Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emission inventory of PFASs and other fluorinated organic substances for the fluoropolymer production industry in Europe†
Joost
Dalmijn
 *a,
Juliane
Glüge
*a,
Juliane
Glüge
 b,
Martin
Scheringer
b,
Martin
Scheringer
 b and
Ian T.
Cousins
b and
Ian T.
Cousins
 a
a
aDepartment of Environmental Science, Stockholm University, SE-10691 Stockholm, Sweden. E-mail: Ian.Cousins@aces.su.se
bInstitute of Biogeochemistry and Pollutant Dynamics, ETH Zürich, 8092 Zürich, Switzerland
First published on 20th December 2023
Abstract
Fluoropolymers are a group of fluorinated polymers within the broad class of substances known as per- and polyfluoroalkyl substances (PFASs). During their production, a wide array of additional fluorinated organic substances (many PFASs and some not defined as PFASs) are used, formed and emitted to air and water. This study aims to assess, and make an inventory of, all emissions of PFASs and other fluorinated organic substances by the fluoropolymer production industry in Europe using available emission databases and permits. Air emissions of the fluorinated gases (i.e., chlorofluorocarbons, hydrofluorocarbons, hydrochlorofluorocarbons and perfluorocarbons (CFCs, H(C)FCs and PFCs)) by this industry have reportedly decreased between 2007 and 2021 from roughly 500 to 150 tonnes per year. Emissions of fluorosurfactants to air and water have also been reduced significantly. However, large uncertainties remain regarding the emissions of substances that are neither fluorinated gases nor fluorosurfactants but are classified as PFASs, such as polymerization by-products, chain transfer agents and fluorinated solvents. The available data indicate that the release of these substances is not decreasing but remains relatively stable. As this inventory probably underestimates emissions, further research, improved data availability and more harmonized reporting of emissions are necessary to obtain more accurate emission data for these substances. Nevertheless, based on the available data, it is clear that the emissions from fluoropolymer production plants to air and water are still significant and that the production of fluoropolymers continues to introduce persistent substances to the environment.
Environmental significanceThe fluorochemical industry argues that fluoropolymers should not be grouped with other PFASs and points out that they meet the Organization for Economic Cooperation and Development (OECD) criteria for “polymers of low concern”. These arguments focus misleadingly on the use phase, while it is known that the largest environmental impacts have been, and remain to be, associated with emissions during production. This study summarizes what is known regarding the many different fluorinated substances released during European production. While there have been some recent emission reductions, there remain emissions of multiple fluorinated substances ongoing with known or probable environmental impacts. It is important not to trivialize the lifecycle impacts of fluoropolymers and be misled that they are inert and safe materials. |
1. Introduction
Fluoropolymers are high-molecular weight synthetic materials that consist of a carbon-fluorine backbone which comprises repeating building blocks that are derived from smaller reactive organic fluorocarbon molecules called monomers.1 The strong carbon–fluorine bond provides fluoropolymers with high chemical and thermal resistance. Furthermore, the unparalleled electronegativity of fluorine leads to low polarizability of the carbon–fluorine bond and thus weak dispersive intermolecular forces, which explains the low surface tension (or surface energy) of these materials.2 Fluoropolymers can be classified as per- and polyfluoroalkyl substances (PFASs) according to the 2021 OECD definition, because they contain at least one perfluoroalkyl (–CF2–) moiety without a chlorine, bromine, iodine or hydrogen group attached.3 Fluoropolymers have many applications including (but not limited to) non-stick coatings in cookware, insulated electrical wiring, battery membranes, building materials, lubricants, personal protection gear and medical devices.4–6 While useful, carbon–fluorine chemistry is associated with environmental impacts, such as global warming, ozone depletion, ecotoxicity and impacts on human health.7–9Fluoropolymers with significant production volumes include polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), fluorinated ethylene propylene (FEP), fluorine Kautschuk materials (FKMs) or fluoroelastomers, ethylene tetrafluoroethylene (ETFE), ethylene chlorotrifluoroethylene (ECTFE), perfluoropolyethers (PFPEs), perfluoroalkoxyalkanes (PFA), and copolymers of tetrafluoroethylene, hexafluoropropylene and vinylidene fluoride (THV).10 The diversity of fluoropolymers reflects different desired characteristics, such as melt-processability, crystallinity, solvability and other physical and chemical properties.
Monomers used in the production of fluoropolymers include tetrafluoroethylene (TFE), trifluoroethylene (TrFE), hexafluoropropylene (HFP), hexafluoropropylene oxide (HFPO), chlorotrifluoroethylene (CTFE), vinylidene fluoride (VDF) and various perfluoroethers (e.g. perfluoropropylvinyl ether (PPVE)).11 Fluoropolymers are either polymers of a single monomer (homopolymer) or multiple monomers (copolymer). The basis of fluoromonomer synthesis is the reaction of hydrofluoric acid (HF) with small organic molecules. HF itself is derived from the reaction of the inorganic mineral fluorspar (CaF2) and sulphuric acid (H2SO4).12 The fluoropolymer production industry is projected to grow from an estimated 3.3 billion USD in 2019 to a projected 4.6 billion USD in 2024.13 This makes fluoropolymers the second largest produced subgroup of PFASs after fluorinated gases, which comprise chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), hydrofluoroolefins (HFOs) and perfluorocarbons (PFCs).14
(Fluoro)polymers cover a grey zone in both classification and regulation in the European Union (EU) as they are currently not regulated under the European regulation on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). However, with the revision of REACH it is planned to establish the obligation to register polymers,15 which would also include fluoropolymers. Also, authorities from the EU member states Denmark, Germany, the Netherlands and Sweden, together with the EU-associated state Norway, submitted a proposal for the restriction of PFASs under Annex XV of REACH.16 This proposal follows a grouping approach, using the OECD 2021 definition of PFASs as a starting point and does include fluoropolymers.3,16 The fluoropolymer production industry, and some downstream users of fluoropolymers, on the other hand, are arguing for fluoropolymers to be exempted from this restriction proposal on the basis of their inert properties and the functions they fulfil in society.17
Many modern fluoropolymers are considered relatively inert due to their high molecular weight and stability and are believed to contain few leachable impurities.18 However, during their production, lower-molecular-weight fluorinated or other halogenated organic molecules are used or emitted. These substances could be persistent, bioaccumulative, toxic, and have high global warming potential (GWP) and/or ozone depleting potential (ODP).11,19,20 These issues necessitate a wide scope for assessments of the sustainability and future of this industry in the context of the restriction proposal.
Historically, PFAS research conducted by environmental scientists has mostly focused on a subset of PFASs called perfluoroalkyl acids (PFAAs), and their precursors. The PFAAs are fluorosurfactants and contain a hydrophobic per- or polyfluoroalkyl chain and a hydrophilic head group (e.g. carboxylate or sulfonate). Fluoropolymers made through the emulsion polymerization process require fluorosurfactants to emulsify and stabilize aqueous dispersions. Initially, salts of long-chain perfluoroalkyl carboxylic acids (PFCAs), such as PFOA and PFNA, were used in this process. These uses were phased out by manufacturers in Europe, North America and Japan between 2002 and 2016 and alternative fluorosurfactants were introduced, mainly perfluoroether carboxylic acids (PFECAs) (with shorter perfluoroalkyl chains connected via ether linkages) that are less bioaccumulative but still toxic and persistent. While the fluoropolymer production industry was a significant historical source of these problematic fluorosurfactant substances in the environment,21 the use and emissions of PFAAs and PFECAs by the fluoropolymer industry in Europe are better controlled today through the introduction of abatement measures, restrictions and regulations and innovations such as fluorosurfactant-free polymerization.12,17 Although these developments seem beneficial for PFAS emissions, some fluorosurfactant-free polymerization techniques make use of chain transfer agents to actually generate fluorinated surfactants in situ and thereby potentially increase the formation of fluorinated polymerization by-products.12,22 Furthermore, fluorosurfactants only represent a part of the PFAS uses and emissions by this industry (see Table S1 in ESI-2† for an overview of fluorosurfactants used by the fluoropolymer production industry in Europe).
As such, removing or remediating fluorosurfactant use and emissions will not eliminate all PFAS emissions during fluoropolymer production, as the emissions of monomers and other fluorinated organic substances such as polymerization by-products will still occur.12 Additionally, some of these emitted PFASs could be transformed into PFAAs in the environment. Relatively little is known about the amounts and the structural identities of the many additional PFASs emitted from fluoropolymer production in comparison to the better-studied fluorosurfactant processing aids.23–25
This study aims to assess the knowledge gaps in the emissions of all substances that can be classified as PFASs or other fluorinated organic substances by the fluoropolymer production industry in Europe. This emission inventory thus not only includes PFAAs, CFCs, H(C)FCs and PFCs but also monomers, building blocks and by-products. The goal is to attempt to provide a first comprehensive inventory of fluorinated organic substance and PFAS emissions from fluoropolymer production and then to critically evaluate the inventory for data gaps. This inventory will allow scientists and regulators to better evaluate the risks associated with emissions from fluoropolymer production going forward and to determine where emission reduction measures are still needed. Hopefully, the identified gaps in the inventory will also provide incentives for regulators and industry to provide or generate the missing data and to make them publicly available in the near future so that the emission estimates provided by this study could be further improved or verified.
2. Methods
2.1 Substance scope
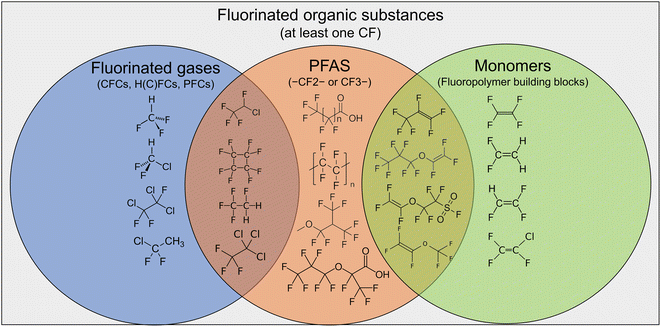
Because fluoropolymer production plants use and emit a wide range of substances, multiple partially overlapping definitions are used in this study, emission permits and the literature (see Fig. 1). The broadest definition of the substances within the scope of this report is fluorinated organic substances. This group was defined as substances containing at least one carbon–fluorine bond. All subsequently defined substance categories in this study are subsets of this broad category which were included because emission permits used this category. The fluorinated organic substances that are mentioned in this study (with their abbreviations) are also listed with their full names and Chemical Abstract Service Registry Numbers® (CAS RN®) in Table 2 in the Appendix. More details about the substances and their properties, including to which subgroup they belong, are provided in ESI-1†.Fluorinated gases were defined within this study as consisting of the substance groups CFCs, HFCs, HCFCs and PFCs that are listed in Annex II of the EC regulation no. 166/2006 (ref. 26), which is the regulation that established the European Pollutant Release and Transfer Register (E-PRTR). The definition for fluorinated gases here differs from the term ‘F-gases’ which is used by industry and regulators to refer to some HFCs, PFCs and some inorganic gases that are fluorinated (e.g., sulphur hexafluoride (SF6)). The same companies that are active in the fluoropolymer production industry also produce and emit a part of these fluorinated gases. However, this study focuses on the emissions associated with fluoropolymer production sites and is thus not an attempt to compile a comprehensive inventory of fluorinated gas emissions.
PFASs are defined according to the definition in the PFAS restriction proposal, which states that substances that contain one fully fluorinated methylene (–CF2–) or methyl (CF3–) group, without any H/Cl/Br/I attached are PFASs, with the exception of X–CF2–X′ or CF3–X “where X = –OR or –NRR′ and X′ = methyl (–CH3), methylene (–CH2–), an aromatic group, a carbonyl group (–C(O)–), –OR′′, –SR′′ or –NR′′R′′′ and where R/R′/R′′/R′′′ is a hydrogen (–H), methyl (–CH3), methylene (–CH2–), an aromatic group or a carbonyl group (–C(O)–)”.16 One key characteristic of substances classified as PFASs is that they are either persistent themselves or degrade to form other persistent PFASs, often PFAAs.
Monomers are defined as the fluorinated organic building blocks that are made into fluoropolymers by polymerization. Although the monomers are also gaseous, we do not group them here as fluorinated gases because there is no reporting obligation for the monomers under EC regulation no. 166/2006. The PFAS definition used overlaps with the definitions of monomers and fluorinated gases (see Fig. 1), but not all monomers and not all fluorinated gases mentioned here are defined as PFASs. For example, HCFC-22 and HCFC-142b, which are very relevant for fluoropolymer production, are not PFASs according to the current definition, because a chlorine atom is attached to the –CF2– moiety, whereas e.g., HCFC-124 is classified as a PFAS. Monomers such as TFE and VDF are not PFASs because they do not contain a –CF2– moiety and are mineralizable in the environment,27,28 while HFP and fluoroether monomers are PFASs. For a comprehensive description of the environmental impact of the European fluoropolymer industry, fluorinated organic substances that are used and emitted in large volumes by this industry were considered within the scope of this study, regardless of their status as PFASs.
2.2 Production sites
Data were collected on fluoropolymer production plants (FPPs) in Europe, which is defined in this study as the European Economic Area (EEA), the United Kingdom (UK) and Switzerland. A site was considered within scope if it produced fluoropolymers by polymerization of monomers and had a production volume above 1000 tonnes per year. As such, six different production sites were assessed in this study (see Fig. S1 in ESI-2† for a map with an overview of production locations). Sites that have ceased the production of fluoropolymers, such as the former Zakłady Azotowe site in Tarnow, Poland, which stopped producing PTFE after the ban of PFOA, were not considered. Furthermore, plants that (only) produce side-chain fluorinated polymers, fluorinated gases, and small organic fluorinated molecules or further process fluoropolymers by e.g., compounding or coating were not considered. On this basis, sites such as the 3M facility in Zwijndrecht, Belgium, were excluded (see Table 1 for data on every site assessed in this study).| Name | Location | Polymers produced (production capacity) | Substances used and/or emitted (excluding fluorosurfactants) | Fluorosurfactants (polymer) (years used) | Abatement | Permitting authority | Permit year (medium) |
|---|---|---|---|---|---|---|---|
| Asahi Glass Chemicals Europe | Thornton-cleveleys, UK | PTFE (4000 t per year) and ETFE (2000 t per year) | TFE, TrFE, HFP, HCFC-22, HFC-23, HFC-125, PFIB, 1H-PFHx, PFBE, and PPVE | EEA (PTFE) (2012-currently) | Thermal oxidation (air) | UK-EPA | 2017 (air and water) |
| APFO (PTFE) (1952–2012) | |||||||
| Arkema France and Daikin Chemical | Pierre-Bénite, FR | PVDF (8000 t per year) and FKM (3650 t per year) | HFP, VDF, HCFC-22, HFC-23, HFC-134a, HCFC-122b, HCFC-132b, HCFC-133a, HCFC-141b HCFC-142b, HFC-143a HFC-125, BTFM, and DIOFB | 6:2 FTS (PVDF) (1973-currently) | System unknown (water) | Géorisques | Permit available |
| PFHxA (FKM) (2008-currently) | |||||||
| Surflon S-111 (PVDF) (2003–2016) | |||||||
| APFO (PTFE) (1965–1986) and (FKM) (2004–2008) | |||||||
| Chemours | Dordrecht, NL | PTFE (8500 t per year), FEP (3500 t per year) and FKM (6800 t per year) | TFE, HFP, VDF, HCFC-22, HFC-23, PFC-318, PFIB, PFAC, PPVE, PMVE, PEVE, HFPO-DA, 6:2 FTS, ether A, ether B, BPAF, E1, and HFC-4310mee | HFPO-DA (PTFE and FEP) (2012-currently) | Activated carbon scrubbing (air and water) | DCMR (air) | 2022 (air) |
| 6:2 FTS (FKM) (unknown-currently) | RWS (water) | 2022 (water) | |||||
| APFO (PTFE and FEP) (1967–2012) | |||||||
| Dyneon (subsidiary of 3M) | Bürgkirchen a/d Alz, DE | PTFE, PFA, PFPEs, ETFE, THV, and FKM (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year, all polymers summed) 000 t per year, all polymers summed) |
TFE, HFP, VDF, HFPO, PPVE, PMVE, DIOFB, HCFC-22, HFC-23, PFC-116, PFC-218, PFC-318, and PFIB | ADONA (all emulsion polymers) (2008-currently) | Unknown | LRA-Alltötting | Permit unavailable |
| MV31 (Co-monomer) (unknown-currently) | |||||||
| APFO (all emulsions) (1968–2008) | |||||||
| Solvay–Solexis | Tavaux, FR | PVDF (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year) 000 t per year) |
HCFC-141b, HCFC-142b, HFC-143a, VDF, and HFC-365mfc | Unknown if and which fluorosurfactants are in use or were used | Thermal oxidation (air) | Géorisques | 2011 (air and water) |
| Solvay Specialty Polymers | Spinetta Marengo, IT | PTFE, PFA, PFPEs, and FKM (production capacities unknown) | TFE, HFP, VDF, DIOFE, DIOFB, DIOFH, FSVE, PMVE, PPVE, PFC-318, and PFIB | cC6O4 (unknown) (2013-currently) | Thermal oxidation (air) | Commune of Alessandria (air) | 2010 (air) |
| ADV (unkown) (1996–2021) | Regione Piemonte (water) | 2021 (water) | |||||
| APFO (unknown) (unknown-2012) | |||||||
| VEFS (co-monomer) (unknown-currently) |
For the purposes of this study, plants that had multiple companies operating on the same premises producing fluoropolymers or monomers and sharing infrastructure, such as Arkema France and Daikin Chemical in Pierre-Bénite, France, and Solvay and Solexis in Tavaux, France, were considered to be a single production site. Additionally, some plants not only produce and process fluoropolymers, but also produce or process fluorinated gases. Aside from producing PVDF, Arkema France produces a mixture of different H(C)FCs, Solvay–Solexis produces the blowing agent HFC-365mfc and in addition to their fluoropolymer portfolio Chemours loads and blends various fluorinated gases in canisters and tanks at their Dordrecht site in the Netherlands. Because the emissions from fluorinated gas production and processing vs. fluoropolymer production are difficult to distinguish or are not distinguished at all during reporting, all emissions from these sites were considered. If more information becomes available in the future, we will be happy to adjust the numbers accordingly.
2.3 Data collection
Exploring the E-PRTR data was done by locating the facility on the European map with all facilities in the European Industrial Emissions Portal and selecting the dot representing the entry in the registry. Some companies had multiple entries or were registered under a different name, necessitating the compilation of multiple entries.31
In addition to the E-PRTR, the national databases Thru for Germany, INERIS for France and the PRTR-UK were used to confirm the E-PRTR data and to gain additional information on the type of emissions.32–34 Data from the Thru and PRTR-UK databases were in the same reporting format as the E-PRTR data. The PRTR-UK additionally differentiates accidental and normal emissions. The INERIS database reports the emissions of some fluorinated gases on a substance level; however the number of substances is limited (e.g., HCFCs are not reported) and smaller than the number of substances included in Annex II of EC regulation no. 166/2006. Additionally, the data are aggregated on a municipality basis (see Tables S2 and S3 in ESI-2†). Thus, even though the plants in France were most probably the main contributors to the fluorinated gas emissions in their respective municipalities, these data can be influenced by other emission sources. The Solvay–Solexis complex in Tavaux is actually situated in two municipalities: Tavaux and Abergement-la-Ronce, so data from these two locations were summed.
Furthermore, REACH and CLP registration dossiers were accessed to determine the applications and use of various substances at the production sites.
Permits with clear emission limits or reported emissions to air and water could be obtained for AGC Europe (UK), Chemours (NL), Solvay Specialty Polymers (Italy) and Solvay–Solexis (FR). LRA-Alltötting responded to our data request, but has up to now not supplied us with an emission permit or any other data for Dyneon (DE). Data for this site were acquired through publicly available sources. Géorisques, the responsible authority for the French fluoropolymer manufacturing plants, referred us to the publicly available texts on their website, but only fragmented information on reported and permitted emissions could be found in these documents.
Data from the emission permits were reported in different formats and with different levels of detail. Some authorities pose limits on single substance emissions (e.g., the United Kingdom Environmental Protection Agency (UK EPA) and Rijnmond Regional Environmental Protection Agency (DCMR)), while others limit the emissions of fluorinated organic substances as a group (e.g., Géorisques and Commune di Alessandria) (Fig. 1). Also, some permits only provide limit values while others also supplement these limit values with reported emissions or estimates of actual yearly emissions. For Solvay Specialty Polymers (IT), emission permits include concentrations (in mg m−3) and flow rates (m3 h−1), which can be used to calculate permitted emissions (e.g., in mg h−1), while AGC Europe (UK) and Chemours (NL) report annual emission limits (in units of kilograms per year). For this study all values were converted to the unit of tonnes per year (t per year). Emission points (e.g. a stack) and/or processes (e.g. PTFE production) were given in most permits. To calculate total emissions from a plant, the emissions from different emission points in the permit were summed. In order to have the most accurate emission volume estimates, actual reported emissions were preferably used. If these data were not available, permitted emissions were used as a worst-case estimate.
3. Results
3.1 E-PRTR inventory
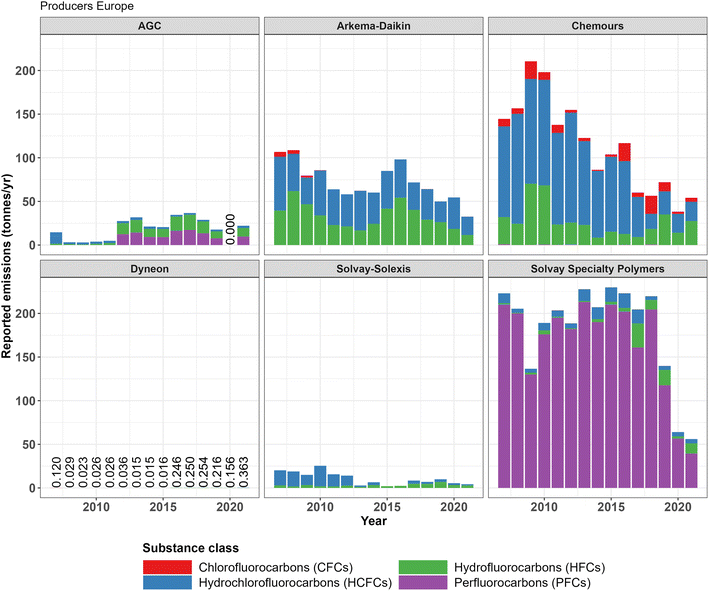
For four out of the six European fluoropolymer producers, data summarized from the E-PRTR show decreasing temporal trends in the reported emissions of fluorinated gases (Fig. 2). However, no temporal trends are visible for AGC and Dyneon. For total fluorinated gas emissions there is a clear downward trend; total emissions of around 500 tonnes of fluorinated gases were reported by the production sites in 2007, while this figure dropped to around 150 tonnes in 2021. The main reason for this decrease in total emissions is the lower reported emissions of HCFCs by Chemours and PFCs by Solvay Specialty Polymers. Reported total emissions of CFCs and HFCs were fairly stable between 2007 and 2021, fluctuating around 100 t per year for HCFCs and between 1 and 10 t per year for CFCs. Dyneon has a very similar production portfolio and capacity as the other producers in this inventory. However, relatively low total emissions are reported to the E-PRTR compared to other producers with a maximum of only 0.2 t per year. Furthermore, Solvay Specialty Polymers emission reports are dominated by PFCs, while for the other companies in this inventory HCFCs and HFCs make up most reported emissions. It is noteworthy that most reported emission values to the E-PRTR are either estimated or calculated and not measured (see Fig. S3 in ESI-2†).The data from the French emission registry on the municipality level of the location of the factories in Tavaux (Solvay-Solexis) and Pierre-Bénite (Arkema-Daikin) also show decreases in fluorinated gas emissions in the reported years 2004, 2007 and 2012 (ref. 34). These data are over a decade old and provide only a few datapoints and thus are not likely to represent current emissions. Additionally, not all fluorinated gases were reported (HCFCs are notably missing) and only three years of data are present in the database. Considering these discrepancies, the emissions of HFCs from Pierre-Bénite reported to this database in 2007 and 2012 match the emissions reported to the E-PRTR of Arkema–Daikin relatively well. In these years, 43.1 tonnes and 25.4 tonnes of HFC emissions were reported to INERIS, while 39.5 tonnes and 21.1 tonnes were reported to the E-PRTR respectively. For Solvay–Solexis the results do not add up as well as in 2007 and 2012; 4.0 and 1.1 tonnes were reported to INERIS, while 2.9 and 2.7 tonnes were reported to the E-PRTR. More details on the results from the INERIS inventory can be found in Tables S2 and S3 in ESI-2.†
3.2 Reported and permitted emissions
AGC has a permit dating from 2017 to emit HCFC-22, HFC-23, HFC-125, TFE, TrFE and HFP to the air.36 The total permitted emissions of these fluorinated organic substances by AGC to air are 28.8 t per year, of which 5.35 t per year (HFC-125 and HFP) are PFASs (Fig. 3).
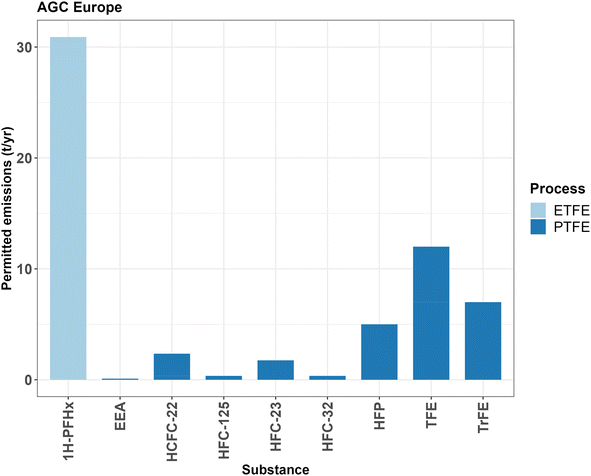 | ||
| Fig. 3 Air emissions of AGC Chemicals Europe at Thornton-Cleveleys, UK. Emissions of EEA and 1H-PFHx are reported yearly emissions from 2023 documents;37,38 all others are permitted emissions from the 2017 permit. For the exact values, see ESI-2 Table S5.† | ||
Reported emissions found in other publicly available documents include the fluorosurfactant processing aid EEA-NH4 (ref. 37) (estimated to be around 0.8 t per year to water and less than 0.1 t per year to air), the fluorinated solvent 1H-PFHx (ref. 38) used for granular ETFE production (0.04 t per year to water and 30.9 t per year to air) and the co-monomers PPVE39 and PFBE40 (0.001 t per year to water). The UK EPA estimates that around 250 tonnes of PFOA were released from this site between 1950 and 2012, of which 75 tonnes were emitted to the river Wyre, 70 tonnes to the atmosphere, 80 tonnes were transferred to downstream users, 15 tonnes were incinerated and less than 5 tonnes were landfilled.41
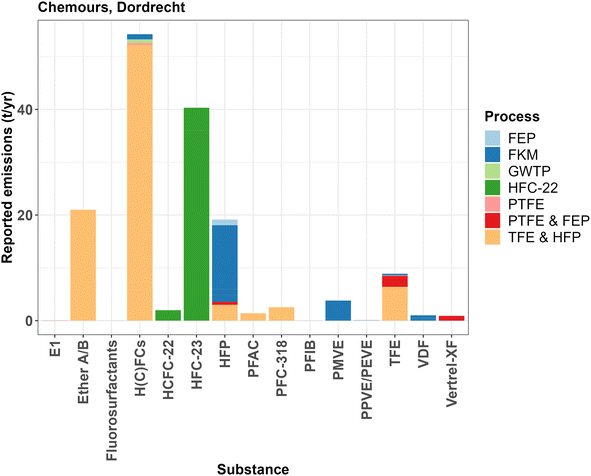 | ||
| Fig. 4 Annual reported estimated emissions of fluorinated organic substances from Chemours, Dordrecht, to the air in 2021–2024. Colors represent different production processes. For the exact emission values, see ESI-2 Table S5.† | ||
The permit shows relatively high reported air emissions of fluorinated gases and other substances that are formed as by-products in the production of the monomers TFE and HFP (light orange and green bars in Fig. 4). These emissions originate from both the production of HCFC-22 and its subsequent pyrolysis to TFE and HFP and result in 127 t per year fluorinated organic substances in total. At least 28 t per year of these emissions are PFASs (HFP, PFC-318, PFAC and Ether A/B). A part of the emissions of the H(C)FCs might also be PFASs. Additionally, the permitted emissions from the production of fluoroelastomers (FKM), 20.6 t per year of mainly HFP, PMVE and VDF, are significantly higher than those from the production of PTFE and FEP combined. The latter amounts to 5.3 t per year of mainly TFE, Vertrel XF (HFC-4310mee), H(C)FCs and HFP. The production capacity of FKM is 6800 t per year, relative to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year of PTFE and FEP, so it seems that FKM production is more prone to emissions to the atmosphere.
000 t per year of PTFE and FEP, so it seems that FKM production is more prone to emissions to the atmosphere.
Notable from the permit data is that fluorosurfactant emissions make up a relatively small amount (only several kilograms) of the total permitted and reported emissions to air and water. The maximally permitted fluorosurfactant emissions to water are 0.005 t per year for HFPO-DA and 0.002 t per year for PFOA from both direct sources (process water) and indirect sources (drainage water). Maximal emissions to air are around 0.004 t per year for HFPO-DA.
These amounts have been reduced significantly over the years. Permitted and reported PFOA and HFPO-DA emissions to air and water between 1998 and 2018 were a few tonnes per year for both compartments, after which the permitted emissions were gradually lowered toward current levels.44
When the yearly reported emissions from the permit are compared to the latest emissions reported to the E-PRTR in 2021, an interesting discrepancy can be observed. Chemours reports air emissions of about 54 t per year of HFCs, HCFCs and CFCs to the E-PRTR in 2021. No data are reported on the emissions of PFCs after 2018. An almost identical number as in the E-PRTR can be found in the emission permit from 2022 when summing the reported emissions of the reported substance category H(C)FCs. However, emissions of the individual substances HCFC-22 (2 t per year), HFC-23 (40 t per year), PFC-318 (2.5 t per year) and HFC-4310mee (Vertrel XF, 0.9 t per year) should also be reported to the E-PRTR. These emissions account for an additional 45 t per year of fluorinated gases that either go unreported, or the reported emissions of which are overestimated in the permit. PFC-318, which should be reported as a PFC to the E-PRTR, does not appear in these data as no PFC emissions have been reported to this registry by Chemours in this period.
The permit of Solvay Specialty Polymers allows for the emission of around 44 t per year of fluorinated organic substances to the air (see Fig. 5) (ref. 45). About 31 t per year of these permitted emissions consist of non-polymeric organic fluorinated substances, of which 7.5 t per year is TFE.45 Furthermore, the company is permitted to emit about 13 t per year of ‘inert particulate fluoropolymers’ to the air. Variations on the permit without clear air limits have been published, following the phaseout of PFOA and the introduction of the fluorosurfactants cC6O4 and ADV.46,47 Other PFASs emitted by this plant according to the permit include the monomers HFP, PMVE, PPVE and FSVE/VEFS, the chain-transfer agents DIOFB and DIOFH and by-products PFIB and PFC-318 (ref. 45 and 47). The permit does not mention certain by-products of TFE, HFP and HCFC-22 production that are mentioned in the Chemours permit, such as HFC-23 or PFAC.42
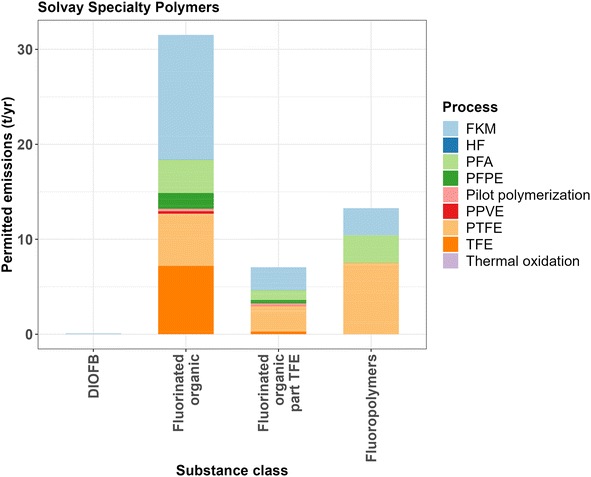 | ||
| Fig. 5 Air emissions limits of Solvay Specialty Polymers in Spinetta Marengo (IT) based on the emission permit from 2010.45 Note that the ‘part TFE’ refers to the part of permitted emissions of fluorinated organic substances that is TFE. | ||
It is notable that in this plant, as is the case with the Chemours plant, FKM production has relatively high permitted emissions of fluorinated organic substances, with 13.1 t per year relative to PTFE (5.5 t per year), PFA (3.5 t per year) and PFPE (1.6 t per year). However, this could be a result of different production capacities, which unfortunately are unknown for this plant. It should be noted though that when comparing the permitted emissions of fluorinated organic substances to ‘inert particulate fluoropolymers’, which might be an indication of production volumes, some interesting differences can be observed. Permitted emissions of the fluorinated organic substances are 4.6 times higher than permitted emissions of the ‘inert particulate fluoropolymers’ in the case of FKM production, while for other fluoropolymers the ratios are closer to one (for PFA it is 1.2 times higher and for PTFE it is 0.73 times lower). For PFPE production, the permitted emissions of the fluorinated organic substances (1.66 t per year) are 145 times higher than the permitted emissions of the ‘inert particulate fluoropolymers’ (0.011 t per year). However, emissions are lower overall and PFPEs are generally not solid but liquid.
Limits on the water emissions of the fluorosurfactants cC6O4 and ADV at the discharge point were published in 2021.46 These have to be reduced from 7 to 3.5 to 0.5 μg L−1 for cC6O4 from 1 February 2022 to 31 January 2023, 1 February 2023 to 31 January 2024 and starting from 1 February 2024, respectively. For ADV the limits are 2 μg L−1 from 1 February to 31 January 2023 and 0.5 μg L−1 from 1 February 2023 onwards.46
Until these limits were implemented, the annual average in the Bormida river downstream of the discharge point could not exceed 0.9 μg L−1 for cC6O4 and 0.3 μg L−1 for ADV. With the implementation of the new limits, these values have to decrease to 0.3 μg L−1 for cC6O4 and 0.1 μg L−1 for ADV between 1 February 2022 and 31 January 2023 and to 0.2 μg L−1 for cC6O4 and 0.06 μg L−1 for ADV from 1 February 2023.46
From comparing the Solvay Specialty Polymer permit to the data reported to the E-PRTR, it becomes clear that a large part of the air emissions reported in the E-PRTR is unaccounted for in the emission permit.31,45 Reported emissions to the E-PRTR show very high emissions of PFCs between 2007 and 2017 (around 200 t per year) while the permit only allows for the emission of around 32 t per year of fluorinated organic substances. The only PFC mentioned in the permit is PFC-318 (octafluorocyclobutane), which is a by-product of the production of TFE by the pyrolysis of HCFC-22. It seems unlikely that the emissions of PFC-318 are the sole cause for these relatively high reported values as PFC-318 is only a minor by-product in the production of TFE and unlikely to reach emission values up to 200 t per year.
More details are reported on the emissions to the E-PRTR for 2017. These consist of CFC-12 and CFC-13 (152 kg), HCFCs 22, 142b and 141b (34.9 tonnes) and HFCs 134a, 23, 125, 143a, and 32 (29 tonnes) (Fig. 6).
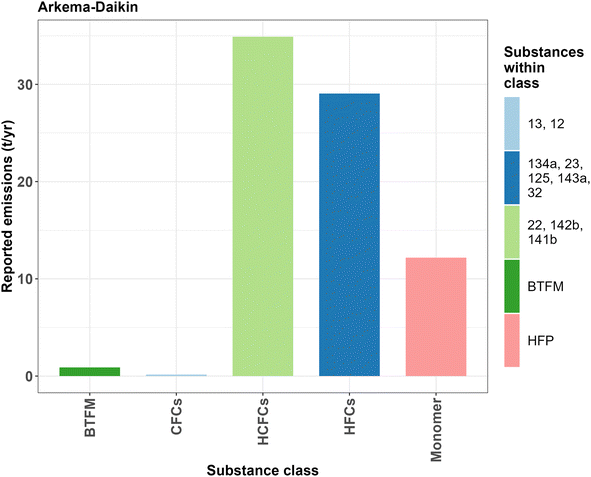 | ||
| Fig. 6 Reported air emissions by Arkema–Daikin in 2017. Note that the HFP emissions are estimates from a separate document published in 2020. For the exact emission values, see ESI-2 Table S5.† | ||
Additionally, one document reports the emissions of HFC-23 by Arkema in the years 2015–2018 with 7.57, 12.23, 15.40 and 6.55 t per year, respectively.48
An additional document for Daikin mentions release of the monomer HFP, which is estimated to be around 12.18 t per year when the maximum production capacity of 2000 t per year is reached and excludes fugitive emissions.50
In 2013, water emissions of PFHxA, PFNA, PFUndA and 6:2 FTS to the river Rhône were estimated to be 0.2, 1, 0.3 and 1.5 t per year, respectively. Surflon S-111, a fluorosurfactant with the ammonium salts of PFNA, PFUnDA and PFTriDA as the main components, was phased out in 2016 and a water treatment station was installed in 2017. In 2022 emissions were significantly reduced; about 600 g of PFHxA and 22 kg of 6:2 FTS were reportedly emitted between June and October 2022, with the other mentioned compounds abated effectively.49
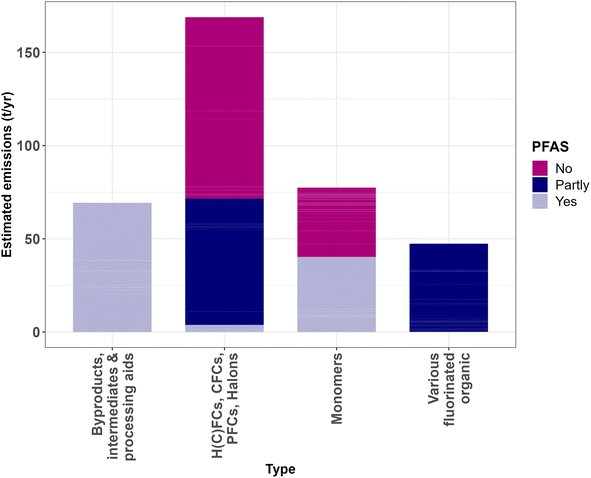
Approximately half of these emissions (169 t per year) are for fluorinated gases and these emissions should also have been reported to the E-PRTR. The figure of 169 t per year seems realistic when it is compared with the actual reported emissions to the E-PRTR in this time period (Fig. 2). From these reported data it is clear that a large fraction of these emissions (97 t per year) consists of low-molecular-weight H(C)FCs, such as HFC-23 and HCFC-22, which are not classified as PFASs in the restriction proposal. Another part (68 t per year) of these emissions are just grouped as H(C)FCs and it is unclear if they can be classified as PFASs or not due to the partly overlapping definitions of these substances and PFASs (Fig. 1). Only around 4 t per year of H(C)FC, CFC and halon emissions could unequivocally be classified as PFASs.
The emissions of monomers (77 t per year) are for a large part made up of TFE (29 t per year), which is not a PFAS, and HFP (36 t per year), which is a PFAS. It should be noted, however, that emissions of monomers were only reported in reports or the permits of AGC, Arkema–Daikin (only HFP), Chemours and Solvay Specialty polymers. Thus, this figure could be significantly higher when the monomer emissions of the other producers are also considered.
Estimated emissions of by-products, processing aids and intermediates (78 t per year) consist for a large part (39.9 t per year) of the fluorinated solvent 1H-PFHx emitted by AGC and of Ether A and B (21 t per year), reportedly emitted by Chemours, and inert particulate fluoropolymers (13 t per year) permitted to be emitted by Solvay Specialty Polymers. All these emissions could be considered PFASs, but data on the emissions of these types for the other plants are relatively scarce. Therefore, this figure is likely also an underestimation.
Lastly, a large part (47 t per year) of mainly permitted emissions could not be classified in any other way than simply fluorinated organic substances and could thus fall in any of the previously mentioned classes.
4. Discussion and implications
4.1 Data availability
Furthermore, some permits seem to be updated and altered regularly, while others are dated. This irregular reporting of emissions makes directly comparing emission figures from different plants a difficult task. From the current compiled emission inventory, Chemours is a major emitter of many different fluorinated organic substances. However, this conclusion is potentially due to the extensive public reporting and permit applications this company has to do relative to the other companies.42
From an analysis of the various emission permits, it becomes clear that companies are obliged to monitor the concentrations of emission fluxes and report these values to the permitting authorities. However, these data are not always available to the public and are still not made available even after inquiry to the permitting authorities. It is therefore recommended that in the future emission data are made publicly available and are reported in a more consistent and transparent way.
The database could also be expanded to include all substances that are classified as PFASs. This would considerably improve the transparency of the impact of this industry. The industrial emission portal of the EC is currently under review and from 2023 onwards, the production volumes of plants will be registered in the E-PRTR as well.56
4.2 Emissions
In 2021, about 52 tonnes of HCFCs, which were supposed to be phased out entirely as end-use substances, were still emitted. This is probably because the production of monomers relies on HCFCs as feedstock substances (HCFC-22 for TFE and HCFC-142b for VDF).12,22 Moreover, the emissions of HFCs remained relatively stable from 2007 to 2021. These substances do not have any ODP because they do not contain chlorine or bromine, but some of them have high GWPs (see ESI-1†). HCFCs, HFCs and PFCs with high GWPs and ODPs have been replaced by various HFCs and hydrofluoroolefins (HFOs), which are more reactive in the atmosphere. Whereas this increased reactivity lowers their GWP, these replacements are precursors to TFA and other ultra-short chain PFCAs, which are steadily increasing in concentration in the environment.59
Climate scientists have monitored the levels of fluorinated gases in the environment, using instruments such as a Medusa-GC-MS60 at remote sampling locations. Atmospheric concentrations of fluoropolymer-production-related gases, such as PFC-318, HFC-4310mee and HCFC-22 have been increasing over the last few decades.61,62 To obtain a more integral picture of the emissions of fluoropolymer production, a higher number of volatile fluorinated organic substances should be analysed. The current analyte list should be expanded to include substances identified in this inventory and other fluoropolymer-production-related substances. Measurements are particularly needed at or close to the point sources to improve the characterization of emissions. Combining these measurements with the sampling and high-resolution analysis of non-volatile ionic PFASs close to the sources and at remote locations would also be valuable to improve the knowledge on the environmental fate of substances emitted by the fluoropolymer production industry.
Only part of the used and emitted substances in monomer production is classified as PFASs in the context of the PFAS restriction proposal. HCFC-22, HFC-23, HCFC-142b, VDF and TFE are not PFASs. However, they remain problematic as they either have a high GWP, a high ODP (H(C)FCs) or degrade to form the highly toxic carbonyl fluoride (COF2) and corrosive HF (TFE and VDF).27,28 By-products from monomer production processes, such as PFIB, PFC-318 and PFAC and the eventual fluoropolymer end products are PFASs. Therefore, emissions of fluorinated gases, PFASs and fluorinated organic substances should be considered and treated as overlapping issues for fluoropolymer production as they all are part of a single synthesis process.
Monomers that are defined as PFASs are HFP and fluoroether monomers such as PMVE, PPVE and PEVE. This class of substances is concerning because the emission volumes are relatively high and these substances are probably precursors to TFA or ultra-short chain PFCAs, which are persistent and mobile in the environment.64–66 One or more of these monomers are used in the production of FEP, PFA, THV, FK(K)M and PFPEs.67 Additionally, small amounts of PMVE are sometimes added to modify PTFE.
From the inventory it becomes clear that manufacturers have reduced the emissions of fluorosurfactants used in the polymerization process to both air and water. However, there are still a lot of uncertainties regarding the formation and emissions of by-products. Specifically the formation of (ultra)short-chain PFAAs is an important aspect because these substances are not removed effectively by sorption techniques and could be classified as persistent, mobile and toxic (PMT). Furthermore, the emissions of chain transfer agents, which are usually perfluorinated substances with a reactive end moiety (e.g., iodine) and polymerization by-products, especially from fluorosurfactant-free emulsion polymerization, could be significant. These emissions have gone relatively understudied and underreported, but the analysis of effluents of fluoropolymer production plants using high-resolution mass spectrometry indicates that these could be relevant components that do not fall in the traditional PFAS analysis suite, such as perfluoroalkyl dicarboxylic acids.68
Furthermore, there could be significant air emissions of understudied fluorinated organic substances from polymerization that do not fall under the fluorinated gas definition, as is observed in the results of the Chemours permit with reported emissions of fluoroether E1 (decarboxylated HFPO-DA) and Ether A and B, which are reaction products of PFIB and methanol (this reaction is carried out to mitigate the emissions of the highly toxic pyrolysis by-product PFIB).42
Even though emissions have been reduced over the last few decades, there seem to be different standards when it comes to the permitted water emissions of fluorosurfactants. AGC is still allowed to discharge an estimated 0.8 t per year of EEA to the River Wyre without a clear emission limit, whereas Chemours is only permitted to emit a few kilograms of HFPO-DA and PFOA. Arkema–Daikin, Dyneon and Solvay Specialty Polymers have been obliged to reduce the concentrations of the fluorosurfactant processing aids in effluents. See Table S1 in ESI-2† for an overview of fluorosurfactants that are and were used in Europe.
Aside from by-products, fluorosurfactants and chain transfer agents used in aqueous emulsion polymerization and fluorinated solvents, such as HFC-4310mee (Vertrel XF) or 1H-PFHx (AC-2000), can be used in granular fluoropolymer production or in different fluoropolymer processing steps such as aggregation and compounding. These fluorinated solvents could subsequently be emitted to the environment, as observed from the Chemours and AGC data.
Lastly, on- or off-site storage or treatment of polymerization waste and abatement material (e.g., activated carbon) could be an additional understudied source of fluorinated organic substances to the environment. For instance, air concentrations of HFPO-DA were reported at an oven of an incineration plant for waste treatment operated by the company Indaver NV, which incinerates the activated carbon used for emission abatement by Chemours in Dordrecht.69
Fluoroelastomers (FK(K)Ms) need to be toughened by polymer cross-linking (curing), which is often achieved by the addition of bisphenols.22 These substances are problematic in and of themselves because of endocrine disrupting properties.71,72 Additionally, the common FKM curing agent bisphenol AF is also a PFAS. As with chain transfer agents and polymerization by-products, relatively little is known about the use and emissions of fluoroelastomer curing agents.
4.3 Implications for the PFAS restriction proposal
The fluoropolymer production industry, and even downstream industrial users of fluoropolymers, are arguing for the exclusion of fluoropolymers from the PFAS restriction proposal currently discussed in the EU. Industry claims that fluoropolymers are inert, not bioavailable and non-toxic. They therefore suggest that fluoropolymers should be considered as “polymers of low concern”73,74 and that they should be exempted from the PFAS restriction proposal. However, the emissions from the production of fluoropolymers are a big part of the whole PFAS problem and it is questionable if these emissions can be avoided in the future.From the inventory, it becomes apparent that fluoropolymer production can have significantly different emission levels of PFASs and environmental impact depending on the type of the produced fluoropolymer and employed (co)monomers. However, information on the reasons for these differences is not readily available. In order to minimize PFAS emissions from fluoropolymer production, it would therefore be relevant to differentiate between fluoropolymer types, production processes and their environmental footprint. More transparency on the identity of emitted substances and emission volumes is needed to complete this assessment.
When it comes to the use of fluorosurfactants, there are roughly two directions that industry is proposing to take. In some companies, fluorosurfactants are to be phased out and replaced by fluorosurfactant-free polymerization, while others intend to continue the use of fluorosurfactants, while maximizing their recovery and emission abatement. Arkema is planning to phase out the use of 6:2 FTS for PVDF production in Pierre-Bénite by the end of 2024 and shift to a fluorosurfactant-free process.75 Solvay will phase out ADV in Spinetta Marengo by 2023, but will continue to use cC6O4 until at least 2026 (ref. 46 and 76). 3M has announced that it will discontinue its PFAS portfolio entirely by 2025.77 One of the implications of this decision is the closure of the Dyneon plant in Gendorf/Bürgkirchen a/d Alz, as Dyneon is a full subsidiary of 3M.78 Chemours wants to continue using fluorosurfactants in their emulsion polymerization processes and is focusing on reducing emissions to a minimum with abatement techniques.79 The intentions of AGC remain unclear as they have not communicated their strategy regarding fluorosurfactants as of the writing of this paper. Although emission reduction might prevent additional contamination, due to historical emissions and the persistence of these substances, the surroundings of fluoropolymer production plants will remain PFAS hotspots in the future. It is currently unclear which of the two industry paths, namely (1) continued emission abatement of fluorosurfactant processing aids (by Chemours) or (2) switching to fluorosurfactant-free processing aids (Arkema and Solvay), is favourable in terms of overall reduction in environmental impacts. On the one hand, it is impossible to achieve zero emissions of fluorosurfactant processing aids,17 and on the other hand, the use of fluorosurfactant-free processing aids appears to lead to the release of additional unwanted fluorinated by-products.79,80
It is further important to emphasize that air emissions of fluorinated organic substances that are neither regulated by the Montreal Protocol nor used as fluorosurfactant processing aids by the fluoropolymer production industry remain relatively high (see Fig. 7). Examples of these substances are feedstock substances, fluorinated monomers, by-products and fluorinated solvents. The emissions of these anthropogenic substances have to be further investigated and, where possible, minimized.
Although regulatory pressure has led to reductions in the emissions of PFASs or other fluorinated organic substances during the lifecycle of fluoropolymers, there remains a wide range of emissions and impacts which are ongoing and should not be trivialized. These emissions need to be consistently reported and documented across Europe. Regulatory pressures should also be equally stringent across Europe ensuring that the best available technologies (BAT) are applied to reduce emissions as much as possible going forward. Restricting fluoropolymers to their essential uses81 would be an additional effective way of reducing the production of fluoropolymers and thus, emissions, further.
Appendix
| Abbreviation | Name | Formula | CAS RN® |
|---|---|---|---|
| 1H-PFHx | Trideca-1,1,1,2,2,3,3,4,4,5,5,6,6-fluorohexane | C6HF13 | 355-37-3 |
| 6:2 FTS | 6:2 fluorotelomersulfonic acid | C8H5F13O3S | 34455-29-3 |
| ADV | 1-Propene, 1,1,2,3,3,3-hexafluoro-, telomer with chlorotrifluoroethene, oxidized, reduced, hydrolyzed | Cl(C3F6O)n(C2F4O)mCF2COOH | 330809-92-2 |
| BPAF | Bisphenol AF | C15H10F6O2 | 1478-61-1 |
| BTFM | Bromotrifluoromethane | CBrF3 | 75-63-8 |
| cC6O4 | (Difluoro{[2,2,4,5-tetrafluoro-5-(trifluoromethoxy)-1,3-dioxolan-4-yl]oxy}acetic acid) | C6HF9O6 | 1190931-27-1 |
| CTFE | Chlorotrifluoroethylene | C2ClF3 | 79-38-9 |
| DIOFB | 1,4-Diiodoperfluorobutane | C4F8I2 | 375-50-8 |
| DIOFE | 1,1,2,2-Tetrafluoro-1,2-diiodoethane | C4F4I2 | 354-65-4 |
| DIOFH | 1,6-Diiodoperfluorohexane | C6F12I2 | 375-80-4 |
| DONA | Perfluoro-4,8-dioxa-3H-nonanoic acid | C7H2F12O4 | 919005-14-4 |
| E1 | Heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether | C5HF11O | 3330-15-2 |
| ECTFE | Ethylene chlorotrifluoroethylene | (C2H4)n(C2ClF3)m | 25101-45-5 |
| EEA | Perfluoro(2-ethoxy-2-fluoroethoxy)-acetic acid | C6HF11O4 | 908020-52-0 |
| ETFE | Ethylene tetrafluoroethylene | (C2H4)n(C2F4)m | 25038-71-5 |
| Ether A | 2-[Difluoro(methoxy)methyl]-1,1,1,3,3,3-hexafluoropropane | C5H4F8O | 382-26-3 |
| Ether B | 1-Methoxy(perfluoro-2-methyl-1-propene) | C5H3F7O | 360-53-2 |
| FC-72 | Perfluorohexane | C6F14 | 355-42-0 |
| FEP | Fluorinated ethylene propylene | (C2F4)n(C3F6)m | 25067-11-2 |
| FEPM | Tetrafluoroethylene propylene | (C2F4)n(C3H6)m | 64706-30-5 |
| FKM | Fluorine Kautschuk material | (C2H2F2)n(C3F6)m | 64706-30-5 |
| HCFC-124 | Tetrafluorochloroethane | C2HClF4 | 359-28-4 |
| HCFC-142b | 1-Chloro-1,1-difluoroethane | C2H3ClF2 | 75-68-3 |
| HCFC-21 | Dichlorofluoromethane | CHCl2F | 75-43-4 |
| HCFC-22 | Chlorodifluoromethane | CHClF2 | 75-45-6 |
| HFC-125 | Pentafluoroethane | C2HF5 | 354-33-6 |
| HFC-134a | 1,1,2,2-Tetrafluoroethane | C2HF4 | 811-97-2 |
| HFC-141b | 1,1-Dichloro-1-fluoroethane | C2H3Cl2F | 1717-00-6 |
| HFC-143a | 1,1,1-Trifluoroethane | C2H3F3 | 420-46-2 |
| HFC-152a | 1,1-Difluoroethane | C2H5F2 | 75-37-6 |
| HFC-23 | Trifluoromethane | CHF3 | 75-46-7 |
| HFC-32 | Difluoromethane | CH2F2 | 75-10-5 |
| HFC-365mfc | 1,1,1,3,3-Pentafluorobutane | C4H5F5 | 406-58-6 |
| HFC-4310mee | 1,1,1,2,3,4,4,5,5,5-Decafluoropentane | C5H2F10 | 138495-42-8 |
| HFP | Hexafluoropropylene | C3F6 | 116-15-4 |
| HFPO | Hexafluoropropylene oxide | C3F6O | 428-59-1 |
| HFPO-DA | Hexafluoropropylene oxide dimer acid | C6HF11O3 | 13252-13-6 |
| MV31 | 2-(3-Trifluoromethoxy-1,1,2,2,3,3-hexafluoropropoxy)-2,3,3,3-tetrafluoropropanoic acid | C7HF13O4 | 496805-64-2 |
| PCTFE | Polychlorotrifluoroethylene | (C2ClF3)n | 9002-83-9 |
| PEVE | Perfluoroethyl vinyl ether | C4F8O | 10493-43-3 |
| PFA | Perfluoroalkoxyalkane | (C2F4)n(C3F6O)m | 26655-00-5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| PFAC | Perfluoroallylchloride | C3ClF5 | 2804-50-4 |
| PFBA | Perfluorobutanoic acid | C4HF7O2 | 375-22-4 |
| PFBE | Perfluorobutyl ethylene | C6H3F9 | 19430-93-4 |
| PFBS | Perfluorobutanesulfonic acid | C4HF9O3S | 19430-93-4 |
| PFC-116 | Hexafluoroethane | C2F6 | 76-16-4 |
| PFC-218 | Octafluoropropane | C3F8 | 76-19-7 |
| PFC-318 | Perfluorocyclobutane | C4F8 | 115-25-3 |
| PFDA | Perfluorodecanoic acid | C10HF19O2 | 335-76-2 |
| PFDoDA | Perfluorododecanoic acid | C12HF23O2 | 307-55-1 |
| PFHpA | Perfluoroheptanoic acid | C7HF13O2 | 75-85-93 |
| PFHxA | Perfluorohexanoic acid | C6HF11O2 | 307-24-4 |
| PFHxDA | Perfluorohexadecanoic acid | C16HF31O2 | 67905-19-5 |
| PFHxS | Perfluorohexanesulfonic acid | C6HF13O3S | 355-46-4 |
| PFIB | Perfluoroisobutylene | C4F8 | 382-21-8 |
| PFNA | Perfluorononanoic acid | C9HF17O2 | 375-95-1 |
| PFOA | Perfluorooctanoic acid | C8HF15O2 | 335-67-1 |
| PFOcDA | Perfluorooctadecanoic acid | C18HF35O2 | 16517-11-6 |
| PFOS | Perfluorooctanesulfonic acid | C8HF17O3S | 1763-23-1 |
| PFPA | Perfluoropropionic acid | C3HF5O2 | 422-64-0 |
| PFPE | Perfluoropolyether | C3F7O(C3F6O)nC2F5 | 60164-51-4 |
| PFPeA | Perfluoropentanoic acid | C5HF9O2 | 2706-90-3 |
| PFTriDA | Perfluorotridecanoic acid | C13HF25O2 | 72629-94-8 |
| PFUnDA | Perfluoroundecanoic acid | C11HF21O2 | 2058-94-8 |
| PMVE | Perfluoromethyl vinyl ether | C3F6O | 1187-93-5 |
| PPVE | Perfluoropropyl vinyl ether | C5F10O | 1623-05-8 |
| PTFE | Polytetrafluoroethylene | (C2F4)n | 9002-84-0 |
| PVDF | Polyvinylidene fluoride | (CH2CF2)n | 24937-79-9 |
| TFA | Trifluoroacetic acid | C2HF3O2 | 76-05-1 |
| TFE | Tetrafluoroethylene | C2F4 | 116-14-3 |
| TFMS | Trifluoromethylsulfonic acid | CF3O3S | 1493-13-6 |
| THV | Copolymers of tetrafluoroethylene, hexafluoropropylene and vinylidene fluoride | (C2F4)n(C3F6)m(CH2CF2)l | 25190-89-0 |
| TrFE | Trifluoroethylene | C2HF3 | 359-11-5 |
| VDF | Vinylidene fluoride | C2H2F2 | 75-38-7 |
| VEFS | Perfluoro-2-(vinyloxy)ethane-1-sulfonic acid | C4F7O3S | 29514-94-1 |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study is part of the PERFORCE3 project which has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 860665. J. G. acknowledges funding from the Tides Foundation (grants TF2101-096992 and TF2304-113820).References
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins and P. de Voogt, et al., Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7, 513–541 CrossRef CAS PubMed.
- R. R. Thomas, Material properties of fluoropolymers and perfluoroalkyl-based polymers, in Fluoropolymers 2: Properties, Springer, 1999, pp. 47–67 Search PubMed.
- Z. Wang, A. M. Buser, I. T. Cousins, S. Demattio, W. Drost and O. Johansson, et al., A New OECD Definition for Per- and Polyfluoroalkyl Substances, Environ. Sci. Technol., 2021, 55, 15575–15578 CrossRef CAS PubMed.
- B. Ameduri, Fluoropolymers: The Right Material for the Right Applications, Chem.–Eur. J., 2018, 24, 18830–18841 CrossRef CAS PubMed.
- J. Glüge, M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman and D. Herzke, et al., An overview of the uses of per- and polyfluoroalkyl substances (PFAS), Environ. Sci.: Processes Impacts, 2020, 22, 2345–2373 RSC.
- N. Karim, S. Afroj, K. Lloyd, L. C. Oaten, D. V. Andreeva and C. Carr, et al., Sustainable Personal Protective Clothing for Healthcare Applications: A Review, ACS Nano, 2020, 14, 12313–12340 CrossRef CAS PubMed.
- Z. Yang and X. Wu, Retrofits and options for the alternatives to HCFC-22, Energy, 2013, 59, 1–21 CrossRef CAS.
- M. Wahlström, E. Pohjalainen, E. Yli-Rantala, D. Behringer, D. Herzke, S. M. Mudge, et al., Fluorinated Polymers in a Low Carbon, Circular and Toxic-Free Economy Technical Report, European Environment Agency, 2021 Search PubMed.
- R. Lohmann and R. J. Letcher, The universe of fluorinated polymers and polymeric substances and potential environmental impacts and concerns, Curr. Opin. Green Sustainable Chem., 2023, 41, 100795 CrossRef CAS PubMed.
- S. Ebnesajjad, Introduction to Fluoropolymers, in Applied Plastics Engineering Handbook, Elsevier, 2017, pp. 55–71 Search PubMed.
- R. Lohmann, I. T. Cousins, J. C. DeWitt, J. Glüge, G. Goldenman and D. Herzke, et al., Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS?, Environ. Sci. Technol., 2020, 54, 12820–12828 CrossRef CAS PubMed.
- K. Hintzer and W. Schwertfeger, Fluoropolymers—Environmental Aspects, in Handbook of Fluoropolymer Science and Technology, ed. D. W. Smith, S. T. Iacono and S. S. Iyer, Wiley, 2014, pp. 495–520 Search PubMed.
- B. Améduri, The Promising Future of Fluoropolymers, Macromol. Chem. Phys., 2020, 221(8), 1900573 CrossRef.
- M. G. Evich, M. J. B. Davis, J. P. McCord, B. Acrey, J. A. Awkerman and D. R. U. Knappe, et al., Per- and polyfluoroalkyl substances in the environment, Science, 2022, 375(6580), 512–526 CrossRef PubMed.
- EC, Chemicals: commission seeks views on revision of REACH, the EU’s chemicals legislation, 2022 [cited 2023], available from: https://environment.ec.europa.eu/news/chemicals-commission-seeks-views-revision-reach-eus-chemicals-legislation-2022-01-20_en.
- ECHA, ECHA publishes PFAS restriction proposal, 2023 [cited 2023 February 23], available from: https://echa.europa.eu/-/echa-publishes-pfas-restriction-proposal.
- B. Ameduri, Fluoropolymers: A special class of per- and polyfluoroalkyl substances (PFASs) essential for our daily life, J. Fluorine Chem., 2023, 267, 110117 CrossRef CAS.
- S. H. Korzeniowski, R. C. Buck, R. M. Newkold, A. E. kassmi, E. Laganis and Y. Matsuoka, et al., A critical review of the application of polymer of low concern regulatory criteria to fluoropolymers II: Fluoroplastics and fluoroelastomers, Integr. Environ. Assess. Manage., 2022, 19, 326–354 CrossRef PubMed.
- M. Clara, S. Scharf, S. Weiss, O. Gans and C. Scheffknecht, Emissions of perfluorinated alkylated substances (PFAS) from point sources—identification of relevant branches, Water Sci. Technol., 2008, 58, 59–66 CrossRef CAS PubMed.
- B. K. Sovacool, S. Griffiths, J. Kim and M. Bazilian, Climate change and industrial F-gases: A critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions, Renewable Sustainable Energy Rev., 2021, 141, 110759 CrossRef CAS.
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, Fate and Transport of Perfluorocarboxylates, Environ. Sci. Technol., 2005, 40, 32–44 CrossRef PubMed.
- J. G. Drobny, Fluoroelastomers Handbook, 2016, includes bibliographical references and index Search PubMed.
- N. Kotlarz, J. McCord, D. Collier, C. S. Lea, M. Strynar and A. B. Lindstrom, et al., Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina, Environ. Health Perspect., 2020, 128(7), 77005 CrossRef CAS PubMed.
- J. McCord and M. Strynar, Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening, Environ. Sci. Technol., 2019, 53, 4717–4727 CrossRef CAS PubMed.
- S. Newton, R. McMahen, J. A. Stoeckel, M. Chislock, A. Lindstrom and M. Strynar, Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama, Environ. Sci. Technol., 2017, 51, 1544–1552 CrossRef CAS PubMed.
- EC, Regulation (EC) No. 166/2006 of the European Parliament and of the Council of 18 January 2006 concerning the establishment of a European Pollutant Release and Transfer Register and amending Council Directives 91/689/EEC and 96/61/EC, 2006 [cited 2023], available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R0166-20200101.
- ECETOC, Vinylidene Fluoride (CAS No. 75-38-7). JACC Report, 2005, Brussels. Report No.: ISSN-0773-6339-49.
- ECETOC, Tetrafluoroethylene (CAS No. 116-14-3). JACC No. 42, 2003 Brussels. Report No.: ISSN-0773-6339-42.
- R. G. Prinn, R. F. Weiss, J. Arduini, T. Arnold, H. L. DeWitt and P. J. Fraser, et al., History of chemically and radiatively important atmospheric gases from the Advanced Global Atmospheric Gases Experiment (AGAGE), Earth System Science Data, 2018, 10, 985–1018 CrossRef.
- D. J. Sheldon and M. R. Crimmin, Repurposing of F-gases: challenges and opportunities in fluorine chemistry, Chem. Soc. Rev., 2022, 51, 4977–4995 RSC.
- EEA, Industrial emissions portal, 2023 [cited 2023], available from: https://industry.eea.europa.eu/explore/explore-data-map/map.
- DEFRA, UK Pollutant Release and Transfer Register (PRTR) data sets, 2023 [cited 2023], available from: https://prtr.defra.gov.uk/facility-details?view=preleases%26q=1139208%26facility_id=2166%26year=2019.
- UBA,Dyneon GmbH, 2023 [cited 2023], available from: https://thru.de/daten.
- INERIS, Inventaire National Spatialisé, 2023 available from: http://emissions-air.developpement-durable.gouv.fr/map.html?name=metropole.
- UK EPA, Permitting decisions (variation number EPR/BU5453IY/V004), 2017.
- UK EPA, Notice of variation and consolidation with introductory note (Permit number EPR/BU5453IY), 2017.
- UK EPA, Environmental risk evaluation report: Perfluoro(2-ethoxy-2-fluoroethoxy)-acetic acid, ammonium salt [EEA-NH4] (CAS no. 908020-52-0), 2023.
- UK EPA, Environmental risk evaluation report: Trideca-1,1,1,2,2,3,3,4,4,5,5,6,6fluorohexane [1H-PFHx] (CAS no. 355-37-3), 2023.
- UK EPA, Environmental risk evaluation report: 1,1,1,2,2,3,3-Heptafluoro-3-[(trifluorovinyl)oxy]propane [PPVE] (CAS no. 1623-05-8), 2023.
- UK EPA, Environmental risk evaluation report: 3,3,4,4,5,5,6,6,6-Nonafluorohexene [Perfluorobutylethylene; PFBE] (CAS no. 19430-93-4), 2023.
- UK EPA, Enquiry regarding emissions of fluorinated organic substances by the fluoropolymer manufacturing industry, 2023.
- DCMR, Ontwerpbeschikking Chemours Netherlands B.V., 2022 April.
- Rechtbank Den Haag. ECLI:NL:RBDHA:2023:3302, 2023 [cited 2023], available from: https://uitspraken.rechtspraak.nl/#!/details?id=ECLI:NL:RBDHA:2023:3302%26showbutton=true%26keyword=ECLI%253aNL%253aRBDHA%253a2023%253a3302%26idx=1.
- M. Beekman, P. Zweers, A. Muller, W. de Vries, P. Janssen and M. Zeilmaker, Evaluation of substances used in the GenX technology by Chemours, Dordrecht, RIVM letter report 2016-0174, 2016 Search PubMed.
- Commune di Alessandria, Autorizzazione integrata - proponente, Solvay Solexis S.P.A., 2010 Search PubMed.
- Regione Piemonte, Modifica Sostanziale Impianto IPPC Autorizzato CON ATTO DDAA2-206-2010 DEL 24/06/10 E S.M.I. PER Produzione ED USO DI cC6O4 - Proponente: Solvay Specialty Polymers Italy SPA - Impianto SITO IN P.ZZA Donegani 5/6 Alessandria Fraz.Spinetta M.GO, 2021.
- Solvay Autorizzazione Integrata Ambientale, Riesame/rinnovo del provvedimento, 2021.
- Géorisques, Installations classées, 2023 [cited 2023], available from: https://www.georisques.gouv.fr/risques/installations/donnees.
- DREAL, Focus sur la situation au Sud de Lyon, 2023 [cited 2023], available from: https://www.auvergne-rhone-alpes.developpement-durable.gouv.fr/focus-sur-la-situation-au-sud-de-lyon-a23562.html.
- Préfet du Rhône, Rapport de contrôle de l’inspection des installations classées, 2020, DREAL, Lyon, report no.: UDR-CRT-2020-406.
- Préfecture du Jura, Installations Classées pour la Protection de l'Environment Société Solvay Electrolyse France, 2011.
- Préfecture du Jura, Recueil des actes administratifs spécial (No. 39-2019-08-002), 2019.
- Préfet du Jura, Arrete Prefectoral No. AP-2019-31 DU 25 JUILLET 2019 Codifiant ET Renforçant, 2019.
- Bourgogne-Franche-Comté M. Avis de la Mission Régionale d'Autorité environnementale de Bourgogne-Franche-Comté sur le projet d’augmentation de capacité de production du site SOLVAY Tavaux sur les communes de Tavaux et d'Abergement-la-Ronce (39), 2023.
- LRA-Alltötting, Antrag auf Erteilung einer gehobenen Erlaubnis für die Einleitung von gereinigtem Abwasser und nicht behandlungsbedürftigem Abwasser in die Alz, 2023.
- European Commission, for Environment, Directorate-General, Industrial emissions – Modernising EU rules for the green transition, 2022, Publications Office of the European Union.
- UNEP, Montreal protocol on substances that deplete the ozone layer, 1987.
- S. O. Andersen, S. Gao, S. Carvalho, T. Ferris, M. Gonzalez and N. J. Sherman, et al., Narrowing feedstock exemptions under the Montreal Protocol has multiple environmental benefits, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(49), e2022668118 CrossRef CAS PubMed.
- H. M. Pickard, A. S. Criscitiello, D. Persaud, C. Spencer, D. C. G. Muir and I. Lehnherr, et al., Ice Core Record of Persistent Short-Chain Fluorinated Alkyl Acids: Evidence of the Impact From Global Environmental Regulations, Geophys. Res. Lett., 2020, 47(10), e2020GL087535 CrossRef CAS.
- B. R. Miller, R. F. Weiss, P. K. Salameh, T. Tanhua, B. R. Greally and J. Mühle, et al., Medusa: A Sample Preconcentration and GC/MS Detector System for in Situ Measurements of Atmospheric Trace Halocarbons, Hydrocarbons, and Sulfur Compounds, Anal. Chem., 2008, 80, 1536–1545 CrossRef CAS PubMed.
- T. Arnold, D. J. Ivy, C. M. Harth, M. K. Vollmer, J. Mühle and P. K. Salameh, et al., HFC-43-10mee atmospheric abundances and global emission estimates, Geophys. Res. Lett., 2014, 41, 2228–2235 CrossRef CAS.
- J. Mühle, L. J. M. Kuijpers, K. M. Stanley, M. Rigby, L. M. Western and J. Kim, et al., Global emissions of perfluorocyclobutane (PFC-318, cC4-F8) resulting from the use of hydrochlorofluorocarbon-22 (HCFC-22) feedstock to produce polytetrafluoroethylene (PTFE) and related fluorochemicals, Atmos. Chem. Phys., 2022, 22, 3371–3378 CrossRef.
- E. L. D’Ambro, H. O. Pye, J. O. Bash, J. Bowyer, C. Allen, C. Efstathiou, R. C. Gilliam, L. Reynolds, K. Talgo and B. N. Murphy, Characterizing the Air Emissions, Transport, and Deposition of Per-and Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility, Environ. Sci. Technol., 2021, 55(2), 862–870 CrossRef PubMed.
- ECETOC, Hexafluoropropylene (CAS no. 116-15-4), JACC no. 48, 2005, report no.: ISSN-0773-6339-48.
- D. Amedro, L. Vereeckena and J. N. Crowley, Kinetics and mechanism of the reaction of perfluoro propyl vinyl ether (PPVE, C3F7OCH=CH2) with OH: assessment of its fate in the atmosphere, Phys. Chem. Chem. Phys., 2015, 17(28), 18558–18566 RSC.
- ECHA, Trifluoro(trifluoromethoxy)ethylene, 2023 [cited 2023 November 20] available from: https://echa.europa.eu/es/registration-dossier/-/registered-dossier/13408/5/1.
- D. Z. Wang, G. Goldenman and T. Tugran, Per- and polyfluoroalkylether substances: identity, production and use. Nordic working paper, 2020, Nordic Council of Ministers, Copenhagen, report no.: ISSN 2311-0562.
- H. Joerss, F. Menger, J. Tang, R. Ebinghaus and L. Ahrens, Beyond the Tip of the Iceberg: Suspect Screening Reveals Point Source-Specific Patterns of Emerging and Novel Per- and Polyfluoroalkyl Substances in German and Chinese Rivers, Environ. Sci. Technol., 2022, 56, 5456–5465 CrossRef CAS PubMed.
- VITO, Case: Monitoring PFAS Schouwemissies UIT Draaitrommeloven (DTO 2) Van Indaver NV, Samenvatting resultaten 2021–2022, Departement Omgeving, 2023.
- P. E. Boon, J. D. te Biesebeek and B. G. H. Bokkers, Herziening van de risicobeoordeling van PFAS in moestuingewassen in Helmond, RIVM letter report, 2021-0071, 2021 Search PubMed.
- B. S. Rubin, Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects, J. Steroid Biochem. Mol. Biol., 2011, 127(1–2), 27–34 CrossRef CAS PubMed.
- S. Kitamura, T. Suzuki, S. Sanoh, R. Kohta, N. Jinno, K. Sugihara, S. Yoshihara, N. Fujimoto, H. Watanabe and S. Ohta, Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds, Toxicol. Sci., 2005, 84(2), 249–259 CrossRef CAS PubMed.
- B. J. Henry, J. P. Carlin, J. A. Hammerschmidt, R. C. Buck, L. W. Buxton and H. Fiedler, et al., A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers, Integr. Environ. Assess. Manage., 2018, 14, 316–334 CrossRef CAS PubMed.
- J. Sales, F. Hernández, D. Kapoor and M. van den Noort, Fluoropolymers: the safe science that society needs, IRCL, 2022, 13 Search PubMed.
- Arkema, Arkema comments on the UE PFAS restriction proposal, 2023 [cited 2023 September 28], available from: https://www.arkema.com/global/en/social-responsibility/innovation-and-sustainable-solutions/responsible-product-management/pfas/arkema-comments-on-the-ue-pfas-restriction-proposal/.
- Solvay, Phasing out Fluorosurfactants at Solvay, 2023 [cited 2023 September 28], available from: https://www.solvay.com/sites/g/files/srpend221/files/2023-03/Phasing-out-Fluorosurfactants-V2.pdf.
- 3M, 3M to Exit PFAS Manufacturing by the End of 2025, 2023 [cited 2023], available from: https://news.3m.com/2022-12-20-3M-to-Exit-PFAS-Manufacturing-by-the-End-of-2025.
- LRA-Altötting, 3M lehnt Stiftungskonzeption zur Fortführung der Fluorpolymerproduktion in Gendorf ab, 2023 [cited 2023], available from: https://www.lra-aoe.de/aktuelles/aktuelle-meldungen/3m-lehnt-stiftungskonzeption-zur-fortfuehrung-der-fluorpolymerproduktion-in-gendorf-ab.
- Chemours. Manufacturing of Fluoropolymers – An Alternative Proposal for Managing The Risk. 2023.
- M. C. Davis, J. Boyle, M. Cervantes Garcia, J. Kramer, P. Morken, A. Smith, et al., Nontarget LC/QToF Interrogation of Fluorinated Residues in a Fluoropolymer Dispersion Prepared with a Hydrocarbon based Processing Aid, 2023 August 30, Poster at Fluoros 2023.
- I. T. Cousins, H. D. GG, M. M. LR, P. S. NCA, T. X. SM and W. Z. VL, The concept of essential use for determining when uses of PFASs can be phased out, Environ. Sci.: Processes Impacts, 2019, 21(11), 1803–1815 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00426k |
| This journal is © The Royal Society of Chemistry 2024 |