Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of Vaccinium berries (blueberries, cranberries and bilberries) on oxidative stress, inflammation, exercise performance, and recovery – a systematic review
Arnold
Prieto Martínez
a,
Michelle
Coutiño Diaz
a,
Lizette
Anaya Romero
a,
Ali
Ali Redha
 *bc,
Reza
Zare
*bc,
Reza
Zare
 de,
Sthefano
Ventura Hernandez
a,
Konstantinos
Prokopidis
de,
Sthefano
Ventura Hernandez
a,
Konstantinos
Prokopidis
 fg and
Tom
Clifford
fg and
Tom
Clifford
 h
h
aSchool of Medicine and Health Sciences, Tecnológico de Monterrey Campus Guadalajara, Jalisco, Mexico
bThe Department of Public Health and Sport Sciences, University of Exeter Medical School, Faculty of Health and Life Sciences, University of Exeter, Exeter, EX1 2LU, UK. E-mail: aa1249@exeter.ac.uk
cCentre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation (QAAFI), The University of Queensland, Brisbane, QLD 4072, Australia
dMeshkat Sports Complex, Karaj, Alborz Province, Iran
eArses Sports Complex, Karaj, Alborz Province, Iran
fDepartment of Musculoskeletal Biology, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, L7 8TX, UK
gSociety of meta-Research and Biomedical Innovation, London, UK
hSchool of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, LE11 3TU, UK
First published on 2nd January 2024
Abstract
Exercise-induced muscle damage is common in athletes and recreational exercisers and can lead to muscle soreness, weakness, and impaired muscle function. The precise mechanisms are unclear but oxidative stress and inflammation are thought to play a role. (Poly)phenols are substances abundant in Vaccinium berries that have been suggested to possess antioxidant and anti-inflammatory effects that could help improve exercise performance and/or recovery from exercise. The objective of this systematic review was to evaluate the benefits of Vaccinium berry supplementation on exercise performance and recovery, as well as on exercise-induced oxidative and inflammatory biomarkers in healthy individuals. A comprehensive search was conducted in PubMed, ProQuest Medline, Web of Science, Cochrane Library, and Scopus. Studies were included if the participants were healthy individuals who were supplemented with any Vaccinium berry or Vaccinium berry-based products in comparison to a control group. Of the 13 articles included in this review, no significant differences in the exercise performance were found and only one study reported benefits for markers of recovery. Interleukins and c-reactive protein were the most frequently reported biomarkers, but there was limited evidence that Vaccinium berry supplementation impacted them post-exercise. Most studies were of high quality and showed a low risk of bias. Vaccinium berry supplementation is not effective in modulating markers of exercise-induced inflammation and oxidative distress in healthy individuals; nevertheless, more studies are required to evaluate their effects on exercise performance and recovery in this population.
1. Introduction
(Poly)phenols are natural compounds that have a variety of potentially beneficial health effects, including antioxidant and anti-inflammatory activities. They are subdivided into four main classes: flavonoids, phenolic acids, lignans, and stilbenes.1 (Poly)phenol-rich foods tend to have vibrant bright colours, such as yellow, blue or red, and have a slight acidity, as well as a bitter and sour taste. Fruits such as grapes, apples, pears, cherries, and berries, vegetables such as broccoli, carrots, spinach, and beetroot, and grains such as corn, wheat, oats, and rice all contain high quantities of (poly)phenols. Nevertheless, a variety of other foods like red wine, tea, coffee, chocolate, dry legumes, herbs, spices, stems, and flowers also contain (poly)phenols.2–4 Various mechanisms have been proposed to explain the antioxidant effects of (poly)phenols: (i) scavenging reactive oxygen and nitrogen species (RONS) production by reducing the catalytic activity of enzymes that participate in the generation of free radicals such as xanthine oxidase;5 (ii) upregulation of antioxidant defences by activating transcription factors such as nuclear factor erythroid 2-related factor 2 (Nrf2);6 and (iii) chelation of metal ions and free radicals, preventing their access into cells.7 While their anti-inflammatory effects are related to their antioxidant capacity, (poly)phenols can also downregulate the activity of cyclooxygenase (COX), lipoxygenase (LOX), and nitric oxide synthase (NOS), as well as inhibit tyrosine protein kinase and inflammasome NLRP3 activation, all of which may contribute to the symptoms of exercise-induced muscle damage (EIMD).8,9EIMD is characterised by inflammation, muscle pain (soreness), muscle weakness, and/or impaired muscle function that develops in hours and days after strenuous physical activity.9 EIMD is especially common in athletes and recreational exercisers after engaging in new or unfamiliar exercise, for example, when learning new techniques or when the intensity or volume of exercise is increased. Several mechanisms of EIMD have been proposed, and inflammation and oxidative stress are thought to play a crucial role in its development. Exercise increases the concentration of key immune cell markers, including several cytokines, white blood cells, and acute phase proteins such as c-reactive protein, in blood and skeletal muscle.10,11 In addition, several markers of oxidative damage, such as the byproducts of RONS-related damage (e.g., protein carbonyls), and/or markers of redox balance (reduced glutathione/oxidised glutathione), are altered post-exercise.12,13 Collectively, exercise-induced inflammation and/or oxidative damage is thought to cause secondary damage to muscle and other tissues in the days after strenuous exercise, and this contributes to the muscle pain and/or decrements in muscle function typically observed.14–18
(Poly)phenols can also influence endothelial function by increasing the skeletal muscle blood flow.19,20 This effect is believed to be caused by an increase in nitric oxide (NO) bioavailability, as (poly)phenols inhibit nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, an enzyme that reduces NO bioavailability.19,20 Increased blood flow may improve exercise performance by increasing the supply of nutrients and oxygen to skeletal muscle, as well as by accelerating the clearance of fatiguing metabolites.19,20
Berries are among the foods with the highest concentration of (poly)phenols.21 As such, Vaccinium berries may also have the potential to increase physical performance and recovery in athletes, by reducing oxidative and inflammatory-related damage to cells that contribute to EIMD. Vaccinium berries, such as blueberries, have been shown to possess comparable antioxidant and anti-inflammatory properties.22–24 They are easily available to the public and have affordable pricing in comparison to other berries, which makes them attractive to consumers. There are other (poly)phenol-rich berries such as chokeberries (Aronia melanocarpa) that have been previously studied as sports supplements,25 yet they might not be accessible and affordable to most consumers. Elderberries (Sambucus nigra) are also known as (poly)phenol-rich berries,26 yet they have not been studied for any potential sports applications.
Around 60% of the (poly)phenols in fresh blueberries are anthocyanins, with amounts ranging from 93 to 280 mg per 100 g.27,28 Other (poly)phenols in blueberries include proanthocyanidins, flavonols, and chlorogenic acid.29,30In vitro findings have shown that blueberry supplementation may suppress fatigue and oxidative stress-induced muscle damage, as well as oxidative distress in several tissues.31 They may also accelerate the recovery of muscle peak isometric strength and enhance exercise performance.32 Since blueberries are low in kilojoules and high in fibre, vitamins C, K, and E, they are also considered healthful foods.33 Fresh bilberries contain 300 to 698 mg per 100 g of anthocyanins, as well as flavonols, proanthocyanidins, and phenolic acids; they are low in kilojoules and high in fibre and vitamin C.34 It has been reported that bilberry supplementation might help inhibit smooth muscle contraction and affect vasoactive properties that could increase aerobic exercise performance.35,36 Additionally, bilberries could also enhance recovery from exercise by inhibiting the expression of pro-inflammatory cells that may contribute to muscle damage.37–39 Cranberries are low in kilojoules and high in fibre, vitamin C, and a variety of minerals such as manganese, potassium, and magnesium.40 Raw cranberries are especially rich in anthocyanins and proanthocyanidins, the latter of which might be the main compound responsible for their anti-inflammatory and antioxidant properties.41 They contain 13.6 to 140 mg per 100 g of anthocyanins,42 as well as benzoic acid, flavonols, and ursolic acid.43 Consuming cranberry and cranberry-based products may improve endothelial function, reduce arterial stiffness, attenuate CRP levels, and decrease inflammatory and oxidative distress markers.44–50
The aforementioned research with blueberries, cranberries, and bilberries suggests that these Vaccinium berries have vasoactive, antioxidant and anti-inflammatory effects and, therefore, the potential to enhance exercise performance and attenuate EIMD. However, studies to date have reported conflicting findings, and there remains no consensus as to the effectiveness of consuming Vaccinium berries on exercise performance and recovery in humans. Therefore, in this systematic review, we evaluated studies examining the effects of Vaccinium berry supplementation on markers of inflammation, oxidative stress, exercise performance, and recovery in healthy individuals.
2. Methodology
This systematic review was pre-registered in the PROSPERO database (ID: CRD42023456233) and was performed according to the recommendations established by the Preferred Reporting Items for Systematic Reviews and meta-Analysis (PRISMA).512.1 Literature search
An extensive search in PubMed, ProQuest Medline, Web of Science, Cochrane Library, and Scopus was conducted on 15th August 2023 to find studies that assessed the effects of Vaccinium berry supplementation on exercise performance and recovery in healthy individuals. The search expression consisted of: (Vaccinium OR blueberry OR cranberry OR bilberry) AND (exercise OR sport OR athletes OR physically active) AND (antioxidant OR oxidative stress OR inflammatory OR anti-inflammatory OR muscle damage OR recovery OR exercise performance OR sports performance OR performance). Articles relevant to our investigation that were referenced in any of the included studies were also taken into consideration. No limitation was made on the publication date or time length of the studies. The search strategy and inclusion/exclusion criteria based on population, intervention, comparison, outcomes and study design (PICOS) are summarised in Table 1.| Databases | Search terms | PICOS | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|
| PubMed, ProQuest Medline, Web of Science, Cochrane, Scopus | (Vaccinium, blueberry, cranberry, or bilberry) AND (exercise, sport, or athletes) AND (antioxidant, “oxidative stress”, inflammatory*, anti-inflammatory*, “muscle damage”, recovery, “exercise performance”, “sports performance”, or performance) | Population | Healthy adults 18 to 65 years old | Unhealthy individuals; individuals below or above the established age range |
| Intervention | Vaccinium berry supplementation | Multiple ingredients used as one intervention | ||
| Comparison | Supplementation vs. no supplementation/placebo | |||
| Outcome | Changes in markers of oxidative stress, inflammatory status, muscle damage, exercise performance and recovery | Alterations in any of the markers before the intervention | ||
| Study design | Randomized, non-randomized, controlled, crossover, and quasi-experimental studies | Meta-analysis, systematic review, cross-sectional, case-control, case reports, animal, and in vitro research studies |
2.2 Study selection
Included studies were randomized and non-randomized controlled trials in humans that had a control group as a comparator to assess the beneficial effects of Vaccinium berry supplementation on markers of exercise performance and recovery. The excluded studies were observational, animal, and in vitro studies. Three reviewers independently assessed the titles and abstracts against the inclusion and exclusion criteria. The eligible full-text articles were retrieved. Full-text screening was completed independently by the three reviewers. Any disagreements were resolved through consulting with all the team members by establishing a consensus.2.3 Data extraction
The following data were retrieved from each study: type of intervention, target population characteristics, outcomes, and analysis of these outcomes. All data were summarised and described as qualitative and quantitative variables. A narrative synthesis was performed for the demographic characteristics of the participants such as age, sex, health, and exercise performance activity, the characteristics of the interventions such as dose, frequency, and intervention time of the supplementation with Vaccinium berries as well as the characteristics of the placebo, and the assessment tools used to determine the antioxidant, anti-inflammatory, and muscle damage markers, as well as recovery time, quantitative and qualitative exercise performance assessments.2.4 Risk of bias assessment
The scientific quality of the studies was assessed independently by three reviewers using the risk of bias 2 tool (RoB2) for randomised and crossover trials,52 and the ROBINS-I tool for non-randomised trials.53 The assessment of randomised trials was based on the following domains: randomisation process, assignment and adherence to intervention, missing data, measurement of outcome, and selection of the reported results. For crossover studies, the risk of bias arising from period and carryover effects was also considered. For non-randomised studies, the assessment also evaluated the bias due to confounding in addition to the domains stated for randomised studies. The studies were then categorised as having a low risk of bias, some concerns, or a high risk of bias. If assessment outcomes were conflicting, reviewers discussed and came to a consensus. Visualisation of the risk of bias assessments was performed using the robvis online tool.543. Results
This systematic review included 13 studies (11 randomised and 2 non-randomised trials). The studies included 9 to 59 participants who were considered healthy and relatively young (18–50 years). Four studies evaluated men only,55–58 one study evaluated women only,59 four studies evaluated both,60–63 and four did not specify gender.64–67 A total of 287 participants were studied, 58 of which were women and 132 were men; the gender of 98 participants was not disclosed. The studies analysed the effects of supplementation after various types of trials such as running, cycling, jumping, rowing, and using exercise machines. No significant differences in exercise performance were found in any of the studies, and only one study found benefits for strength recovery after exercise.59 The changes in the relevant biomarkers with each berry are discussed below.3.1 Study selection
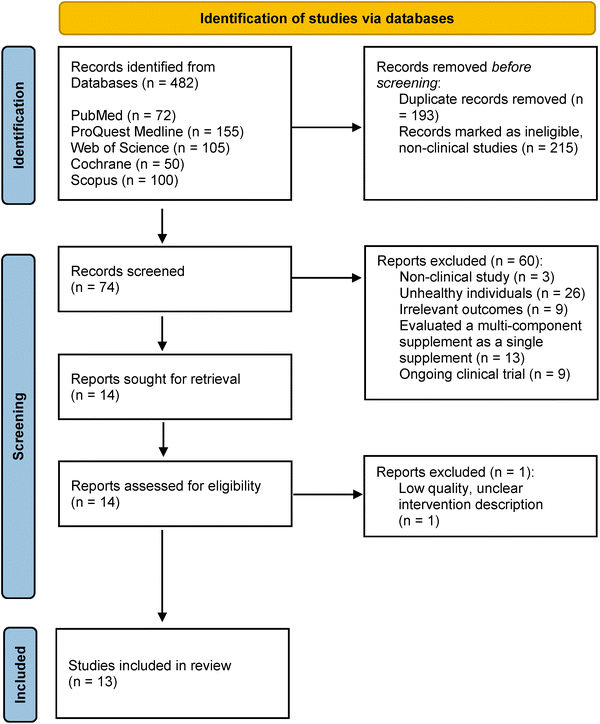
The review identified 482 records by searching five databases. After removing duplicates (n = 193), 289 articles remained, from which 215 non-clinical trial articles were identified and removed before the screening. 74 articles were then screened by title, abstract, and keywords by all reviewers independently. A total of 61 articles were excluded for different reasons: the study did not evaluate supplementation in humans (n = 3); the study targeted sick individuals (n = 26); the study did not evaluate markers of inflammation, oxidative stress, exercise performance, or EIMD (n = 9); the study evaluated multiple ingredients as a single supplementation (n = 13), ongoing clinical trial (n = 9), and unclear intervention description (n = 1). The excluded study due to unclear intervention description evaluated the effects of blueberry supplementation on exercise performance, total antioxidant status, inflammatory markers and other biomarkers; however, the study did not provide sufficient details regarding the type of supplementation, dosage, total duration of supplementation, nor the (poly)phenol content in its intervention.68 A total of 13 articles were assessed for eligibility: 10 articles reviewed the effects of blueberry supplementation; two studies evaluated the effects of cranberry supplementation, and one study analysed the effects of bilberry supplementation. The details of the study selection process are shown in Fig. 1.3.2 Characteristics of the included studies
| Berry | Study | Study design | Participants | Supplemented group | Placebo/control group | Duration | Training load | Key findings |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: 5-OHMU, 5-hydroxymethyl-2′-deoxyuridine; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; ACNs, anthocyanins; ARA-CYP, arachidonic acid cytochrome p450; CI, confidence interval; CK, creatine kinase; CMVJ, countermovement vertical jump; CRP, c reactive protein; DHA-LOX, docosahexaenoic acid and lipoxygenase; diHETrEs, dihydroxy-eicosatetraenoic acids; DJ, drop jump; DNA DI, DNA damage index; DOMS, delayed onset muscle soreness; EPA, eicosapentaenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE/HETEs, hydroxy-eicosatetraenoic acids; hs-CRP, high sensitivity CRP; IL, interleukin; MnSOD, manganese superoxide dismutase; N/S, not specified; NK, natural killer; OR, odds ratio; PA, proanthocyanidins; ROOH, lipid hydroperoxides; RSI, reactive strength index; SPM, specialized pro-resolving lipid mediators; TAC, total antioxidant capacity; TAD, total anthocyanin dose; TPAD, total proanthocyanidin dose; TT, treadmill time; and VO2 max, maximum rate of oxygen consumption.a The concentration of TAD/TPAD was estimated using the anthocyanin/proanthocyanidin content reported in other studies.56,57 | ||||||||
| Blueberry | McLeay, Y. et al., 201159 | Randomized, crossover trial | n = 10 physically active participants | n = 10 | n = 10 | 3 days | 300 maximal eccentric repetitions using the quadriceps | Faster rate of recovery in the blueberry group (p = 0.047). Faster rate of decrease in oxidative stress was observed in the blueberry group (p > 0.05) |
| Female = 10 | Beverage with 200 g of blueberries (96.6 g of ACNs per 100 ml) three times a day for 1 day and once a day for 2 days | Placebo beverage | ||||||
| Age = 21–23 years | TAD = 580 mg on the first day, 193 mg for the next days, and 966 mg for full intervention | |||||||
| Blueberry | Brandenburg et al., 202161 | Randomized double-blind, placebo-controlled, crossover trial | n = 11 physically active participants | n = 11 | n = 11 | 4 days | 30-minute run in normobaric hypoxia | Blood lactate was significantly lower in the blueberry group (p = 0.02) |
| Male = 4 | 24 g of blueberry powder (336 mg of ACNs per serving) 3 times a day | Placebo powder | ||||||
| Female = 7 | TAD = 1008 mg per day, 4032 mg for full intervention | |||||||
| Age = 20–34 years | ||||||||
| Blueberry | Nieman, D. et al., 202060 | Randomized double-blind placebo-controlled, four-group parallel trial | n = 59 trained cyclists | n = 30 | n = 29 | 2 weeks | 75-km cycling time trial | Banana and blueberry ingestion were independently associated with significant post-exercise reductions in 10 ARA-CYP pro-inflammatory oxylipins: six HETEs and four diHETrEs (p = 0.003) |
| Male = 40 | 26 g of blueberry powder (345 mg of ACNs per serving) once a day TAD = 345 mg per day, 4830 mg for full intervention | Carbohydrate and fiber-matched placebo powder | ||||||
| Female = 19 | ||||||||
| Age = 37–41 years | ||||||||
| Blueberry | McAnulty, S. et al., 200465 | Randomized double-blind, placebo-controlled, crossover trial | n = 9 physically active participants | n = 9 | n = 9 | 1 week | 70% VO2 max run in a hyperthermic environment until a core temperature of 39.5 °C was reached | Significantly lower ROOH concentration in the blueberry group (p = 0.005) |
| Male = N/S | Session I, 150 g per d of blueberries. Session II, 1250 mg per d vitamin C supplement. TAD = 250 mg per day, and 1750 mg for full intervention.a | Blueberry-flavored shake | ||||||
| Female = N/S | ||||||||
| Age = 18–43 years. | ||||||||
| Blueberry | Brandenburg, J., & Giles, L. 201967 | Randomized, double-blind, placebo-controlled, crossover trial | n = 14 physically active participants | n = 14 | n = 14 | 4 or 2 days | CMVJ, DJ and a 8-km run TT on a non-motorized treadmill | There was an interaction effect observed for blood lactate, with lower post-TT concentrations in the 4-day supplementation group (p = 0.027). A decline in RSI was lower following the 4-day supplementation group. |
| Male = N/S | 24 g of blueberry powder (336 mg of ACNs per serving) 3 times a day. TAD = 1008 mg per day, 4032 mg for 4 days, 2016 mg for 2 daysa | Placebo powder | ||||||
| Female = N/S | ||||||||
| Age = 21–41 years | ||||||||
| Blueberry | McAnulty, L. et al. 201166 | Randomized, double-blind, parallel-group trial | n = 25 physically active participants | n = 13 | n = 12 | 6 weeks | 2.5-hour treadmill run | The blueberry group had significantly lower levels of F2-isoprostanes (p = 0.016) and 5-OHMU (p = 0.028). IL-10 (p = 0.045) and NK cell counts (p = 0.003) were significantly higher. |
| Male = N/S. | 250 g of fresh blueberries per day and an acute dose of 375 g of blueberries within 1 hour before exercise testing. TAD = 491 mg per day, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 378 mg for full intervention.a 378 mg for full intervention.a |
Control group | ||||||
| Female = N/S | ||||||||
| Age = 18–49 years | ||||||||
| Blueberry | Pilolla, K. et al., 202356 | Non-randomized, quasi-experiment, free-living trial | n = 11 physically active participants | n = 11 | n = 11 | 2 weeks | 65% of VO2max-cycle for 40 minutes | Lactate was lower in the blueberry group (p = 0.0053) |
| Male = 11 | 12.5 g of blueberry powder twice a day. TAD = 350 mg per day, 4900 mg for full intervention. | "C diet” | ||||||
| Age = 18–34 years | ||||||||
| Blueberry | Nieman, D. et al., 202364 | Randomized, double-blinded, placebo-controlled, parallel-group trial | n = 49 healthy untrained participants. | n = 23 | n = 26 | 2 weeks | Muscle function tests, 60-yard shuttle run test, and anaerobic power through the 30-s Wingate cycling test. Then a 90-min eccentric exercise. | Linoleic acid oxylipins were lower in the blueberry group (p = 0.051). Nine plasma HDoHEs were significantly higher (p = 0.008). The composite variables of 14-HDoHE, 17-HDoHE, and the EPA-derived 18-HEPE were significantly higher in the blueberry group (p = 0.014). A positive relationship between post-exercise DHA-LOX HDoHEs and SPM intermediates with urine blueberry gut-derived phenolics was found (p = 0.023 and p = 0.015, respectively). |
| Male = N/S | 25 g of freeze-dried blueberries (280 mg of ACNs per serving) per day | 25 g per day of a placebo powder | ||||||
| Female = N/S | TAD = 280 mg per day, 3920 mg for full intervention | |||||||
| Age = 18–50 years | ||||||||
| Blueberry | Nyberg, S. et al., 201363 | Randomized, group controlled, crossover trial | n = 32 healthy untrained individuals | n = 32 | n = 32 | 4 weeks | 5-km run at the fastest possible speed at the beginning and end, and a 5-km run per day, 5 times a week | No significant difference in CK, hs-CRP or troponin-T levels between groups |
| Male = 15 | 150 g of frozen blueberries on each day of running. TAD = 250 mg per day, 7000 mg for full intervention.a | Eating habits unchanged | ||||||
| Female = 17 | ||||||||
| Age = 21–48 years | ||||||||
| Blueberry | Bloedon, T. et al., 201555 | Non-randomized, group controlled, crossover trial | n = 10 healthy untrained individuals | n = 10 | n = 10 | 8 weeks | 1-hour treadmill exercise at 70% of their VO2 max | Plasma MnSOD had a significant interaction between pre- and post-interventions over time (p ≤ 0.014) in the blueberry group; it also showed a decreasing tendency 30 min after exercise (p = 0.072). |
| Male = 10 | 300 g per d of blueberries. TAD = 600 mg per day, 33600 mg for full intervention.a | Control group | ||||||
| Age = 21–30 years | ||||||||
| Cranberry | Skarpańska Stejnborn et al., 201757 | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 16 trained rowers | n = 9 | n = 7 | 6 weeks | 2000-m test on a rowing ergometer at the beginning and end | Significantly higher resting, post-exercise, and post-recovery levels of TAC (p < 0.001) in the cranberry group |
| Male = 16 | 360 mg of cranberry extract (36 mg of PA), per day. TPAD = 36 mg per day, 1512 mg for full intervention. | Dyed gelatin placebo capsules containing “Poznańska” flour | ||||||
| Age = 20–22 years | ||||||||
| Cranberry | Meihua et al., 201858 | Randomized, placebo-controlled, parallel-group trial | n = 20 physically active participants | n = 10 | n = 10 | 2 weeks | Bruce test | Significant increase of DNA DI (p < 0.01) and 8-OHdG (p < 0.01) in the control group in comparison with the supplementation group. |
| Male = 20 | Cranberry soft chews, one daily with a meal | One dextrose capsule a day | ||||||
| 500 mg dried TPAD = 50 mg per day, 700 mg for full intervention.a | ||||||||
| Age = 19–22 years | ||||||||
| Bilberry | Lynn, A. et al., 201862 | Randomized, single-blind, placebo-controlled, parallel-group trial | n = 21 trained runners.Male = 16Female = 5Age = 18–42 years | n = 11 200 mL of bilberry juice twice a day (≈160 mg/d of ACNs). TAD = 160 mg/day, 1280 for full intervention. | n = 10 200- mL of fruit-flavored maltodextrin powder. | 8 days | Half-marathon | Possibly harmful effect (OR 11.6%, 90% CI ± 14.7%) of bilberry juice on DOMS in comparison with placebo. The possibly harmful effect on post-race DOMS was mirrored by a likely harmful effect (OR 0.56, 90% CI ± 0.72) on CRP at 24 h post-race and a possibly harmful effect (OR 0.12, 90% CI ± 0.69) at 48 h post-race. |
3.3 General findings
3.3.1.1 Effects on oxidative stress and inflammation biomarkers. The most commonly reported biomarkers were interleukins, especially IL-6, which was reported in five studies. There were no statistically significant differences between the supplementation and control group in any subtype of interleukins.55,59,61,65 CRP was measured in three studies, none of which reported significant differences between the two groups.61,63,67 F2-isoprostanes were reported in three studies, without significant differences between groups.56,65,66 The ferric-reducing antioxidant potential (FRAP) was measured in three studies,59,65,66 one of which reported that the assessments of plasma antioxidant capacity between the pre-treatment and the 60-hour recovery time point revealed a significant treatment-in-time interaction for the supplementation group.59 Manganese superoxide dismutase (Mn-SOD), TNF-α, and DNA damage were analysed in one study, where a significant interaction between pre- and post-intervention over time was reported for Mn-SOD, but no significant changes were reported for the others.55 Gut phenolic data showed significant group differences in blueberry-related metabolite perturbation between the two groups, as well as significant time per treatment effect in 45 out of 165 gut-derived phenolics, with 24 being associated with blueberry intake.60 One study reported that 5-hydroxymethyl-2′-deoxyuridine (5-OHMU), a urinary marker of oxidative damage, had a significant decrease post-exercise in the supplemented group, while 8-OHDG did not significantly change.66
3.3.1.2 Effects on muscle damage, exercise performance and recovery. All three studies that assessed CK levels reported a large rise after exercise, however, no significant between-group differences were found.59,63,64 Serum troponin-T increased after a running race but there were no significant differences between groups.63 No significant group differences in creatinine, free/total carnitine, and myoglobin were found.56,64 Lastly, only one study that assessed exercise performance as isometric, concentric and eccentric torque found a significant reduction at post-exercise and a significantly faster rate of recovery in the blueberry supplementation group.58 None of the other studies found a significant difference between groups.55,56,59–61,63–67
3.3.1.3 Effects on other outcomes. Other outcomes include lactate, oxylipins, and natural killer (NK) cells. One study reported that lactate levels were significantly elevated at post-exercise, and higher in the placebo group.61 In another study, plasma lactate levels were significantly lower in the supplemented group.64 Lastly, one study reported that lactate levels had a significant interaction between conditions over time at 20, 30, and 40 min post-exercise in the supplemented group.56 There was a significant increase in plasma levels of arachidonic acid (ARA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) in two trials, as well as significant increases in 64 out of 67 identified oxylipins, 15 of which had significant time-for-treatment effects.56,60 NK cells exhibited significant group, time, and interaction effects in the supplementation group and the mitogen-induced lymphocyte proliferative response decreased post-exercise for both groups in a similar pattern.63
3.4 Risk of bias assessment
A total of 11 studies were randomised controlled trials while two were non-randomised trials.55–67 All but one of the randomised controlled trials was associated with a low risk of bias (Fig. 2 and 3). This one study raised some concerns regarding bias in the randomisation process and deviations from intended interventions due to unclear information; however, the baseline difference between intervention groups did not suggest a problem and the deviations were unlikely to affect the outcome.58 Other than the lack of randomisation, both non-randomised studies were deemed to have a low risk of bias.55,56 Finally, four out of five crossover studies had a low risk of bias.59,61,65,67 One study raised some concerns regarding deviations from intended interventions and measurement of outcome; nevertheless, these concerns were deemed not likely to affect the final outcome.63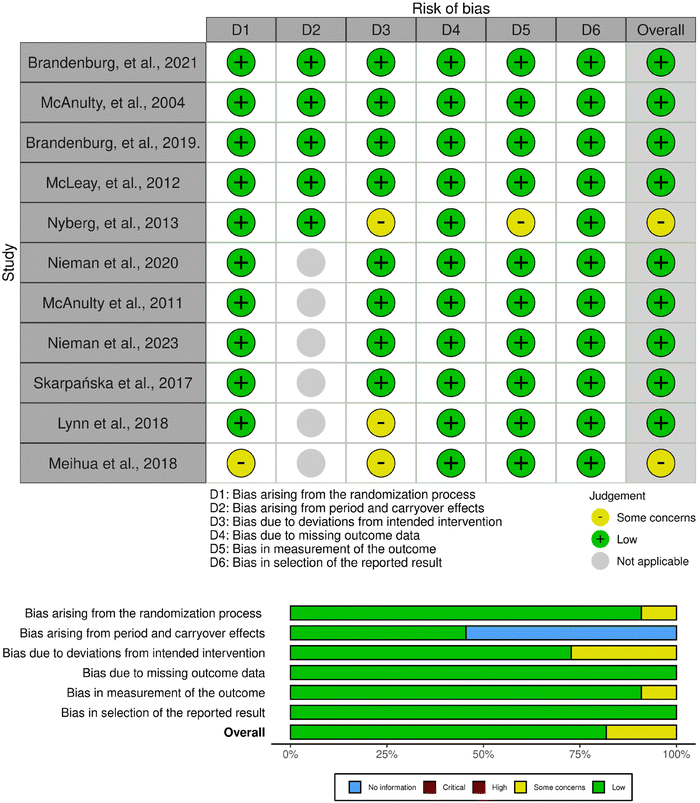 | ||
| Fig. 2 Assessment of bias of the randomized studies according to the RoB 2 tool: the traffic light plot and summary plot. | ||
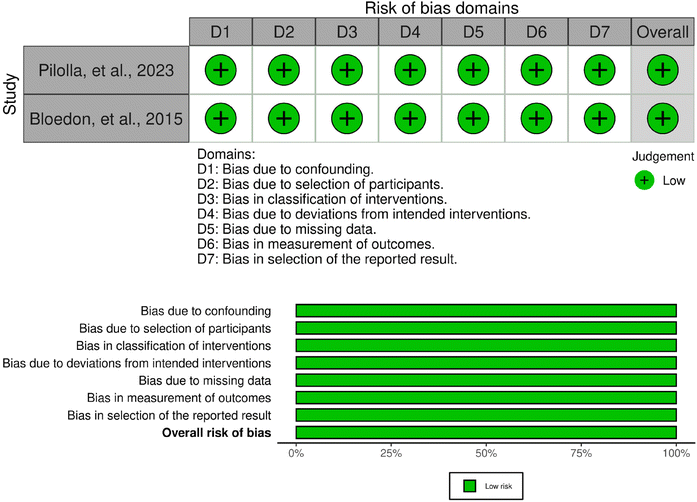 | ||
| Fig. 3 Assessment of bias of the non-randomised studies according to the ROBINS-I tool: the traffic light plot and summary plot. | ||
4. Discussion
This systematic review evaluated the effect of Vaccinium berry supplementation on inflammatory, muscle damage, and oxidative stress biomarkers. We found in most studies Vaccinium berry supplementation did not significantly reduce the concentration of these biomarkers. Only one study reported some benefit for strength recovery, and no studies reported significant benefits for exercise performance.4.1 Blueberries
Interleukins were the most frequently measured biomarkers of inflammation in studies evaluating the effects of blueberry intake. However, there were no statistically significant differences between groups, suggesting that the effects of blueberries at that dose, frequency and time of supplementation, were not effective at modulating cytokines. IL-6 was the most frequently measured cytokine; it has been proposed that modulating this cytokine, and other immune cell markers, could help facilitate the recovery of damaged muscle.69 It has also been reported that the plasma concentration of IL-6 increases during physical activity and peaks post-exercise.70 The rise in IL-6 may occur in response to muscle damage, however, muscle damage is not required to increase the plasma concentrations of IL-6 during exercise.71 The main source of IL-6 in response to exercise is skeletal muscle.69 Additionally, it is believed that the muscle glycogen content is partly responsible for the expression of IL-6 mRNA, so this biomarker is probably more indicative of changes in energy metabolism than muscle damage.72 Thus, IL-6 is probably not a reliable biomarker to assess post-exercise-induced inflammation, which may partly explain why supplementation had no significant effect on IL-6 levels in most of the studies.Another biomarker that was frequently measured and that increased post-exercise was F2-isopropanes, but no significant differences were reported compared to a placebo.56,65,66 These compounds are produced from arachidonic fatty acids esterified in phospholipids and are released as free isoprostanes by the action of phospholipase A2. F2-isoprostanes cause muscle vasoconstriction, and platelet aggregation, and are considered reasonably good markers of oxidative damage.73 Since they come from an extravascular origin, short supplementation periods might not be sufficient to modify these compounds in the extravascular compartment.74 While the exact consequences of increased F2-isoprostanes are unknown, higher levels are reported after cardiac arrest, in diabetics, and smokers.66
The most common muscle damage marker measured in studies with blueberries was CK, an enzyme normally found in skeletal muscle cells but leaks into the circulation after muscle-damaging exercise.56,59,63 CK levels typically increase post-exercise, which was a common finding in all included studies.56,59,63 There were no significant differences in CK levels between groups, however, the changes in this biomarker vary greatly between individuals and it has been suggested that this may in part be due to single nucleotide polymorphisms, which may not only limit the efficacy of supplementation, but also the interpretation of post-exercise changes.56
Regarding other biomarkers, levels of ARA, DHA and EPA increased significantly after exercise. A correlation between blueberry supplementation and increased intermediate oxylipins generated by DHA and EPA (which have anti-inflammatory effects) was found.56 The underlying mechanisms are not completely understood, however, it has been suggested that increases in intestinal-derived phenolics and changes in the microbiota caused by the ingestion of (poly)phenols can influence the production of oxylipins through enzymatic stimulation.75 Likewise, there is a theory that lipid mediators generated from DHA and EPA play a role in muscle regeneration by regulating the inflammatory response.74,76
The training status did not appear to affect the effectiveness of blueberry supplementation across the included studies. Three studies with untrained individuals were included, however, most of the investigated biomarkers differed from those reported in other studies, for example, they analysed insulin, triglycerides, HDL-c, and MnSOD; DHA, ARA, EPA, and IL-6 levels were the only variables that were shared between these two groups, showing no significant differences when compared to physically active people.55,56,63
4.2 Cranberries
A study in rowers analysed the effect of supplementation on various markers of inflammation and oxidative distress. While there were no statistically significant differences in most of the biomarkers, the lack of post-exercise increases in the supplemented group suggests a possible benefit of cranberry extract components, such as the stimulation of antioxidant capacity and reduction of iron availability via chelation.57 The study in runners evaluated the effect of supplementation on oxidative stress and muscle damage markers.58 While there were significant differences in oxidative stress markers, the overall description of the intervention and the presentation of the results raised some bias concerns, for example, CK, BUN, and MDA levels of two participants were excluded from the results without explanation.4.3 Bilberries
A study in runners analysed the effect of supplementation on DOMS, CK and CRP but did not measure any markers of muscle strength. No benefit was found in exercise performance, recovery or any of the markers. It is also worth noting that the two-day follow-up after the race might not have been enough to fully capture the effects of BJ supplementation on both CRP and CK levels, since both markers had not returned to baseline levels for any of the participants. In addition, there were no diet restrictions in this study, except to avoid NSAIDs and antioxidant vitamins, and this could have influenced the results. Finally, the number of participants in the study was likely insufficient to detect small between-group differences in performance and recovery.624.4 Limitations and future perspectives
One limitation of this review is the heterogeneity of the studies; indeed, the studies differed considerably, not only in the type of supplementation, administered dose, frequency, and duration of administration, but also in terms of the included participants, (i.e., sedentary individuals in one study, Olympic athletes in the other), as well as the type of physical activity performed. It is perhaps therefore expected that there would be large variation in results between the different studies. While most of the trials reported that oxidative stress, inflammatory status, and muscle damage increased after strenuous physical activity, supplementation did not significantly affect most of these markers.59,61,65,67 It is also worth noting that, although a small number of studies were included, six out of the thirteen articles were written by the same three authors.56,59–61,65,67We propose several methods to improve research in this area:
(i) studies should ensure that they are sufficiently powered to detect statistically significant group differences by performing a priori sample size calculations;
(ii) studies should include measures of exercise performance and recovery, alongside biological markers that interrogate the underpinning mechanisms of action;
(iii) studies should measure the bioavailability of (poly)phenols from consuming these berries. Less than 2% of anthocyanins maintain their original structure after ingestion.77 Anthocyanins exist in different chemical structures depending on their pH.77 Additionally, they undergo sulfation, methylation, and enzymatic and microbiota catabolism, which alters their structure producing anthocyanin glucuronides, phenolic acids, and aldehydes.77 It would be reasonable to assume that any bioactive effects could be related to these metabolites, and not the intact compounds. Nonetheless, only a few studies have evaluated bioavailability alongside their primary outcomes.49,77–82 Among all of the polyphenols, gallic acid has the highest absorption. Gallic acid, quercetin glucoside, tea catechins, and free hydroxycinnamic acids, which are absorbed in the small intestine and the stomach, reached plasma max concentration (Cmax) at 1.5 h, whereas rutin, hesperidin, naringin, which are absorbed after the release of the aglycones by the microflora, reached Cmax at 5.5 h. Likewise, the most well-absorbed flavonoids are isoflavones with Cmax values of 2 μmol L−1 after a 50 mg intake.83 When considering colonic metabolites, the bioavailability of anthocyanins and proanthocyanidins may range from 12% to 18%.84
(iv) Among the Vaccinium berries included in our systematic review, bilberries are the richest in anthocyanins since they contain 300 to 698 mg per 100 g,34 while blueberries contain 93 to 280 mg per 100 g,27,28 and cranberries contain 13.6 to 140 mg per 100 g;40 therefore, to consume a minimum dose of 80 mg, based on the midrange concentration of anthocyanins in each berry, a minimum of 31 g per d of blueberries, 65 g per d of cranberries, and 16 g per d of bilberries would be required.85 This recommendation is based on a study in people with risk factors for cardiovascular disease and dyslipidemic subjects, where a dose of ≥80 mg per d of anthocyanins was effective in lowering inflammation markers such as TNF-a;77 regarding cranberries, this amount has a midrange concentration of approximately 76 mg per d of proanthocyanidins,43,50 more than 1.5 times the amount used in a study in which reduced levels of cholesterol and LDL were observed after supplementation.77 However, we acknowledge that due to the limited data available on the most efficacious dose of anthocyanins or other (poly)phenols in Vaccinium berries, these amounts are only reasonable estimations based on the limited data available.
(v) A minimum wash-out period of 1 week should be used in crossover studies to guarantee that the interventions do not interfere with each other.
(vi) The supplementation period should continue until all relevant plasma biomarkers or exercise performance assessments are expected to return to their baseline levels.
(vii) To gain better mechanistic insights, participants are required to avoid foods/herbs rich in anthocyanins,83 and other (poly)phenols with similar effects, and avoid the use of medications that are known to affect oxidative stress and immune function.
(viii) No specific or individual biomarkers can validly capture inflammation or oxidative stress, and therefore a wide range of markers should be measured to assess possible effects on these pathways.84,86 At a minimum, we encourage that the following biomarkers are measured to characterise: (a) inflammation: CRP, TNF-α, interleukin-1b, interleukin-4, interleukin-10 and interleukin-6, as these are key markers from which the Dietary Inflammatory Index was developed, based on their association with food intake; (b) oxidative stress: antioxidants (e.g., glutathione, glutathione peroxidase, catalase, and superoxide dismutase), oxidation products (e.g., protein carbonyls, 8-hydroxyguanosine (8-OHdG), and isoprostanes), and redox balance (e.g., glutathione to oxidised glutathione ratio) (c) muscle damage: CK, LDH, and myoglobin. The wide range of biomarkers employed in the individual studies of this review meant that we were unable to perform a meta-analysis in this study.
(ix) We recommend further investigation regarding the effects of bilberry supplementation on exercise performance. Out of the three Vaccinium berries, bilberries not only have the highest anthocyanin content,34 but their effects on exercise performance/recovery have only been examined in one human study.
5. Conclusion
Based on the findings from the 13 studies in this review, the results suggest that Vaccinium berry supplementation has neither beneficial nor harmful effects on exercise performance or recovery, while the effects on the markers of oxidative stress and inflammation were inconsistent. While most studies had a low risk of bias, many had low sample sizes and did not evaluate the most appropriate outcomes; for example, few studies measured changes in muscle strength following exercise, the most valid marker of EIMD. Future studies are needed to examine whether Vaccinium berries can enhance exercise performance and recovery.Author contributions
Conceptualisation: A. A. R. and R. Z. Methodology: A. A. R., A. P. M., M. A. C. D., and L. A. R. Investigation, data curation and formal analysis: A. P. M., M. A. C. D., and L. A. R. Writing – original draft: A. P. M., M. A. C. D., and L. A. R. Writing – review & editing: A. A. R., R. Z., S. V. H., K. P., and T. C. Project administration: A. A. R. and A. P. M. Supervision: T. C. and A. A. R.Conflicts of interest
All authors declare that they have no conflict of interest relevant to the content of this review.Acknowledgements
The authors would like to thank the Cambridge University ReachSci Society for their support and assistance. This project was an outcome of the Mini-PhD Global Programme 2023: Food Science & Sports Nutrition.References
- T. Hussain, B. Tan, Y. Yin, F. Blachier, M. C. Tossou and N. Rahu, Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?, Oxid. Med. Cell. Longevity, 2016, 2016, 7432797 CrossRef PubMed.
- J. P. Spencer, M. M. Abd El Mohsen, A. M. Minihane and J. C. Mathers, Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research, Br. J. Nutr., 2008, 99, 12–22 CrossRef CAS PubMed.
- G. R. Beecher, Overview of dietary flavonoids: nomenclature, occurrence and intake, J. Nutr., 2003, 133, 3248S–3254S CrossRef CAS PubMed.
- A. Ali Redha, S. Anusha Siddiqui, R. Zare, D. Spadaccini, S. Guazzotti, X. Feng, N. A. Bahmid, Y. S. Wu, F. Z. Ozeer and R. E. Aluko, Blackcurrants:A Nutrient-Rich Source for the Development of Functional Foods for Improved Athletic Performance, Food Rev. Int., 2022, 1–23, DOI:10.1080/87559129.2022.2162076.
- S. Kumar, U. K. Sharma, A. K. Sharma and A. K. Pandey, Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect, Cell. Mol. Biol., 2012, 58, 174–181 CAS.
- L. F. Cardozo, L. M. Pedruzzi, P. Stenvinkel, M. B. Stockler-Pinto, J. B. Daleprane, M. Leite Jr. and D. Mafra, Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2, Biochimie, 2013, 95, 1525–1533 CrossRef CAS PubMed.
- K. E. Heim, A. R. Tagliaferro and D. J. Bobilya, Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships, J. Nutr. Biochem., 2002, 13, 572–584 CrossRef CAS PubMed.
- B. S. Cheon, Y. H. Kim, K. S. Son, H. W. Chang, S. S. Kang and H. P. Kim, Effects of prenylated flavonoids and biflavonoids on lipopolysaccharide-induced nitric oxide production from the mouse macrophage cell line RAW 264.7, Planta Med., 2000, 66, 596–600 CrossRef CAS PubMed.
- X. Zhang, G. Wang, E. C. Gurley and H. Zhou, Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages, PLoS One, 2014, 9, e107072 CrossRef PubMed.
- J. M. Peake, O. Neubauer, P. A. Della Gatta and K. Nosaka, Muscle damage and inflammation during recovery from exercise, J. Appl. Physiol., 2017, 122, 559–570 CrossRef CAS PubMed.
- G. Paulsen, U. R. Mikkelsen, T. Raastad and J. M. Peake, Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise?, Exerc. Immunol. Rev., 2012, 18, 42–97 Search PubMed.
- D. J. Owens, C. Twist, J. N. Cobley, G. Howatson and G. L. Close, Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions?, Eur. J. Sport Sci., 2019, 19, 71–85 CrossRef PubMed.
- T. Kawamura and I. Muraoka, Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint, Antioxidants, 2018, 7, 119 CrossRef PubMed.
- L. L. Smith, Acute inflammation: the underlying mechanism in delayed onset muscle soreness?, Med. Sci. Sports Exercise, 1991, 23, 542–551 CAS.
- J. M. P. F. X. Pizza, J. H. Baas and T. J. Koh, Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice, J. Appl. Physiol., 2002, 562, 899–913 Search PubMed.
- J. F. B. M. Lapointe and C. H. Cote, Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion, J. Appl. Physiol., 2002, 92, 1995–2004 CrossRef PubMed.
- H. Toumi and T. M. Best, The inflammatory response: friend or enemy for muscle injury?, Br. J. Sports Med., 2003, 37, 284–286 CrossRef CAS PubMed.
- S. Uchiyama, H. Tsukamoto, S. Yoshimura and T. Tamaki, Relationship between oxidative stress in muscle tissue and weight-lifting-induced muscle damage, Pflugers Arch., 2006, 452, 109–116 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, C. Rendeiro, T. Bergillos-Meca, S. Tabatabaee, T. W. George, C. Heiss and J. P. Spencer, Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity, Am. J. Clin. Nutr., 2013, 98, 1179–1191 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, R. Del Pino-Garcia, T. W. George, A. Vidal-Diez, C. Heiss and J. P. Spencer, Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols, Mol. Nutr. Food Res., 2014, 58, 1952–1961 CrossRef CAS PubMed.
- J. Perez-Jimenez, V. Neveu, F. Vos and A. Scalbert, Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database, Eur. J. Clin. Nutr., 2010, 64(Suppl 3), S112–S120 CrossRef CAS PubMed.
- J. Kang, K. M. Thakali, G. S. Jensen and X. Wu, Phenolic acids of the two major blueberry species in the US Market and their antioxidant and anti-inflammatory activities, Plant Foods Hum. Nutr., 2015, 70, 56–62 CrossRef CAS PubMed.
- O. G. L. C. J. B. Ana Paula Silva Caldas, Cranberry antioxidant power on oxidative stress, inflammation and mitochondrial damage, Int. J. Food Prop., 2018, 21, 582–592 CrossRef.
- A. Sharma and H. J. Lee, Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.), Curr. Issues Mol. Biol., 2022, 44, 4570–4583 CrossRef CAS PubMed.
- R. Zare, R. Kimble, A. Ali Redha, G. Cerullo and T. Clifford, How can chokeberry (Aronia) (poly)phenol-rich supplementation help athletes? A systematic review of human clinical trials, Food Funct., 2023, 14, 5478–5491 RSC.
- A. G.-M. Andrzej Sidor, Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food – a review, J. Funct. Foods, 2015, 18, 941–958 CrossRef.
- W. Kalt, A. Cassidy, L. R. Howard, R. Krikorian, A. J. Stull, F. Tremblay and R. Zamora-Ros, Recent Research on the Health Benefits of Blueberries and Their Anthocyanins, Adv. Nutr., 2020, 11, 224–236 CrossRef PubMed.
- A. M. Connor, J. J. Luby, J. F. Hancock, S. Berkheimer and E. J. Hanson, Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage, J. Agric. Food Chem., 2002, 50, 893–898 CrossRef CAS PubMed.
- L. Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, S. Gebhardt and R. L. Prior, Concentrations of proanthocyanidins in common foods and estimations of normal consumption, J. Nutr., 2004, 134, 613–617 CrossRef CAS PubMed.
- M. J. Cho, L. R. Howard, R. L. Prior and J. R. Clark, Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry, J. Sci. Food Agric., 2004, 84, 1771–1782 CrossRef.
- R. D. Hurst, R. W. Wells, S. M. Hurst, T. K. McGhie, J. M. Cooney and D. J. Jensen, Blueberry fruit polyphenolics suppress oxidative stress-induced skeletal muscle cell damage in vitro, Mol. Nutr. Food Res., 2010, 54, 353–363 CrossRef CAS PubMed.
- C. H. Park, Y. S. Kwak, H. K. Seo and H. Y. Kim, Assessing the Values of Blueberries Intake on Exercise Performance, TAS, and Inflammatory Factors, Iran. J. Public Health, 2018, 47, 27–32 Search PubMed.
- U. S. D. A. U.S Department of Agriculture, Blueberries, Raw, FoodData Central, https://fdc.nal.usda.gov/fdc-app.html#/food-details/171711/nutrients, (accessed 09/10, 2023).
- R. Upton, Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics, in American Herbal Pharmacopoeia, Santa Cruz, CA, 2001, no. 14 Search PubMed.
- S. W. Chan and B. Tomlinson, Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk, Molecules, 2020, 25, 1653 CrossRef CAS PubMed.
- M. Yarahmadi, G. Askari, M. Kargarfard, R. Ghiasvand, M. Hoseini, H. Mohamadi and A. Asadi, The effect of anthocyanin supplementation on body composition, exercise performance and muscle damage indices in athletes, Int. J. Prev. Med., 2014, 5, 1594–1600 Search PubMed.
- I. F. F. B. A. S. Wachtel-Galor, Herbal Medicine: Biomolecular and Clinical Aspects, CRC Press/Taylor & Francis., Boca Raton (FL), 2nd edn, 2011 Search PubMed.
- S. Triebel, H. L. Trieu and E. Richling, Modulation of inflammatory gene expression by a bilberry (Vaccinium myrtillus L.) extract and single anthocyanins considering their limited stability under cell culture conditions, J. Agric. Food Chem., 2012, 60, 8902–8910 CrossRef CAS PubMed.
- L. Arevstrom, C. Bergh, R. Landberg, H. Wu, A. Rodriguez-Mateos, M. Waldenborg, A. Magnuson, S. Blanc and O. Frobert, Freeze-dried bilberry (Vaccinium myrtillus) dietary supplement improves walking distance and lipids after myocardial infarction: an open-label randomized clinical trial, Nutr. Res., 2019, 62, 13–22 CrossRef PubMed.
- U. S. D. A. U.S Department of Agriculture, Cranberries, Raw, FoodData Central, https://fdc.nal.usda.gov/fdc-app.html#/food-details/171711/nutrients (accessed 09/10, 2023).
- B. V. Nemzer, F. Al-Taher, A. Yashin, I. Revelsky and Y. Yashin, Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview, Molecules, 2022, 27, 1503 CrossRef CAS PubMed.
- E. Pappas and K. M. Schaich, Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability, Crit. Rev. Food Sci. Nutr., 2009, 49, 741–781 CrossRef CAS PubMed.
- J. B. Blumberg, T. A. Camesano, A. Cassidy, P. Kris-Etherton, A. Howell, C. Manach, L. M. Ostertag, H. Sies, A. Skulas-Ray and J. A. Vita, Cranberries and their bioactive constituents in human health, Adv. Nutr., 2013, 4, 618–632 CrossRef CAS PubMed.
- I. T. Lee, Y. C. Chan, C. W. Lin, W. J. Lee and W. H. Sheu, Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes, Diabetic Med., 2008, 25, 1473–1477 CrossRef CAS PubMed.
- M. M. Dohadwala, M. Holbrook, N. M. Hamburg, S. M. Shenouda, W. B. Chung, M. Titas, M. A. Kluge, N. Wang, J. Palmisano, P. E. Milbury, J. B. Blumberg and J. A. Vita, Effects of cranberry juice consumption on vascular function in patients with coronary artery disease, Am. J. Clin. Nutr., 2011, 93, 934–940 CrossRef CAS PubMed.
- C. H. Wang, C. C. Fang, N. C. Chen, S. S. Liu, P. H. Yu, T. Y. Wu, W. T. Chen, C. C. Lee and S. C. Chen, Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials, Arch. Intern. Med., 2012, 172, 988–996 Search PubMed.
- M. A. Maher, H. Mataczynski, H. M. Stefaniak and T. Wilson, Cranberry juice induces nitric oxide-dependent vasodilation in vitro and its infusion transiently reduces blood pressure in anesthetized rats, J. Med. Food, 2000, 3, 141–147 CrossRef CAS PubMed.
- R. C. Khanal, T. J. Rogers, S. E. Wilkes, L. R. Howard and R. L. Prior, Effects of dietary consumption of cranberry powder on metabolic parameters in growing rats fed high fructose diets, Food Funct., 2010, 1, 116–123 RSC.
- S. J. Duthie, A. M. Jenkinson, A. Crozier, W. Mullen, L. Pirie, J. Kyle, L. S. Yap, P. Christen and G. G. Duthie, The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers, Eur. J. Nutr., 2006, 45, 113–122 CrossRef CAS PubMed.
- J. A. Novotny, D. J. Baer, C. Khoo, S. K. Gebauer and C. S. Charron, Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults, J. Nutr., 2015, 145, 1185–1193 CrossRef CAS PubMed.
- J. E. M. M. J. Page, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, J. M. Tetzlaff, E. A. Akl, S. E. Brennan, R. Chou, J. Glanville, J. M. Grimshaw, A. Hrobjartsson, M. M. Lalu, T. Li, E. W. Loder, E. Mayo-Wilson, S. McDonald, L. A. McGuinness, L. A. Stewart, J. Thomas, A. C. Tricco, V. A. Welch, P. Whiting and D. Moher, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, Br. Med. J., 2021, 372 Search PubMed.
- J. S. J. A. C. Sterne, M. J. Page, R. G. Elbers, N. S. Blencowe, I. Boutron, C. J. Cates, H. Y. Cheng, M. S. Corbett, S. M. Eldridge, J. R. Emberson, M. A. Hernan, S. Hopewell, A. Hrobjartsson, D. R. Junqueira, P. Juni, J. J. Kirkham, T. Lasserson, T. Li, A. McAleenan, B. C. Reeves, S. Shepperd, I. Shrier, L. A. Stewart, K. Tilling, I. R. White, P. F. Whiting and J. P. T. Higgins, RoB 2: a revised tool for assessing risk of bias in randomized trials, Br. Med. J., 2019, 366, l4898 CrossRef PubMed.
- M. A. H. J. A. Sterne, B. C. Reeves, J. Savovic, N. D. Berkman, M. Viswanathan, D. Henry, D. G. Altman, M. T. Ansari, I. Boutron, J. R. Carpenter, A. W. Chan, R. Churchill, J. J. Deeks, A. Hrobjartsson, J. Kirkham, P. Juni, Y. K. Loke, T. D. Pigott, C. R. Ramsay, D. Regidor, H. R. Rothstein, L. Sandhu, P. L. Santaguida, H. J. Schunemann, B. Shea, I. Shrier, P. Tugwell, L. Turner, J. C. Valentine, H. Waddington, E. Waters, G. A. Wells, P. F. Whiting and J. P. Higgins, ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions, Br. Med. J., 2016, 355, i4919 CrossRef PubMed.
- J. P. T. H. L. A. McGuinness, Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments, Res. Synth. Methods, 2021, 12, 55–61 CrossRef PubMed.
- T. Bloedon, S. Vendrame, J. Bolton, R. Lehnhard, P. Riso and D. Klimis-Zacas, The effect of wild blueberry (Vaccinium angustifolium) consumption on oxidative stress, inflammation, and DNA damage associated with exercise, Comp. Exerc. Physiol., 2015, 11, 173–181 Search PubMed.
- K. D. Pilolla, J. Armendariz, B. M. Burrus, D. S. Baston, K. A. McCarthy and T. K. Bloedon, Effects of Wild Blueberries on Fat Oxidation Rates in Aerobically Trained Males, Nutrients, 2023, 15, 1339 CrossRef CAS PubMed.
- A. Skarpanska-Stejnborn, P. Basta, J. Trzeciak, A. Michalska, M. E. Kafkas and D. Woitas-Slubowska, Effects of cranberry (Vaccinum macrocarpon) supplementation on iron status and inflammatory markers in rowers, J. Int. Soc. Sports Nutr., 2017, 14, 7 CrossRef PubMed.
- S. Meihua, Y. DuoDuo and Z. Shuilian, Preventive effect of cranberry consumption against DNA damage after exhaustive exercise in athlete men, Acta Medica Mediterr., 2018, 34, 499–506 Search PubMed.
- Y. McLeay, M. J. Barnes, T. Mundel, S. M. Hurst, R. D. Hurst and S. R. Stannard, Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage, J. Int. Soc. Sports Nutr., 2012, 9, 19 CrossRef PubMed.
- D. C. Nieman, N. D. Gillitt, G. Y. Chen, Q. Zhang, W. Sha, C. D. Kay, P. Chandra, K. L. Kay and M. A. Lila, Blueberry and/or Banana Consumption Mitigate Arachidonic, Cytochrome P450 Oxylipin Generation During Recovery From 75-Km Cycling: A Randomized Trial, Front. Nutr., 2020, 7, 121 CrossRef PubMed.
- J. P. Brandenburg and L. V. Giles, Blueberry supplementation reduces the blood lactate response to running in normobaric hypoxia but has no effect on performance in recreational runners, J. Int. Soc. Sports Nutr., 2021, 18, 26 CrossRef CAS PubMed.
- A. Lynn, S. Garner, N. Nelson, T. N. Simper, A. C. Hall and M. K. Ranchordas, Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: a randomised, placebo-controlled trial, J. Int. Soc. Sports Nutr., 2018, 15, 22 CrossRef PubMed.
- S. Nyberg, E. Gerring, S. Gjellan, M. Vergara, T. Lindstrom and F. H. Nystrom, Effects of exercise with or without blueberries in the diet on cardio-metabolic risk factors: an exploratory pilot study in healthy subjects, Upsala J. Med. Sci., 2013, 118, 247–255 CrossRef PubMed.
- D. C. Nieman, C. A. Sakaguchi, A. M. Omar, K. L. Davis, C. E. Shaffner, R. C. Strauch, M. A. Lila and Q. Zhang, Blueberry intake elevates post-exercise anti-inflammatory oxylipins: a randomized trial, Sci. Rep., 2023, 13, 11976 CrossRef CAS PubMed.
- S. R. McAnulty, L. S. McAnulty, D. C. Nieman, C. L. Dumke, J. D. Morrow, A. C. Utter, D. A. Henson, W. R. Proulx and G. L. George, Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C, Nutr. Res., 2004, 24, 209–221 CrossRef CAS.
- L. S. McAnulty, D. C. Nieman, C. L. Dumke, L. A. Shooter, D. A. Henson, A. C. Utter, G. Milne and S. R. McAnulty, Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running, Appl. Physiol., Nutr., Metab., 2011, 36, 976–984 CrossRef CAS PubMed.
- J. P. Brandenburg and L. V. Giles, Four Days of Blueberry Powder Supplementation Lowers the Blood Lactate Response to Running But Has No Effect on Time-Trial Performance, Int. J. Sport Nutr. Exercise Metab., 2019, 29, 636–642 CAS.
- C. H. Park, Y. S. Kwak, H. K. Seo and H. Y. Kim, Assessing the Values of Blueberries Intake on Exercise Performance, TAS, and Inflammatory Factors, Iran. J. Public Health, 2018, 47, 27–32 Search PubMed.
- B. K. Pedersen and F. Edward, Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines, J. Appl. Physiol., 2009, 107, 1006–1014 CrossRef CAS PubMed.
- B. K. Pedersen, M. Kappel, M. Klokker, H. B. Nielsen and N. H. Secher, The immune system during exposure to extreme physiologic conditions, Int. J. Sports Med., 1994, 15(Suppl 3), S116–S121 CrossRef PubMed.
- B. K. Pedersen and M. A. Febbraio, Muscle as an endocrine organ: focus on muscle-derived interleukin-6, Physiol. Rev., 2008, 88, 1379–1406 CrossRef CAS PubMed.
- A. Steensberg, M. A. Febbraio, T. Osada, P. Schjerling, G. van Hall, B. Saltin and B. K. Pedersen, Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content, J. Physiol., 2001, 537, 633–639 CrossRef CAS PubMed.
- G. L. Milne, H. Yin, J. D. Brooks, S. Sanchez, L. Jackson Roberts 2nd and J. D. Morrow, Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress, Methods Enzymol., 2007, 433, 113–126 CAS.
- S. Parthasarathy, N. Santanam, S. Ramachandran and O. Meilhac, Potential role of oxidized lipids and lipoproteins in antioxidant defense, Free Radical Res., 2000, 33, 197–215 CrossRef CAS PubMed.
- A. Bouyahya, N. E. Omari, N. El Hachlafi, M. E. Jemly, M. Hakkour, A. Balahbib, N. El Menyiy, S. Bakrim, H. Naceiri Mrabti, A. Khouchlaa, M. F. Mahomoodally, M. Catauro, D. Montesano and G. Zengin, Chemical Compounds of Berry-Derived Polyphenols and Their Effects on Gut Microbiota, Inflammation, and Cancer, Molecules, 2022, 27, 3286 CrossRef CAS PubMed.
- S. Jannas-Vela, A. Espinosa, A. A. Candia, M. Flores-Opazo, L. Penailillo and R. Valenzuela, The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review, Nutrients, 2023, 15, 871 CrossRef CAS PubMed.
- P. Ockermann, L. Headley, R. Lizio and J. Hansmann, A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health, Nutrients, 2021, 13, 2831 CrossRef CAS PubMed.
- L. Xie, T. Vance, B. Kim, S. G. Lee, C. Caceres, Y. Wang, P. A. Hubert, J. Y. Lee, O. K. Chun and B. W. Bolling, Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial, Nutr. Res., 2017, 37, 67–77 CrossRef CAS PubMed.
- Y. Jin, D. Alimbetov, T. George, M. H. Gordon and J. A. Lovegrove, A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects, Eur. J. Clin. Nutr., 2011, 65, 849–856 CrossRef CAS PubMed.
- P. E. Milbury, J. A. Vita and J. B. Blumberg, Anthocyanins are bioavailable in humans following an acute dose of cranberry juice, J. Nutr., 2010, 140, 1099–1104 CrossRef CAS PubMed.
- L. Giordano, W. Coletta, C. Tamburrelli, M. D'Imperio, M. Crescente, C. Silvestri, P. Rapisarda, G. Reforgiato Recupero, A. De Curtis, L. Iacoviello, G. de Gaetano, D. Rotilio, C. Cerletti and M. B. Donati, Four-week ingestion of blood orange juice results in measurable anthocyanin urinary levels but does not affect cellular markers related to cardiovascular risk: a randomized cross-over study in healthy volunteers, Eur. J. Nutr., 2012, 51, 541–548 CrossRef CAS PubMed.
- P. Riso, F. Visioli, C. Gardana, S. Grande, A. Brusamolino, F. Galvano, G. Galvano and M. Porrini, Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress, J. Agric. Food Chem., 2005, 53, 941–947 CrossRef CAS PubMed.
- G. Williamson, The role of polyphenols in modern nutrition, Nutr. Bull., 2017, 42, 226–235 CrossRef CAS PubMed.
- M. C. Gomez-Cabrera, A. Carretero, F. Millan-Domingo, E. Garcia-Dominguez, A. G. Correas, G. Olaso-Gonzalez and J. Vina, Redox-related biomarkers in physical exercise, Redox Biol., 2021, 42, 101956 CrossRef CAS PubMed.
- R. Domitrovic, The molecular basis for the pharmacological activity of anthocyans, Curr. Med. Chem., 2011, 18, 4454–4469 CrossRef CAS PubMed.
- P. C. Calder, N. Ahluwalia, R. Albers, N. Bosco, R. Bourdet-Sicard, D. Haller, S. T. Holgate, L. S. Jonsson, M. E. Latulippe, A. Marcos, J. Moreines, C. M'Rini, M. Muller, G. Pawelec, R. J. van Neerven, B. Watzl and J. Zhao, A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies, Br. J. Nutr., 2013, 109(Suppl 1), S1–34 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |