIP | Fiber membranes containing SIPs in the triggering solution achieved a triggered release of encapsulated functional molecules. The SIP-containing fiber membrane in non-triggering solution provides minimal release of the encapsulated material. And when the trigger condition is met, it releases the encapsulated material immediately | 132 |
2.5. Electrospun nanofiber dressings for exudate management and wound-monitoring functions
When damaged skin is in the inflammatory phase, histamine increases the capillary permeability, leading to excessive exudate production from the wound.176 Excessive wound exudate tends to lead to severe infection, which, in turn, hinders the wound-healing process.177 Although the widely used hydrophilic wet dressings have some wound exudate absorption function, when the wound exudate is excessive or is not removed in time, the wet dressings are prone to become a source of contamination, resulting in the hydration and infection of the wound tissue.150 For this reason, electrostatically spun nanofiber dressings with the function of absorbing or pumping the wound exudate have emerged. Yang et al.178 added ε-polylysine (EPL) as a low-cost and safe natural antimicrobial agent to hyaluronic acid (HA) nanofiber dressings, and the prepared EPL/HA composite nanofiber mats were extremely hydrophilic, absorbing 26.3 times its own mass of wound exudate. However, the hydrophilic-absorbent dressing made it difficult to retain the exudate, especially under pressure, and was prone to rewet the wound.
Researchers electrospun hydrophobic substances onto a hydrophilic fiber network to construct a self-pumping dressing with a biofluid pump that unidirectionally drains excess biofluid from the hydrophobic side to the hydrophilic side, preventing the biofluid from wetting the wound, which is referred to as a “self-pumping dressing”,157 and has promising potential in wound exudate management.149,150 In contrast to hydrophilic dressings, self-pumping dressings prevent rewetting of the wound while managing the wound exudate. Currently, the construction of hydrophilic/hydrophobic asymmetric (Janus) structures72 is an important method for the preparation of self-pumping exudate management dressings.179 The Janus structure confers different properties to each side of the material, such as hydrophilicity on one side and hydrophobicity on the other. This asymmetric wettability allows the transport of liquid from the hydrophobic layer to the hydrophilic layer and prevents exudate from flowing back from the hydrophilic layer to the hydrophobic layer.180
Shi et al.181 who electrostatically spun a hydrophobic PU nanofibrous membrane on the surface of cotton medical gauze found that PU/cotton dressings could exclude excessive wound exudate from the wound by a self-pumping action and showed faster healing than conventional dressings. In addition to the micro-/nanofiber-composite-based Janus self-pumping dressing, researchers also developed a pure nanofiber Janus self-pumping dressing with antimicrobial properties.
Qi et al.182 embedded the macromolecular antimicrobial agent poly(hexamethylene guanidine hydrochloride) (PHGC) into multilayered nanofiber membranes (polyurethane/polyacrylonitrile/sodium polyacrylate PU/PAN/SPA) with a hydrophobic/hydrophilic gradient structure and self-pumping effect by ES to prepare unidirectional water-transporting antimicrobial wound dressings. It was found that when the mass fraction of PHGC was 0.06%, the dressing could achieve nearly 100% antimicrobial activity against E. coli and S. aureus. Yang et al.183 fabricated Janus membranes based on hydrophilic PVP and hydrophobic ethylcellulose (EC) polymers by side-by-side ES and prepared antimicrobial wound exudate management dressings by loading ciprofloxacin (CIP) and AgNPs on the PVP side and the EC side, respectively. It was found that when the PVP side was dissolved, the CIP was rapidly released to kill the bacteria, and at the same time, due to the insolubility of EC and AgNPs, the EC/AgNP side could play an antimicrobial role when the Janus fibers were in contact with the wound exudate. The development of Janus electrostatically spun nanofiber dressings that combine wound exudate management with the antimicrobial function will be an effective way to address wound overhydration and bacterial infection.
Currently, the effectiveness of wound care primarily relies on (semi-)blind assessment methods,40 which involve visually observing the wound to judge its healing status. However, this approach is unable to provide an accurate assessment of wound healing, and the frequent removal of dressings can lead to secondary damage and cause pain to patients.188 Therefore, researchers have increasingly paid attention to the incorporation of sensors or stimuli-responsive materials into dressings, which provide real-time monitoring and diagnostic capabilities with respect to wounds.185 A variety of physical, chemical, or biological markers have been loaded into nanofiber dressings that can be used to monitor indicators such as bacteria, pH, temperature, ROS, and blood glucose at the wound site.189,190 The information provided by the detection metrics can enable clinicians to monitor the wound status in real time and provide patients with optimal wound-care protocols. Singh et al.184 designed a medical dressing (TH-WD) for the simultaneous monitoring and treatment of bacterial infections. TH-WD consists of a blend of PU, the antimicrobial agent ciprofloxacin precursor (Pro-Cip), and hemicyanin reactive dyes (H-Cy), and is prepared via the ES technology. When encountering a bacterial infection, TH-WD releases H-Cy and hydrolyzes Pro-Cip to ciprofloxacin, and the wound undergoes a color change from yellow to green to red, enabling rapid wound monitoring and care. Furthermore, Gong et al.191 utilized electrospinning technology to fabricate a nanofiber membrane composed of conductive patterns and a load of moxifloxacin hydrochloride (MOX) thermosensitive polymer, known as the Smart Conductive–Polymer Nanofiber Hydrogel Membrane (SC-PNHM). This membrane enables the real-time monitoring of wound temperature: an infection leads to an increase in the wound temperature. Once the temperature rises, the thermal-responsive fibers are triggered to release MOX, effectively eliminating the bacterial infection. Combining the monitoring sensor with electrospun nanofibers, the development of nanofiber dressings with wound-monitoring capabilities has emerged as a new trend in the field of medical materials (Table 10 summarizes the main features of this subsection).
| Polymer matrix | Active ingredient | Results | References |
|---|---|---|---|
| HA | EPL | EPL/HA composite nanofiber pads are extremely hydrophilic and can absorb 26.3 times their own mass of wound exudate | 136 |
| PU | Cotton medical gauze | Absorbs excess wound exudate from the wound through a self-pumping action and demonstrates faster healing than conventional dressings | 139 |
| LA | IBU | Photothermal antimicrobial and synergistic antimicrobial properties of NIR irradiation. Janus nanofiber dressings have asymmetric wettability for targeted water transport to drain excess wound exudate | 142 |
| PU/PAN/SPA | PHGC | Unidirectional water delivery properties that drive the spontaneous flow of wound exudate from the inside out. Powerful antimicrobial capabilities | 140 |
| PVP and EC | CIP and AgNPs | When the PVP side is dissolved, CIP is rapidly released to kill bacteria, while the EC/AgNPs side can still exert antimicrobial effects when Janus fibers come into contact with wound exudates due to the insolubility of EC and AgNPs | 141 |
| PU | Pro-Cip | When bacterial infection is encountered, TH-WD releases H-Cy and hydrolyzes Pro-Cip to ciprofloxacin, and the wound undergoes a color change from yellow to green to red, enabling rapid wound monitoring and care | 150 |
| Heat-responsive polymers | MOX | Enables real-time monitoring of wound temperature, and infection causes an increase in wound temperature, which triggers the release of MOX from thermo-responsive fibers to eliminate bacterial infection | 151 |
2.6. Refractory wound dressing
Difficult-to-heal wounds, also known as chronic wounds, refer to the condition where wounds are unable to heal or heal slowly due to various reasons. This is considered to be a significant public health issue that severely affects both health and quality of life.82 Acute wounds typically heal within 2 to 4 weeks, whereas chronic wounds fail to fully heal even after subjected to standard care for 4 weeks.176Common chronic wounds include burns, lower extremity venous ulcers (VLUs),192,193 pressure ulcers (PUs),194 diabetic foot ulcers (DFUs),195 and ischemic ulcers (IUs),196 which continue to present a daunting challenge to clinicians and researchers alike.197
The utilization of electrospun nanomaterials to facilitate the healing process of burn wounds is a subject of significant interest. Electrospun nanomaterials can be fabricated into various forms according to specific application requirements, allowing for a tailored selection based on factors such as burn-related wound size and severity.200 Furthermore, these nanomaterials possess diverse properties that can be harnessed to achieve distinct functionalities, such as promoting cell growth, enhancing cell adhesion, or facilitating cell proliferation. Of utmost importance, these nanomaterials exhibit excellent biocompatibility, ensuring their safe implementation.201
Pandey et al.202 developed a novel composite electrostatically spun nanofiber antimicrobial dressing (PVP–Ce–CurNF) consisting of PVP, cerium nitrate hexahydrate (Ce(NO3)3·6H2O), and curcumin for addressing burn-related wound infection and scar formation. In the experiment, a PVP–Ce–CurNF dressing was directly applied to a full circular excision wound in rats. It showed complete healing and reepithelialization within 20 days without any scarring.
Hadisi et al.91 investigated the preparation of a novel wound dressing made of HA–filiprotein/ZnO nucleosheath structure nanofibers for the treatment of burns. ZnO, the nuclear fluid antimicrobial agent in the core–sheath structure, can be continuously released and also maintain its biological activity. The results of in vivo studies showed that loading 3 wt% of ZnO in a wound dressing significantly improved the wound-healing process and significantly reduced the inflammatory response at the wound site.
In diabetic trauma, macrophage polarization from proinflammatory (M1) to antiinflammatory (M2) types becomes very difficult due to the specific high-glucose environment that interferes with the physiological process of wound healing.205 Large numbers of M1 macrophages accumulate at the trauma site, releasing proinflammatory cytokines such as tumor necrosis factor (TNF-α)111 and producing ROS.206 Diabetes mellitus – because of the specificity of the condition – considerably increases the rate of infection in chronic wounds, leading to a prolonged inflammatory phase and vasculopathy, which ultimately leads to difficult-to-heal wounds and increasing the risk of infection: therefore, it creates a vicious circle. It is very probable to cause a variety of complications, including cardiovascular and cerebrovascular diseases,207 renal diseases,204 and retinopathy,208 as well as affect the function of various tissues and organs throughout the body.
Diabetic skin injury (DSI)209 usually occurs on the legs and feet, referred to as the “diabetic foot.” The wound-healing process in DSI can last for more than 12 weeks; often, this process can be impeded by a harsh hypoxic microenvironment (HME), which consists of a range of endogenous and exogenous factors, such as localized hemorrhage, bacterial infection, and microangiopathy–hypoxia feedback loops.
Zhao et al.210 used charged quaternized chitosan (QCS) and HA to self-assemble black phosphorus (BP) nanosheets and hemoglobin (Hb) in a layer-by-layer manner onto electrostatically spun poly(L-propylene-alpha-lipoic acid ester) (PLLA) nanofibers. Hb is a natural oxygen carrier with excellent thermoresponsiveness. On the basis of NIR-assisted oxygen delivery combined with biopolymer bioactive properties, BP was utilized to convert NIR radiation into heat and stimulate oxygen release from Hb. QCS is a hemostatic and broad-spectrum antimicrobial material. Photothermal therapy increases the bacterial susceptibility to QCS and has great potential for diabetes-related wound healing.
Berger et al.211 assembled nanofiber coatings containing anti-miR by ES to package and control the release of anti-miR-92a (92ai). Experimental tests showed that the dressing of 92ai enhanced healing and the release of anti-miR from the dressing exhibited efficacy in promoting endothelial cell angiogenesis and overall healing in a relevant model of diabetic ulcers. Davani et al.39 adopted ES to prepare a coating consisting of PEO, chitosan, and vancomycin shells and PVP, gelatin, and imipenem/cilastatin of novel core–shell double-drug delivery nanofibers. The results showed that the nanofiber dressing exhibited significant antimicrobial activity against S. aureus, methicillin-resistant S. aureus (MRSA), and Pseudomonas aeruginosa (Gram-negative bacteria) without cytotoxicity. It can be used as a suitable drug delivery device for DFU infections in clinical practice, as well as for other chronic wounds165 (Table 11 summarizes the main features of this subsection).
| Polymer matrix | Active ingredient | Results | References |
|---|---|---|---|
| PVP | CurNF | Addresses infection and scar formation in burn wounds | 193 |
| HA-SF | ZnO | ZnO can continuously release and maintain its bioactivity, 3 wt% ZnO wound dressing significantly improved the wound healing process and reduced the inflammatory response at the wound site for the treatment of burns | 90 |
| PLLA | QCS and BP | Using BP to convert NIR radiation into heat and stimulate oxygen release from Hb. QCS is a hemostatic and broad-spectrum antimicrobial material. Photothermal therapy increases bacterial susceptibility to QCS and has great potential for diabetic wound healing | 201 |
| miR-92a | Release of anti-miR in diabetic ulcers has efficacy in promoting endothelial cell angiogenesis and overall healing | 202 | |
| PEO/CS/PVP | Vancomycin/imipenem/cilastatin | Nanofiber dressings can be used as a suitable drug delivery device for diabetic foot ulcer infections in clinical care, as well as for other chronic wounds | 71 |
3. Conclusions and prospects
At present, with the demand for a healthy and comfortable life, the research of ES as wound dressing has made great progress in ES technology and material design, but in order to meet the needs of specific biomedical needs, multifunctionally coordinated and facilitated wound dressings with certain advantages, both multifunctional and precisely prepared as mentioned before, such as antimicrobial, antiinflammatory, antioxidant, controlled drug release, impulse response, wound exudate management, wound monitoring, and other multifunctional nanofiber dressings will be the main research direction in the future. Research on burn-related wound healing and diabetes-related wound healing in recent years is also presented. Functional electrostatically spun nanofiber dressings provide a suitable microenvironment for wound healing.Although electrostatically spun nanofibers show good potential for wound therapy, more practical studies and experimental proof are still needed. Electrostatically spun nanofibrous membranes have structural advantages, but electrostatically spun nanofibers still need to consider a series of problems such as the interference of nozzle and recycling-agent evaporation during spinning, solvent toxicity, and fiber deposition uniformity. Therefore, there are still significant challenges in the selection of suitable polymers and solvents and the optimization of existing spinning technologies to achieve stable, safe, and large-scale greening of nanofiber dressings for preparation and application. The further development of efficient electrostatically spun multifunctional dressings still needs to be urgently addressed.
In the future, with the continuous development and improvement of nanotechnology, it is believed that functional nanofibers and electrospun nanofibers will be more widely used in the field of biomedicine.
Author contributions
Conceptualization, Y. X.; writing – original draft preparation, Q. Z.; data collection, Q. Z. and Y. X.; writing – review and editing, Y. X. and Y. W.; project administration and funding acquisition, Y. W. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.References
- C. Xue and L. D. Wilson, Carbohydr. Polym., 2022, 275, 118751 CrossRef CAS PubMed.
- A. M. Al-Dhahebi, J. Ling, S. G. Krishnan, M. Yousefzadeh, N. K. Elumalai, M. S. M. Saheed, S. Ramakrishna and R. Jose, Appl. Phys. Rev., 2022, 9, 011319 CAS.
- D. Hu, J. Aust. Ceram. Soc., 2022, 58, 1509–1517 CrossRef CAS.
- A. I. Kamil and M. M. Munir, Powder Technol., 2023, 430, 118978 CrossRef CAS.
- C. Drosou, M. Krokida and C. G. Biliaderis, Food Hydrocolloids, 2022, 133, 107949 CrossRef CAS.
- F. Topuz and T. Uyar, Food Res. Int., 2020, 130, 108927 CrossRef CAS.
- S. R. Falsafi, H. Rostamabadi, K. Nishinari, R. Amani and S. M. Jafari, Food Chem., 2022, 374, 131826 CrossRef CAS PubMed.
- B. S. Isik, F. Altay and E. Capanoglu, Food Chem., 2018, 265, 260–273 CrossRef CAS PubMed.
- C. Shi, A. Zhou, D. Fang, T. Lu, J. Wang, Y. Song, L. Lyu, W. Wu, C. Huang and W. Li, Chem. Eng. J., 2022, 445, 136746 CrossRef CAS.
- T. Min, L. Zhou, X. Sun, H. Du, Z. Zhu and Y. Wen, Food Chem., 2022, 391, 133239 CrossRef CAS PubMed.
- Y. Tang, Y. Zhou, X. Lan, D. Huang, T. Luo, J. Ji, Z. Mafang, X. Miao, H. Wang and W. Wang, J. Agric. Food Chem., 2019, 67, 2227–2234 CrossRef CAS.
- T. S. M. Kumar, K. S. Kumar, N. Rajini, S. Siengchin, N. Ayrilmis and A. V. Rajulu, Composites, Part B, 2019, 175, 107074 CrossRef.
- P. Müller and M. Schmid, Foods, 2018, 7(10), 170 CrossRef PubMed.
- A. H. Behroozi, M. Al-Shaeli and V. Vatanpour, Desalination, 2023, 558, 116638 CrossRef CAS.
- H. Lv, Y. Liu, Y. Bai, H. Shi, W. Zhou, Y. Chen, Y. Liu and D.-G. Yu, Catalysts, 2023, 13(4), 758 CrossRef CAS.
- L. Luan, X. Ji, B. Guo, J. Cai, W. Dong, Y. Huang and S. Zhang, Biotechnol. Adv., 2023, 63, 108098 CrossRef CAS PubMed.
- N. Yuan, K. Shao, S. Huang and C. Chen, Int. J. Biol. Macromol., 2023, 240, 124321 CrossRef CAS PubMed.
- W. Zheng, Z. Zhang, Y. Li, L. Wang, F. Fu, H. Diao and X. Liu, Chem. Eng. J., 2022, 447, 137482 CrossRef CAS.
- I. Armentano, N. Bitinis, E. Fortunati, S. Mattioli, N. Rescignano, R. Verdejo, M. A. Lopez-Manchado and J. M. Kenny, Prog. Polym. Sci., 2013, 38, 1720–1747 CrossRef CAS.
- W. Zheng, Z. Zhang, Y. Li, L. Wang, F. Fu, H. Diao and X. Liu, Chem. Eng. J., 2022, 447, 137482 CrossRef CAS.
- H. Bi, T. Feng, B. Li and Y. Han, Polymers, 2020, 12(4), 839 CrossRef CAS PubMed.
- B. Kong, R. Liu, J. Guo, L. Lu, Q. Zhou and Y. Zhao, Bioact. Mater., 2023, 19, 328–347 CAS.
- V. Shadman-Manesh, A. Gholipour-Kanani, N. Najmoddin and S. Rabbani, Sci. Rep., 2023, 13, 919 CrossRef CAS PubMed.
- T. Yao, H. Chen, R. Wang, R. Rivero, F. Wang, L. Kessels, S. M. Agten, T. M. Hackeng, T. G. A. M. Wolfs, D. Fan, M. B. Baker and L. Moroni, Bioact. Mater., 2023, 20, 306–317 CAS.
- J. Fernández-Pérez, K. E. Kador, A. P. Lynch and M. Ahearne, Mater. Sci. Eng., C, 2020, 108, 110415 CrossRef.
- M. M. Demir, I. Yilgor, E. Yilgor and B. Erman, Polymer, 2002, 43, 3303–3309 CrossRef CAS.
- Z. Feng, Z. Zhao, Y. Liu, Y. Liu, X. Cao, D.-G. Yu and K. Wang, Adv. Mater. Technol., 2023, 8, 2300021 CrossRef CAS.
- A. D. Dalgic, E. Koman, A. Karatas, A. Tezcaner and D. Keskin, Biomater. Adv., 2022, 134, 112554 CrossRef PubMed.
- M. Kouhi, M. P. Prabhakaran, M. Shamanian, M. Fathi, M. Morshed and S. Ramakrishna, Compos. Sci. Technol., 2015, 121, 115–122 CrossRef CAS.
- R. S. Bhattarai, R. D. Bachu, S. H. S. Boddu and S. Bhaduri, Pharmaceutics, 2019, 11(1), 5 CrossRef CAS PubMed.
- D. M. Dos Santos, D. S. Correa, E. S. Medeiros, J. E. Oliveira and L. H. C. Mattoso, ACS Appl. Mater. Interfaces, 2020, 12, 45673–45701 CrossRef CAS.
- S. Hu, J. Wu, Z. Cui, J. Si, Q. Wang and X. Peng, J. Appl. Polym. Sci., 2020, 137, 49077 CrossRef CAS.
- C. Hwang, S. Park, I.-G. Kang, H.-E. Kim and C.-M. Han, Mater. Sci. Eng., C, 2020, 115, 111112 CrossRef CAS.
- Y. Liu, X. Liang, R. Zhang, W. Lan and W. Qin, Polymers, 2017, 9(10), 464 CrossRef PubMed.
- G. Patel, K.-S. Na, H. J. Lee and W.-G. Koh, Curr. Ophthalmol. Rep., 2021, 9, 146–157 CrossRef.
- S. V. Langwald, A. Ehrmann and L. Sabantina, Membranes, 2023, 13(5), 488 CrossRef CAS PubMed.
- K. C. Castro, M. G. N. Campos and L. H. I. Mei, Int. J. Biol. Macromol., 2021, 173, 251–266 CrossRef CAS PubMed.
- P. Zhao, W. Chen, Z. Feng, Y. Liu, P. Liu, Y. Xie and D.-G. Yu, Int. J. Nanomed., 2022, 17, 4137–4162 CrossRef CAS PubMed.
- F. Davani, M. Alishahi, M. Sabzi, M. Khorram, A. Arastehfar and K. Zomorodian, Mater. Sci. Eng., C, 2021, 123, 111975 CrossRef CAS.
- E. R. Ghomi, M. Niazi and S. Ramakrishna, Polym. Adv. Technol., 2023, 34(2), 520–530 CrossRef.
- I. Cacciotti, M. Ciocci, E. Di Giovanni, F. Nanni and S. Melino, Int. J. Mol. Sci., 2018, 19(8), 2368 CrossRef.
- T. J. Sill and H. A. von Recum, Biomaterials, 2008, 29, 1989–2006 CrossRef CAS PubMed.
- N. Bhardwaj and S. C. Kundu, Biotechnol. Adv., 2010, 28, 325–347 CrossRef CAS PubMed.
- B. Pant, M. Park and A. A. Kim, Pharmaceutics, 2023, 15(5), 1347 CrossRef CAS PubMed.
- Z. Zhao, J. Li, X. Yuan, X. Li, Y. Zhang and J. Sheng, J. Appl. Polym. Sci., 2005, 97, 466–474 CrossRef CAS.
- A. K. Haghi and M. Akbari, Phys. Status Solidi A, 2007, 204, 1830–1834 CrossRef CAS.
- C. S. Ki, D. H. Baek, K. D. Gang, K. H. Lee, I. C. Um and Y. H. Park, Polymer, 2005, 46, 5094–5102 CrossRef CAS.
- Q. Wang, Y. Li, X. Zhou, T. Wang, L. Qiu, Y. Gu and J. Chang, Polymers, 2019, 11(9), 1413 CrossRef PubMed.
- R. Tonndorf, D. Aibibu and C. Cherif, Mater. Sci. Eng., C, 2020, 106, 110105 CrossRef CAS.
- B. Pant, M. Park and S.-J. Park, Pharmaceutics, 2019, 11(7), 305 CrossRef CAS PubMed.
- J. Li, Y. Liu and H. E. Abdelhakim, Molecules, 2022, 27(6), 1803 CrossRef CAS PubMed.
- R. Boda, I. Lázár, A. Keczánné-Üveges, J. Bakó, F. Tóth, G. Trencsényi, I. Kálmán-Szabó, M. Béresová, Z. Sajtos, E. D. Tóth, Á. Deák, A. Tóth, D. Horváth, B. Gaál, L. Daróczi, B. Dezső, L. Ducza and C. Hegedűs, Int. J. Mol. Sci., 2023, 24(8), 7562 CrossRef CAS PubMed.
- S. S. Ganesh, R. Anushikaa, V. S. Swetha Victoria, K. Lavanya, A. Shanmugavadivu and N. Selvamurugan, J. Funct. Biomater., 2023, 14(5), 288 CrossRef CAS PubMed.
- N. Angel, S. Li, F. Yan and L. Kong, Trends Food Sci. Technol., 2022, 120, 308–324 CrossRef CAS.
- Y. Yang, W. Chen, M. Wang, J. Shen, Z. Tang, Y. Qin and D.-G. Yu, Polymers, 2023, 15(10), 2237 CrossRef CAS PubMed.
- G. Xu, X. Chen, Z. Zhu, P. Wu, H. Wang, X. Chen, W. Gao and Z. Liu, Adv. Compos. Hybrid Mater., 2020, 3, 98–113 CrossRef CAS.
- G. Zheng, J. Jiang, X. Wang, W. Li, W. Zhong and S. Guo, Appl. Phys. A, 2018, 124, 473 CrossRef.
- A. G. Thite, R. D. Kale, P. K. Panda and D. M. More, Cellulose, 2023, 30, 4873–4888 CrossRef CAS.
- M. Hwang, M. O. Karenson and Y. A. Elabd, ACS Appl. Polym. Mater., 2019, 1, 2731–2740 CrossRef CAS.
- M. P. Falaschetti, F. Rondina, E. Maccaferri, L. Mazzocchetti, L. Donati, A. Zucchelli and L. Giorgini, Compos. Struct., 2023, 311, 116845 CrossRef CAS.
- T. Dai, Z. Qin, S. Wang, L. Wang, J. Yao, G. Zhu, B. Guo, J. Militky, M. Venkataraman and M. Zhang, Polym. Adv. Technol., 2022, 33, 4062–4071 CrossRef CAS.
- Y. Zhao, C. Tian, Y. Liu, Z. Liu, J. Li, Z. Wang and X. Han, Biomaterials, 2023, 295, 122029 CrossRef CAS PubMed.
- F. Topuz and T. Uyar, Food Res. Int., 2020, 130, 108927 CrossRef CAS PubMed.
- N. Zhan, Y. Li, C. Zhang, Y. Song, H. Wang, L. Sun, Q. Yang and X. Hong, J. Colloid Interface Sci., 2010, 345, 491–495 CrossRef CAS PubMed.
- N. S. A. Halim, M. D. H. Wirzal, S. M. Hizam, M. R. Bilad, N. A. H. M. Nordin, N. S. Sambudi, Z. A. Putra and A. R. M. Yusoff, J. Environ. Chem. Eng., 2021, 9, 104613 CrossRef.
- S. Noamani, S. Niroomand, M. Rastgar and M. Sadrzadeh, npj Clean Water, 2019, 2, 20 CrossRef CAS.
- X. Han, P. Huo, Z. Ding, P. Kumar and B. Liu, Pharmaceutics, 2019, 11(9), 449 CrossRef CAS PubMed.
- L. Shahreen and G. G. Chase, J. Eng. Fibers Fabr., 2015, 10, 155892501501000308 Search PubMed.
- A. Koski, K. Yim and S. Shivkumar, Mater. Lett., 2004, 58, 493–497 CrossRef CAS.
- X. Jiao, J. Xie, H. Du, X. Bian, C. Wang, L. Zhou and Y. Wen, Food Packag. Shelf Life, 2023, 37, 101066 CrossRef CAS.
- S. Zhao, X. Wu, L. Wang and Y. Huang, J. Appl. Polym. Sci., 2004, 91, 242–246 CrossRef CAS.
- J. Xing, M. Zhang, X. Liu, C. Wang, N. Xu and D. Xing, Mater. Today Bio, 2023, 100710 CrossRef CAS PubMed.
- A. Refate, Y. Mohamed, M. Mohamed, M. Sobhy, K. Samhy, O. Khaled, K. Eidaroos, H. Batikh, E. El-Kashif, S. El-Khatib and S. Mehanny, Heliyon, 2023, 9, e17051 CrossRef CAS PubMed.
- P. Kewlani, L. Singh, B. Singh and I. D. Bhatt, Sustainable Chem. Pharm., 2022, 29, 100791 CrossRef CAS.
- L. Barnum, M. Samandari, T. A. Schmidt and A. Tamayol, Expert Opin. Drug Delivery, 2020, 17, 1767–1780 CrossRef CAS PubMed.
- S. Jacob, A. B. Nair, J. Shah, N. Sreeharsha, S. Gupta and P. Shinu, Pharmaceutics, 2021, 13(3), 357 CrossRef CAS PubMed.
- A. Zimmerman, L. Bai and D. D. Ginty, Science, 2014, 346, 950–954 CrossRef CAS PubMed.
- B. S. Kim, G. Gao, J. Y. Kim and D.-W. Cho, Adv. Healthcare Mater., 2019, 8, 1801019 CrossRef PubMed.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed.
- M. B. Buechler, R. N. Pradhan, A. T. Krishnamurty, C. Cox, A. K. Calviello, A. W. Wang, Y. A. Yang, L. Tam, R. Caothien, M. Roose-Girma, Z. Modrusan, J. R. Arron, R. Bourgon, S. Müller and S. J. Turley, Nature, 2021, 593, 575–579 CrossRef CAS PubMed.
- J. Koehler, F. P. Brandl and A. M. Goepferich, Eur. Polym. J., 2018, 100, 1–11 CrossRef CAS.
- M. Sadeghi-Aghbash, M. Rahimnejad, H. Adeli and F. Feizi, Curr. Pharm. Biotechnol., 2023, 24, 1079–1093 CAS.
- G. Selvakumar and S. Lonchin, Int. J. Biol. Macromol., 2020, 164, 1592–1599 CrossRef CAS PubMed.
- X. Zhang, Y. Wang, Z. Gao, X. Mao, J. Cheng, L. Huang and J. Tang, J. Appl. Polym. Sci., 2024, 141(1), e54746 CrossRef CAS.
- A. Memic, T. Abudula, H. S. Mohammed, K. J. Navare, T. Colombani and S. A. Bencherif, ACS Appl. Bio Mater., 2019, 2, 952–969 CrossRef CAS.
- Z. Norouzi and M. Abdouss, Int. J. Biol. Macromol., 2023, 233, 123518 CrossRef CAS PubMed.
- L. Bardoňová, A. Kotzianová, K. Skuhrovcová, O. Židek, H. Vágnerová, J. Kulhánek, T. Hanová, M. Knor, J. Starigazdová, K. M. Kutláková and V. Velebný, Int. J. Biol. Macromol., 2022, 194, 726–735 CrossRef PubMed.
- B. Tao, C. Lin, Z. Yuan, Y. He, M. Chen, K. Li, J. Hu, Y. Yang, Z. Xia and K. Cai, Chem. Eng. J., 2021, 403, 126182 CrossRef CAS.
- F. Zhou, X. Jia, Y. Yang, Q. Yang, C. Gao, Y. Zhao, Y. Fan and X. Yuan, Mater. Sci. Eng., C, 2016, 68, 623–631 CrossRef CAS PubMed.
- H.-S. Jung, M. H. Kim, J. Y. Shin, S. R. Park, J.-Y. Jung and W. H. Park, Carbohydr. Polym., 2018, 193, 205–211 CrossRef CAS PubMed.
- Z. Hadisi, M. Farokhi, H. R. Bakhsheshi-Rad, M. Jahanshahi, S. Hasanpour, E. Pagan, A. Dolatshahi-Pirouz, Y. S. Zhang, S. C. Kundu and M. Akbari, Macromol. Biosci., 2020, 20, 1900328 CrossRef CAS PubMed.
- C. T. Chasapis, P.-S. A. Ntoupa, C. A. Spiliopoulou and M. E. Stefanidou, Arch. Toxicol., 2020, 94, 1443–1460 CrossRef CAS PubMed.
- Y. Zhu, J. Li, J. Kim, S. Li, Y. Zhao, J. Bahari, P. Eliahoo, G. Li, S. Kawakita, R. Haghniaz, X. Gao, N. Falcone, M. Ermis, H. Kang, H. Liu, H. Kim, T. Tabish, H. Yu, B. Li, M. Akbari, S. Emaminejad and A. Khademhosseini, Biomaterials, 2023, 296, 122075 CrossRef CAS PubMed.
- K. Shameli, M. Bin Ahmad, W. M. Z. W. Yunus, N. A. Ibrahim, R. A. Rahman, M. Jokar and M. Darroudi, Int. J. Nanomed., 2010, 5, 573–579 CrossRef CAS PubMed.
- L. Song, Y. Guo, J. Fan, X. Fan, Y. Xie, Z. Xiao, H. Wang, D. Liang and Y. Wang, Composites, Part A, 2022, 163, 107231 CrossRef CAS.
- M. Cui, S. Li, X. Ma, J. Wang, X. Wang, N. E. Stott, J. Chen, J. Zhu and J. Chen, Int. J. Biol. Macromol., 2023, 128088 Search PubMed.
- N. Chow, K. Fain, J. Truitt and C. Stetson, Proc. - Bayl. Univ. Med. Cent., 2022, 35, 382–384 CrossRef PubMed.
- F. Zhou, C. Cui, S. Sun, S. Wu, S. Chen, J. Ma and C. M. Li, Carbohydr. Polym., 2022, 282, 119131 CrossRef CAS PubMed.
- M. Sun, T. Hou, J. Zhou, L. Zhou, Z. Zhang, X. Li and B. Yang, ACS Appl. Nano Mater., 2023, 6, 9707–9717 CrossRef CAS.
- I. Cacciotti, F. Pallotto, V. Scognamiglio, D. Moscone and F. Arduini, Mater. Sci. Eng., C, 2020, 111, 110744 CrossRef CAS PubMed.
- I. Cacciotti, E. Fortunati, D. Puglia, J. M. Kenny and F. Nanni, Carbohydr. Polym., 2014, 103, 22–31 CrossRef CAS PubMed.
- J. J. Ahire, D. D. Robertson, A. J. van Reenen and L. M. T. Dicks, Biomed. Pharmacother., 2017, 86, 143–148 CrossRef CAS PubMed.
- A. Kyzioł, J. Michna, I. Moreno, E. Gamez and S. Irusta, Eur. Polym. J., 2017, 96, 350–360 CrossRef.
- B. S. Alotaibi, M. Shoukat, M. Buabeid, A. K. Khan and G. Murtaza, J. Drug Delivery Sci. Technol., 2022, 74, 103502 CrossRef CAS.
- G. Selvakumar and S. Lonchin, Biomater. Adv., 2022, 140, 213078 CrossRef CAS PubMed.
- I. Esparza, N. Jimenez-Moreno, F. Bimbela, C. Ancin-Azpilicueta and L. Gandia, J. Environ. Manage., 2020, 265, 110510 CrossRef CAS PubMed.
- Z. Feng, S. Chen, A. Ahmad, L. Chen and W. Bai, Carbohydr. Polym., 2022, 295, 119836 CrossRef CAS PubMed.
- B. Bernier, Can. J. Microbiol., 1958, 4, 195–204 CrossRef CAS PubMed.
- H. Bender, J. Lehmann and K. Wallenfels, Biochim. Biophys. Acta, 1959, 36, 309–316 CrossRef CAS PubMed.
- F. Zhou, Z. Gu, Z. Zeng, X. Tang, C. Li, Z. Fang, B. Hu, H. Chen, C. Wang, S. Chen, H. Wu, W. Wu and Y. Liu, Food Biosci., 2022, 49, 101898 CrossRef CAS.
- Y. Peng, Y. Ma, Y. Bao, Z. Liu, L. Chen, F. Dai and Z. Li, Int. J. Biol. Macromol., 2021, 183, 68–78 CrossRef CAS PubMed.
- Z. Zeng, S. Deng, Y. Liu, C. Li, Z. Fang, B. Hu, H. Chen, C. Wang, S. Chen, W. Wu and Y. Liu, Food Hydrocolloids, 2023, 142, 108781 CrossRef CAS.
- A. W. Jatoi, H. Ogasawara, I. S. Kim and Q.-Q. Ni, Mater. Lett., 2019, 241, 168–171 CrossRef CAS.
- T. Dai, Z. Qin, S. Wang, L. Wang, J. Yao, G. Zhu, B. Guo, J. Militky, M. Venkataraman and M. Zhang, Polym. Adv. Technol., 2022, 33, 4062–4071 CrossRef CAS.
- A. D. Dalgic, E. Koman, A. Karatas, A. Tezcaner and D. Keskin, Biomater. Adv., 2022, 134, 112554 CrossRef PubMed.
- P. M. Tyubaeva, I. A. Varyan, E. D. Nikolskaya, M. R. Mollaeva, N. G. Yabbarov, M. B. Sokol, M. V. Chirkina and A. A. Popov, Nanomaterials, 2023, 13(2), 236 CrossRef CAS PubMed.
- Y. Wang, J. Qian, T. Liu, W. Xu, N. Zhao and A. Suo, Mater. Sci. Eng., C, 2017, 76, 313–318 CrossRef CAS PubMed.
- J. He, G. Ye, H. Ma, S. Jia, J. Ma, J. Lv, D. Jia, Y. Song, F. Liu, P. Li, J. Wang, K. Gyal, K. Gou, M. La and R. Zeng, Int. J. Biol. Macromol., 2023, 240, 124487 CrossRef CAS PubMed.
- H.-J. Shu, C.-X. Wu, K. Yang, T.-W. Liu, C. Li and C.-L. Cao, J. Mater. Eng., 2019, 47, 124–129 CrossRef.
- W.-L. Luo, X. Qiu, J. Zhang, P.-Y. Hu, X.-F. Liu, J.-J. Liu, M. Yu, S. Ramakrishna and Y.-Z. Long, Mater. Sci. Eng., C, 2019, 101, 380–386 CrossRef CAS PubMed.
- R. Chen, Z. Liu, T. Cui, X. Zhang, C.-F. Wang, G.-X. Li, G. Wang and S. Chen, ACS Appl. Mater. Interfaces, 2023, 15, 46322–46332 CrossRef CAS PubMed.
- V. Deineka, O. Sulaieva, N. Pernakov, J. Radwan-Praglowska, L. Janus, V. Korniienko, Y. Husak, A. Yanovska, I. Liubchak, A. Yusupova, M. Piatkowski, A. Zlatska and M. Pogorielov, Mater. Sci. Eng., C, 2021, 120, 111740 CrossRef CAS PubMed.
- W. Liang, Q. Lu, F. Yu, J. Zhang, C. Xiao, X. Dou, Y. Zhou, X. Mo, J. Li and M. Lang, Biomater. Sci., 2021, 9, 7124–7133 RSC.
- F. Jiang, D. Yan, J. Lin, H. Kong and Q. Yao, Mater. Today Chem., 2021, 21, 100494 CrossRef CAS.
- Y. Xi, J. Ge, M. Wang, M. Chen, W. Niu, W. Cheng, Y. Xue, C. Lin and B. Lei, ACS Nano, 2020, 14, 2904–2916 CrossRef CAS PubMed.
- I. Solaberrieta, A. Jimenez, I. Cacciotti and M. Carmen Garrigos, Polymers, 2020, 12(6), 1323 CrossRef CAS PubMed.
- P. Kewlani, L. Singh, B. Singh and I. D. Bhatt, Sustainable Chem. Pharm., 2022, 29, 100791 CrossRef CAS.
- F. Wahid, L.-H. Huang, X.-Q. Zhao, W.-C. Li, Y.-Y. Wang, S.-R. Jia and C. Zhong, Biotechnol. Adv., 2021, 53, 107856 CrossRef CAS PubMed.
- Z. Ding, X. Chang, X. Fu, H. Kong, Y. Yu, H. Xu, Y. Shan and S. Ding, Int. J. Biol. Macromol., 2022, 219, 121–137 CrossRef CAS PubMed.
- E. Shekarforoush, A. C. Mendes, V. Baj, S. R. Beeren and I. S. Chronakis, Molecules, 2017, 22(10), 1708 CrossRef PubMed.
- E. Subroto, R. Andoyo and R. Indiarto, Antioxidants, 2023, 12, 633 CrossRef CAS PubMed.
- J. L. C. de Queiroz, I. Medeiros, A. C. Trajano, G. Piuvezam, A. C. F. Nunes, T. S. Passos and A. H. A. Morais, Food Chem., 2022, 385, 132593 CrossRef PubMed.
- R. K. Deshmukh, P. Kumar, R. Tanwar and K. K. Gaikwad, Food Bioprocess Technol., 2022, 15, 2839–2853 CrossRef CAS.
- P. Pusporini, D. Edikresnha, I. Sriyanti, T. Suciati, M. M. Munir and K. Khairurrijal, Mater. Res. Express, 2018, 5, 5 Search PubMed.
- D. A. Locilento, L. A. Mercante, R. S. Andre, L. H. C. Mattoso, G. L. F. Luna, P. Brassolatti, F. F. Anibal and D. S. Correa, J. Nanomater., 2019, 2019, 2472964 Search PubMed.
- W.-F. Lai, O. S. Reddy, D. Zhang, H. Wu and W.-T. Wong, Sci. Technol. Adv. Mater., 2023, 24, 2167466 CrossRef PubMed.
- O. S. Fenton, K. N. Olafson, P. S. Pillai, M. J. Mitchell and R. Langer, Adv. Mater., 2018, 30, 1705328 CrossRef PubMed.
- C. Zhang, Z. Liu, Y. Zheng, Y. Geng, C. Han, Y. Shi, H. Sun, C. Zhang, Y. Chen, L. Zhang, Q. Guo, L. Yang, X. Zhou and L. Kong, Small, 2018, 14, 1703306 CrossRef PubMed.
- N. Wang, C. Liu, W. Yao, H. Zhou, S. Yu, H. Chen and W. Qiao, Colloids Surf., B, 2021, 205, 111866 CrossRef CAS PubMed.
- A. Luraghi, F. Peri and L. Moroni, J. Controlled Release, 2021, 334, 463–484 CrossRef CAS PubMed.
- Y. Montoya, J. Cardenas, J. Bustamante and R. Valencia, Biomater. Res., 2021, 25, 38 CrossRef CAS PubMed.
- H. Luo, J. Hu, Y. Dou, Y. Yang and J. Hou, Compos. Commun., 2020, 22, 100516 CrossRef.
- Y. Huang, Y. Jin, B. Wang, H. Tian, Y. Weng and S. Men, J. Polym. Environ., 2022, 30, 1758–1771 CrossRef CAS.
- M.-L. Wang, D.-G. Yu and S. W. A. Bligh, Appl. Mater. Today, 2023, 31, 101766 CrossRef.
- J. Wang, M. Wu, Y. Zhu, Z. Wang, H. Cao, X. Li, Y. Yin, X. Ren, Y. Tian, Z. Guo and X. Zeng, Tissue Eng., Part A, 2022, 28, 958–967 CrossRef CAS PubMed.
- G. Yang, L. Zhou, X. Zhang, D. Pan, M. Wang, S. Luo, F. Su, Y. Ji and C. Liu, Compos. Commun., 2023, 37, 101450 CrossRef.
- J. Li, Y. Liu and H. E. Abdelhakim, Molecules, 2022, 27(6), 1803 CrossRef CAS PubMed.
- M. Duan, J. Sun, S. Yu, Z. Zhi, J. Pang and C. Wu, Int. J. Biol. Macromol., 2023, 233, 123433 CrossRef CAS PubMed.
- Z. Xu, J. Fan, W. Tian, X. Ji, Y. Cui, Q. Nan, F. Sun and J. Zhang, Adv. Funct. Mater., 2024, 34(3), 2307449 CrossRef CAS.
- X. Zhang, R. Lv, L. Chen, R. Sun, Y. Zhang, R. Sheng, T. Du, Y. Li and Y. Qi, ACS Appl. Mater. Interfaces, 2022, 14, 12984–13000 CrossRef CAS PubMed.
- P. E. Alves, B. G. Soares, L. C. Lins, S. Livi and E. P. Santos, Eur. Polym. J., 2019, 117, 1–9 CrossRef CAS.
- I. Cacciotti, L. Chronopoulou, C. Palocci, A. Amalfitano, M. Cantiani, M. Cordaro, C. Lajolo, C. Calla, A. Boninsegna, D. Lucchetti, P. Gallenzi, A. Sgambato, G. Nocca and A. Arcovito, Nanotechnology, 2018, 29(28), 285101 CrossRef PubMed.
- D. A. Locilento, L. A. Mercante, R. S. Andre, L. H. C. Mattoso, G. L. F. Luna, P. Brassolatti, F. D. F. Anibal and D. S. Correa, J. Nanomater., 2019, 2019, 1–11 CrossRef.
- S. Goreninskii, N. Danilenko, E. Bolbasov, A. Evtina, M. Buldakov, N. Cherdyntseva, M. Saqib, N. Beshchasna, J. Opitz, V. Filimonov and S. Tverdokhlebov, J. Appl. Polym. Sci., 2021, 138, app50535 CrossRef CAS.
- Y. Yang, W. Li, D.-G. Yu, G. Wang, G. R. Williams and Z. Zhang, Carbohydr. Polym., 2019, 203, 228–237 CrossRef CAS PubMed.
- Y. Zhang, X. Gao, X. Tang, L. Peng, H. Zhang, S. Zhang, Q. Hu and J. Li, Int. J. Biol. Macromol., 2023, 253, 126693 CrossRef CAS PubMed.
- Y. Liu, S. Huang, S. Liang, P. Lin, X. Lai, X. Lan, H. Wang, Y. Tang and B. Gao, ACS Appl. Mater. Interfaces, 2023, 15(45), 52244–52261 CAS.
- A. Khalf and S. V. Madihally, Eur. J. Pharm. Biopharm., 2017, 112, 1–17 CrossRef CAS PubMed.
- Y. Ma and J. Li, Mater. Lett., 2022, 325, 132804 CrossRef CAS.
- M. Zhu, J. Yu, Z. Li and B. Ding, Angew. Chem., Int. Ed., 2022, 61, e202208949 CrossRef CAS PubMed.
- M. Zhu, J. Li, J. Yu, Z. Li and B. Ding, Angew. Chem., Int. Ed., 2022, 61, e202200226 CrossRef CAS PubMed.
- H. Zhang, Y. Shao, B. Gao and J. Li, Eur. Polym. J., 2023, 198, 112429 CrossRef CAS.
- A. K. Brooks, R. G. Ramsey, N. Zhang and V. K. Yadavalli, ACS Biomater. Sci. Eng., 2023, 9, 5793–5803 CrossRef CAS PubMed.
- J. Zhao, Q. Shu, S. H. Jia and J. Tian, Chin. J. Burns, 2022, 38, 870–873 CAS.
- Z. Ge, W. Guo, Y. Tao, H. Sun, X. Meng, L. Cao, S. Zhang, W. Liu, M. L. Akhtar, Y. Li and Y. Ren, Adv. Mater., 2023, 35(47), 2304005 CrossRef CAS PubMed.
- T. Wang, B. Ma, G. Hao, Z. Ding, P. Liu, Y. Zhang and J. Liu, J. Drug Delivery Sci. Technol., 2023, 88, 104961 CrossRef CAS.
- G. R. Nirmal, C.-C. Liao, Z.-C. Lin, A. Alshetaili, E. Hwang, S.-C. Yang and J.-Y. Fang, Drug Delivery, 2023, 30(1), 2245169 CrossRef CAS PubMed.
- O. A. Oyebode, S. W. Jere and N. N. Houreld, J. Diabetes Res., 2023, 2023, 1359537 Search PubMed.
- B. Wang, M. Wang, A. Mikhailovsky, S. Wang and G. C. Bazan, Angew. Chem., Int. Ed., 2017, 56(18), 5031–5034 CrossRef CAS PubMed.
- Z. Chen, J. Yao, J. Zhao and S. Wang, Int. J. Biol. Macromol., 2023, 225, 1235–1245 CrossRef CAS PubMed.
- J. Tang, W. Yi, J. Yan, Z. Chen, H. Fan, D. Zaldivar-Silva, L. Aguero and S. Wang, Int. J. Biol. Macromol., 2023, 247, 125754 CrossRef CAS PubMed.
- Y. Gong, P. Wang, R. Cao, J. Wu, H. Ji, M. Wang, C. Hu, P. Huang and X. Wang, ACS Nano, 2023, 17(22), 22355–22370 CrossRef CAS PubMed.
- L. Sun, L. Song, X. Zhang, R. Zhou, J. Yin and S. Luan, Mater. Sci. Eng., C, 2020, 113, 110936 CrossRef CAS PubMed.
- D. Han, X. Yu, Q. Chai, N. Ayres and A. J. Steckl, ACS Appl. Mater. Interfaces, 2017, 9, 11858–11865 CrossRef CAS PubMed.
- D.-L. Lam, Y.-T. Cheng and C.-J. Huang, ACS Appl. Mater. Interfaces, 2023, 15, 53297–53309 CrossRef CAS PubMed.
- Y. Gao, Z. Qiu, L. Liu, M. Li, B. Xu, D. Yu, D. Qi and J. Wu, J. Polym. Sci., 2022, 60, 2191–2212 CrossRef CAS.
- Y. Yang and H. Hu, Polymers, 2018, 10(2), 210 CrossRef PubMed.
- Q. Yang, Z. Xie, J. Hu and Y. Liu, Mater. Sci. Eng., C, 2021, 128, 112319 CrossRef CAS PubMed.
- S. Yang, K. Yin, D. Chu, J. He and J.-A. Duan, Appl. Phys. Lett., 2018, 113(20), 203701 CrossRef.
- J. Chen, Z.-X. Low, S. Feng, Z. Zhong, W. Xing and H. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 60763–60788 CrossRef CAS PubMed.
- L. Shi, X. Liu, W. Wang, L. Jiang and S. Wang, Adv. Mater., 2019, 31(5), 1804187 CrossRef PubMed.
- L. Qi, K. Ou, Y. Hou, P. Yuan, W. Yu, X. Li, B. Wang, J. He, S. Cui and X. Chen, Mater. Des., 2021, 201, 109461 CrossRef CAS.
- J. Yang, K. Wang, D.-G. Yu, Y. Yang, S. W. A. Bligh and G. R. Williams, Mater. Sci. Eng., C, 2020, 111, 110805 CrossRef CAS PubMed.
- H. Singh, W. Li, M. R. Kazemian, R. Yang, C. Yang, S. Logsetty and S. Liu, ACS Appl. Bio Mater., 2019, 2, 2028–2036 CrossRef CAS PubMed.
- B. Mirani, Z. Hadisi, E. Pagan, S. M. H. Dabiri, A. van Rijt, L. Almutairi, I. Noshadi, D. G. Armstrong and M. Akbari, Adv. Healthcare Mater., 2023, 12(18), 2203233 CrossRef CAS PubMed.
- N. Dehghani, F. Haghiralsadat, F. Yazdian, F. Sadeghian-Nodoushan, N. Ghasemi, F. Mazaheri, M. Pourmadadi and S. M. Naghib, Int. J. Biol. Macromol., 2023, 238, 124078 CrossRef CAS PubMed.
- C. Li, X. Luo, L. Li, Y. Cai, X. Kang and P. Li, Int. J. Biol. Macromol., 2022, 209, 344–355 CrossRef CAS PubMed.
- J. Xia, H. Zhang, F. Yu, Y. Pei and X. Luo, ACS Appl. Mater. Interfaces, 2020, 12, 24370–24379 CrossRef CAS PubMed.
- M. He, F. Ou, Y. Wu, X. Sun, X. Chen, H. Li, D. Sun and L. Zhang, Mater. Des., 2020, 194, 108913 CrossRef CAS.
- Q. Zeng, X. Qi, G. Shi, M. Zhang and H. Haick, ACS Nano, 2022, 16, 1708–1733 CrossRef CAS PubMed.
- M. Gong, P. Wan, D. Ma, M. Zhong, M. Liao, J. Ye, R. Shi and L. Zhang, Adv. Funct. Mater., 2019, 29(26), 1902127 CrossRef.
- M. Kletter, J. Griffiths, C. Arundel, J. Dumville and On behalf of the VenUS 6 Collaborators, Trials, 2023, 24, 727 CrossRef PubMed.
- O. Krizanova, A. Penesova, A. Hokynkova, A. Pokorna, A. Samadian and P. Babula, Int. Wound J., 2023, 1–9 Search PubMed.
- E. Cereda, N. Veronese and R. Caccialanza, Curr. Opin. Clin. Nutr. Metab. Care, 2024, 27(1), 3–8 CrossRef PubMed.
- M. J. Carter, J. DaVanzo, R. Haught, M. Nusgart, D. Cartwright and C. E. Fife, J. Med. Econ., 2023, 26, 894–901 CrossRef PubMed.
- C. W. Curry, S. M. Sturgeon, B. J. O'Grady, A. Yates, A. Kjar, H. Paige, L. S. Mowery, K. A. Katdare, R. Patel, K. Mlouk, M. R. Stiefbold, S. Vafaie-Partin, A. Kawabata, R. McKee, S. Moore-Lotridge, A. Hawkes, J. Kusunose, K. N. Gibson-Corley, J. Schmeckpeper, J. G. Schoenecker, C. F. Caskey and E. S. Lippmann, Biomaterials, 2023, 303, 122397 CrossRef CAS PubMed.
- C. K. Sen, Adv. Wound Care, 2023, 12, 657–670 CrossRef PubMed.
- L. Zhou, F. Liu, J. You, B. Zhou, W. Guo, W. Qu, X. Ren and G. Gao, Adv. Healthcare Mater., 2023, e2303460 CrossRef PubMed.
- B. Wang, J. Chen, C. Zhang, Q. Zhang, Z. Zhu, L. Qiu, J. Yan, Z. Li, X. Zhu, Y. Zhang and Y. Jiang, Int. Immunopharmacol., 2023, 125, 111164 CrossRef CAS PubMed.
- M. A. M. Ahmed, M. F. Ali, N. M. Mohamed, S. A. L. Bayoumi, A. M. Zahran and K. I. Elsayh, J. Ethnopharmacol., 2024, 319, 117174 CrossRef CAS PubMed.
- O. Ramirez, F. Pomareda, B. Olivares, Y.-L. Huang, G. Zavala, J. Carrasco-Rojas, S. Alvarez, C. Leiva-Sabadini, V. Hidalgo, P. Romo, M. Sanchez, A. Vargas, J. Martinez, S. Aguayo and C. M. A. P. Schuh, Phytomedicine, 2024, 122, 155108 CrossRef CAS PubMed.
- V. K. Pandey, G. Ajmal, S. N. Upadhyay and P. K. Mishra, Int. J. Pharm., 2020, 589, 119858 CrossRef CAS PubMed.
- N. Xu, S. Liu, Y. Zhang, Y. Chen, Y. Zuo, X. Tan, B. Liao, P. Li and J. Feng, Redox Rep., 2023, 28(1), 2246720 CrossRef PubMed.
- X. Chen, C. Chen, X. Tian, L. He, E. Zuo, P. Liu, Y. Xue, J. Yang, C. Chen and X. Lv, Talanta, 2024, 266, 125052 CrossRef CAS PubMed.
- Y.-J. Fu, Y.-F. Shi, L.-Y. Wang, Y.-F. Zhao, R.-K. Wang, K. Li, S.-T. Zhang, X.-J. Zha, W. Wang, X. Zhao and W. Yang, Adv. Sci., 2023, 10, 2206771 CrossRef CAS PubMed.
- P. Chandra, N. Sachan, N. Saraswat and N. Vyawahare, Curr. Mol. Pharmacol., 2024, 17(1), e280323215026 Search PubMed.
- M. Oishi, K. Tanaka, K. Ishihara, K. Iino, A. Ito and Y. Yokoyama, J. Matern.-Fetal Neonat. Med., 2023, 36(1), 2183757 CrossRef PubMed.
- S. Vujosevic, E. Chew, L. Labriola, S. Sivaprasad and E. Lamoureux, Ophthalmol. Sci., 2024, 4, 100378 CrossRef PubMed.
- S. Zhu, B. Zhao, M. Li, H. Wang, J. Zhu, Q. Li, H. Gao, Q. Feng and X. Cao, Bioact. Mater., 2023, 26, 306–320 CAS.
- Y. Zhao, C. Tian, Y. Liu, Z. Liu, J. Li, Z. Wang and X. Han, Biomaterials, 2023, 295, 122029 CrossRef CAS PubMed.
- A. G. Berger, E. Deiss-Yehiely, C. Vo, M. G. McCoy, S. Almofty, M. W. Feinberg and P. T. Hammond, Biomaterials, 2023, 300, 122188 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |