Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reverse thiophosphorylase activity of a glycoside phosphorylase in the synthesis of an unnatural Manβ1,4GlcNAc library†
Tessa
Keenan‡
 a,
Natasha E.
Hatton‡
a,
Jack
Porter
b,
Jean-Baptiste
Vendeville
a,
Natasha E.
Hatton‡
a,
Jack
Porter
b,
Jean-Baptiste
Vendeville
 c,
David E.
Wheatley
c,
David E.
Wheatley
 c,
Mattia
Ghirardello
d,
Alice. J. C.
Wahart
c,
Mattia
Ghirardello
d,
Alice. J. C.
Wahart
 b,
Sanaz
Ahmadipour
b,
Julia
Walton
b,
Sanaz
Ahmadipour
b,
Julia
Walton
 a,
M. Carmen
Galan
a,
M. Carmen
Galan
 d,
Bruno
Linclau§
ce,
Gavin J.
Miller
d,
Bruno
Linclau§
ce,
Gavin J.
Miller
 *b and
Martin A.
Fascione
*b and
Martin A.
Fascione
 *a
*a
aDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: martin.fascione@york.ac.uk
bSchool of Chemical and Physical Sciences and Centre for Glycosciences, Keele University, Keele, Staffordshire, ST5 5BG, UK. E-mail: g.j.miller@keele.ac.uk
cSchool of Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK
dSchool of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, UK
eDepartment of Organic and Macromolecular Chemistry, Ghent University, Campus Sterre, Krijgslaan 281-S4, Ghent, 9000, Belgium
First published on 29th September 2023
Abstract
β-Mannosides are ubiquitous in nature, with diverse roles in many biological processes. Notably, Manβ1,4GlcNAc a constituent of the core N-glycan in eukaryotes was recently identified as an immune activator, highlighting its potential for use in immunotherapy. Despite their biological significance, the synthesis of β-mannosidic linkages remains one of the major challenges in glycoscience. Here we present a chemoenzymatic strategy that affords a series of novel unnatural Manβ1,4GlcNAc analogues using the β-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase, BT1033. We show that the presence of fluorine in the GlcNAc acceptor facilitates the formation of longer β-mannan-like glycans. We also pioneer a “reverse thiophosphorylase” enzymatic activity, favouring the synthesis of longer glycans by catalysing the formation of a phosphorolysis-stable thioglycoside linkage, an approach that may be generally applicable to other phosphorylases.
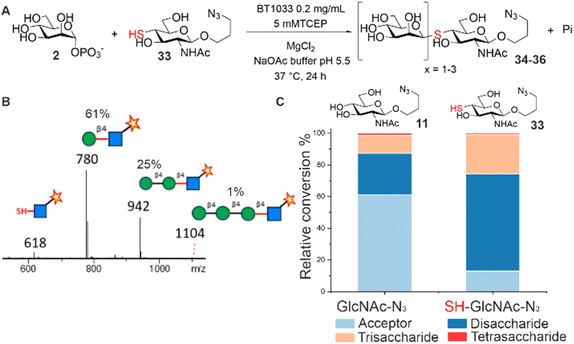
Glycoside phosphorylases (GPs) naturally catalyse the breakdown of glycosidic bonds between glycans (phosphorolysis).1 However, these useful biocatalysts can also be harnessed in a synthetic “reverse phosphorolysis” direction (Fig. 1A) requiring only simple sugar-1-phosphate donors for the synthesis of diverse glycosides.2,3 Yet the inherent reversibility of GPs can limit their utility in the synthetic direction. Herein we explore the use of unnatural substrates to favour “reverse phosphorolysis” using a GP active on β-mannosides and in the process pioneer “reverse thiophosphorylase” enzymatic activity, wherein formation of a phosphorolysis-stable thioglycoside linkage (Fig. 1B) facilitates the synthesis of longer glycans.
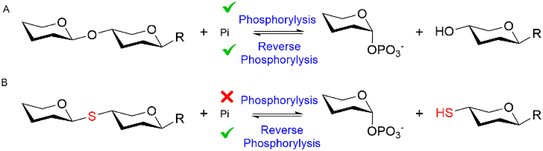 | ||
| Fig. 1 The reversible GP catalyzed reaction (A). Proposed irreversible “reverse thiophosphorylase” activity with a 4SH-thiol acceptor (B). | ||
β-Mannosides are highly prevalent in nature,4 with diverse roles in biological processes including energy storage5 and cell wall biosynthesis.6 Notably the ubiquitous ManGlcNAc2 motif within eukaryotic N-glycans7 contains a Manβ1,4GlcNAc disaccharide, which was recently identified as a novel immune modulator in autoimmune disease.8,9 Manβ1,4GlcNAc has shown potential as a new activator of STING (stimulator of interferon genes pathway) triggering a broad immune response in macrophages.8 STING is a component of the innate immune system and a key mediator of inflammation.10 Therefore small molecule activators are emerging as a promising strategy in cancer immunotherapy.11 Despite its striking biological significance and recent advances in the chemical synthesis of such linkages,12 the efficient assembly of β-mannosides still remains one of the major challenges in glycoscience.
Herein we utilize a GP-mediated chemoenzymatic approach13 for the synthesis of β-mannosides in the form of an unnatural library of Manβ1,4GlcNAc-based glycans, including a number of extended glycans. We incorporate unnatural functionality into the enzymatic building blocks through chemical synthesis and show that when a 4-SH nucleophile or 6F group are present in the GlcNAc acceptor, this facilitates the extension of Manβ1,4GlcNAc producing longer β-mannan like glycans. This approach not only affords access to a series of novel, unnatural Manβ1,4-GlcNAcs which may have potential in immunotherapy, but also represents a benchmark for the utility of GPs for thioglycoside synthesis. With more than 190 GPs that have been characterized to date, if this “reverse thiophosphorylase” activity was observed more broadly in other GPs, it could provide straightforward access to a wide range of thioglycosides.14
For the synthesis of our Manβ1,4GlcNAc analogues we investigated the inverting β-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase from Bacteroides thetaiotaomicron (BT1033).13 BT1033 is a GH130 family phosphorylase, previously shown to catalyse the transfer of Man from α-D-mannose-1-phosphate (Man1-P) onto N-acetyl-D-glucosamine (GlcNAc) to produce Manβ1,4GlcNAc by reverse phosphorolysis. To investigate the substrate promiscuity of BT1033, we screened a series of chemically synthesised Man1-P donors (3–10) and GlcNAc acceptors (11–14) (Fig. 2). The GlcNAc acceptors were designed with an azido-propyl handle to provide an accessible point for bioconjugation and this was exploited in our glycan detection methodology (Fig. 2A). Imidazolium-based ionic liquid tags (ITags) are highly sensitive mass spectrometry (MS) probes that enable low detection limits, due to their dominant ionizability by MS.16 To facilitate the semi-quantitative detection of the Manβ1,4GlcNAc products in our reactions, as well as any unreacted acceptor, the reaction products were labelled with an alkyne-functionalised ITag 1 using a copper-catalysed alkyne–azide cycloaddition (CuAAC) reaction and analysed by liquid-chromatography coupled to mass spectrometry (LC-MS). The relative conversion of starting material to product was determined by comparing the ionisation intensities of the unreacted azido-propyl linked GlcNAc (GlcNAc-N3) acceptor to the azido-propyl linked Manβ1,4GlcNAc products (Fig. 1B and ESI Section 5†). First, we assessed the suitability of GlcNAc-N311 as an acceptor mimic for BT1033, with Man1-P 2 as a donor. In preliminary studies (data not shown) we observed some enzyme-mediated hydrolysis of Man1-P 2. Therefore, in reactions containing Man1-P 2, the donor was supplied in excess (5–10 eq.) relative to the acceptor. Additionally, the donor was supplied in significant excess (5 × 104–1 × 105 eq.) relative to the enzyme to drive the reaction in favour of the synthetic “reverse phosphorolysis” reaction. LC-MS analysis showed an ion consistent with the mass of the Manβ1,4GlcNAc-ITag disaccharide (m/z 764) as expected (Fig. S11†). Additionally, we observed an ion consistent with the mass of the Manβ1,4GlcNAc-ITag disaccharide + 162 Da (m/z 926). BT1033 was previously shown to have weak synthetic activity with D-mannose as an acceptor when using Man-1P as a donor13 whilst able to use chitobiose as an acceptor, demonstrating that it is capable of producing longer-glycans. Therefore, we proposed that the product at m/z 926 was a Man2β1,4GlcNAc-ITag trisaccharide. Overall, we observed 74% conversion to disaccharide 15 and 4% to trisaccharide 16 (Fig. 2B). Next, we screened BT1033 for activity towards eight unnatural Man-1P analogues (3–10) with acceptor 11 (Fig. 2B and S3–S10†). C6-Chloro Man-1P 4 was best tolerated by BT1033, with 61% conversion to disaccharide observed after 24 h (Fig. S3†). Moderate conversions of C5-methyl Man-1P 3 and C6-methyl Man-1P 5 to disaccharide were also observed at 51% and 44%, respectively (Fig. S4 and S5†). Conversion of C6-fluoro Man-1P 6 and C6-azido Man-1P 7 to disaccharide were lower at 16% and 11% respectively (Fig. S6 and S8†), suggesting that these were poor substrates for the enzyme. No conversion of C6-gem-difluoro Man-1P 8 was observed, which was not surprising considering the poor turnover of 6. Additionally, no turnover of C6-hydroxamic acid Man-1P 9 or C6-amine Man-1P 10 were observed (Fig. S7, S9 and S10†). There was no evidence of longer glycan chain formation when using any of the unnatural Man-1Ps. In summary, the results of the unnatural Man-1P substrate screen suggest that BT1033 has little or no activity towards C6-modified analogues with groups larger than the native CH2OH. Whilst poor turnover of C6-azido 7 and C6-amine 10 Man-1Ps was observed, the chlorine in disaccharide 18 could allow for further derivatization at the C6-position to an azide or amine.
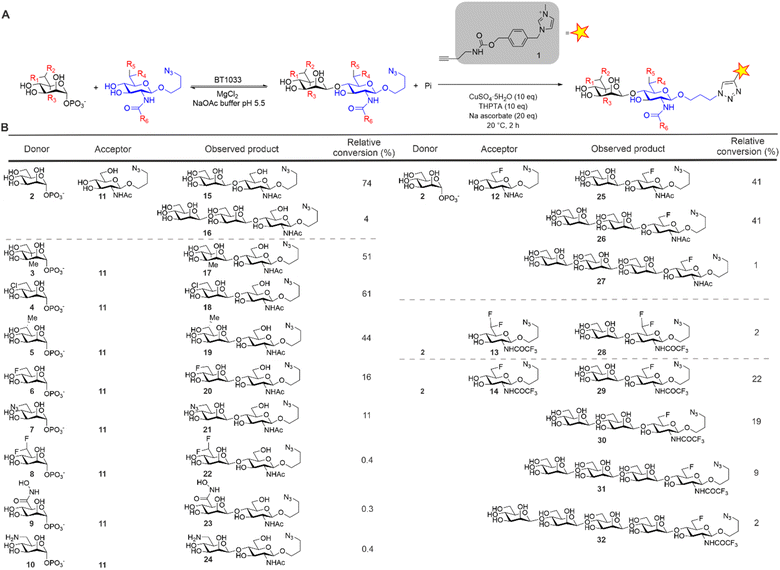 | ||
| Fig. 2 (A) ITag screening methodology for BT1033 reactions. Reaction mechanism depicted in reverse phosphorolysis direction. (B) BT1033 activity towards unnatural donors and acceptors.3,15 | ||
Next, we screened for activity towards fluorinated GlcNAc-N3 acceptors 12–14, with Man-1P 2 (Fig. 2B). Fluorination, whilst having little effect on the overall conformation of a glycan,17 is known to affect stereo-electronic properties and can therefore modulate biological function.18 6F-GlcNAc-N312 and 6F-GlcNTFA-N314 were tolerated by the enzyme, producing 83% and 52% total conversion to product respectively. For both acceptors, not only were the anticipated disaccharide products observed at conversions of 41% (12) and 22% (14) respectively, but also masses consistent with the production of longer Mannan-type glycans. For example, with 12 we observed products consistent with disaccharide (m/z 766, Manβ1,4-6F-GlcNAc-ITag, 41%), trisaccharide (m/z 928, Man2β1,4-6F-GlcNAc-ITag, 41%) and tetrasaccharide (m/z 1090, Man3β1,4-6F-GlcNAc-ITag, 1%) formation (Fig. S12†). With 14, in addition to the expected disaccharide (m/z 820, Manβ1,4-6F-GlcNTFA-ITag, 22%) we observed trisaccharide (m/z 982, Man2β1,4-6F-GlcNTFA-ITag, 19%), tetrasaccharide (m/z 1144, Man3β1,4-6F-GlcNTFA-ITag, 9%) and pentasaccharide (m/z 1306, Man4β1,4-6F-GlcNTFA-ITag, 2%, Fig. S14†). In contrast, only low levels of conversion of 6,6-diF-GlcNTFA 13 to disaccharide (2%) was observed (Fig. S13†). Taken together, this data indicates BT1033 can tolerate acceptors with fluorination at C6 position and within the NAc substituent. Increasing the number of fluorines at C6 in the acceptor resulted in poorer turnover by BT1033, with such presence in carbohydrate substrates previously shown to reduce the catalytic efficiency of some enzymes.19 However, the presence of a single fluorine in the acceptor interestingly appeared to facilitate the formation of longer glycans by BT1033, when compared to GlcNAc-N311. We hypothesized that fluorination in the acceptors may reduce the rate of the competing phosphorolysis reaction, altering the reaction equilibrium and resulting in an accumulation of the reverse phosphorolysis disaccharide product, which could subsequently serve as an acceptor for further mannosylation using 2. To investigate this further, we tested BT1033 for activity with a 4-SH-GlcNAc-N3 analogue 33 and compared this to its activity towards 11 under the same conditions (Fig. 3). We anticipated that the reaction would yield a Manβ1,4-S-GlcNAc-N334 thioglycoside (Fig. 3A). Thioglycosides are carbohydrate mimetics that are often resistant to hydrolysis and have elicited significant interest in recent years as probes for structural and biological studies, and as enzyme inhibitors.20,21 We hypothesized that if BT1033 was able to use a thiol as an acceptor with 2 (in the synthetic direction) the reaction may become irreversible due to the stability of the resultant thioglycoside to phosphorolysis. Following LC-MS analysis of reactions with 33 under disulfide reducing conditions, we observed masses consistent with the expected disaccharide (m/z 780, Manβ1,4-S-GlcNAc-ITag), as well as trisaccharide (m/z 942, Man2β1,4-S-GlcNAc-ITag) and tetrasaccharide (m/z 1104, Man3β1,4-S-GlcNAc-ITag) formation (Fig. 3B). Overall, there was a greater proportion of reverse phosphorolysis product at the end of the reaction using 33, compared with 11 (Fig. 3C).
Using 11, we observed mostly acceptor (61%), some disaccharide (26%) and trisaccharide (11%), and low-level tetrasaccharide (1%). Comparatively, for 33 the majority of the product observed was disaccharide (61%), with some trisaccharide (25%) and tetrasaccharide (1%). These findings are consistent with the accumulation of phosphorolysis resistant Manβ1,4-S-GlcNAc-N334. Although the specific activity of BT1033 towards GlcNAc-N311 (3.20 μmol min−1 mg−1) was ∼2-fold higher than towards SH-GlcNAc-N333 (1.59 μmol min−1 mg−1), enzyme titration curves with the respective acceptors highlight the beneficial effect that the thiol has on the final conversion to product in the competing phosphorolysis reaction, with near full conversion of SH-GlcNAc-N333 achieved in 30 min, with only a 3-fold excess of Man-1P donor relative to acceptor and 0.33 mg mL−1 of enzyme (Fig. S35A†). In contrast, under the same conditions only ∼20% conversion of GlcNAc-N311 was observed, while ∼ 40% was observed with 0.04 mg mL−1 of enzyme (Fig. S35B†).
To further showcase the utility of BT1033 for chemoenzymatic β-mannosylation we assembled a library of unnatural azidopropyl-linked Manβ1,4-GlcNAc glycans on a semi-preparative scale, including thioglycoside di, tri and tetrasaccharides (34–36) and fluorinated di, tri, tetra and pentasaccharides (25–27, 37), in isolated yields ranging from 5% to 68%, (Table 1, ESI Section 8†). The structures of synthesised glycans were validated by 1D and 2D NMR and HRMS (ESI Sections 8 and 9†), with β-glycosidic linkages confirmed using IPAP HSQC, which measures each anomeric carbons 1JCH coupling constant (Tables S3 and S4†).22 Although similar trends were observed the isolated product yields differed from the relative conversions measured by MS, which is likely a reflection of the change in scale and challenges associated with purification of longer oligosaccharides. To validate BT1033 was able to operate irreversibly as a “reverse thiophosphorylase” we investigated the stability of our purified glycan library to BT1033 catalysed phosphorolysis (Fig. 4). As expected Manβ1,4-GlcNAc 15 underwent rapid phosphorolysis, with ∼50% breakdown to acceptor 11 observed after 2 min and ∼65% after 24 h. Intriguingly, although Manβ1,4-6F-GlcNAc 25 showed a greater proportion of phosphorolysis over 24 h compared to 15 (78% vs. 70% breakdown to acceptor respectively), a lower amount of phosphorolysis was observed at 2 min (15% vs. 50% breakdown to acceptor respectively). This slower rate of phosphorolysis may therefore account for the observed formation of C6-fluorinated tri-, tetra-, and pentasaccharide by reverse phosphorolysis. The presence of the 6F-GlcNAc moiety appeared to have minimal effect on breakdown of fluorinated trisaccharide 26 to disaccharide 25, when compared to trisaccharide 16, which contains the natural GlcNAc moiety. Notably, 6Cl-Man β1,4-GlcNAc 18 also showed a much lower proportion (∼20%) of phosphorolysis-mediated product after 24 h compared to 15 (∼65%). Again, potentially accounting for the accumulation of 18 in the reverse phosphorolysis reaction when using 4. As hypothesised Manβ1,4-S-GlcNAc 34 proved resistant to phosphorolysis, with no cleavage of the thioglycoside observed after 24 h indicating that the replacement of the alcohol nucleophile with a thiol in the acceptor does enable the phosphorolase to operate irreversibly in the synthetic direction. However, the presence of the Manβ1,4-S-GlcNAc thioglycoside linkage appears to have no effect on the extent of phosphorolysis of trisaccharide 35 to thioglycoside 34, compared to the natural trisaccharide 16, similar to observations for 26. Tetrasaccharides containing the 6F functionality 27 and the thioglycoside linkage 36 respectively, were subjected to phosphorolysis and showed the expected breakdown to trisaccharide after 24 h. Whilst the phosphorolysis of 27 afforded a distribution of products (from acceptor to even longer glycans, indicating reverse phosphorolysis was occurring), the reaction with 36 halted as disaccharide accumulated due to the stability of the thioglycoside linkage. Finally, the 6F pentasaccharide 37, similarly to 27, afforded a distribution of products from acceptor to hexasaccharide, indicative of reverse phosphorolysis having occurred.
| Donor | Acceptor | Product | Yield (%) | Amount (mg) |
|---|---|---|---|---|
| 4 | 11 | 6Cl-Manβ1,4-GlcNAc 18 | 68 | 2.6 |
| 2 | 11 | Manβ1,4-GlcNAc-N315 | 56 | 6.5 |
| Manβ1,4-Manβ1,4-GlcNAc-N316 | 15 | 2.4 | ||
| 2 | 12 | Manβ1,4-6F-GlcNAc-N325 | 12 | 1.4 |
| Manβ1,4-Manβ1,4-6F-GlcNAc-N326 | 13 | 2.1 | ||
| Manβ1,4-Manβ1,4-Manβ1,4-6F-GlcNAc-N327 | 7 | 1.4 | ||
| Manβ1,4-Manβ1,4-Manβ1,4-Manβ1,4-6F-GlcNAc-N337 | 5 | 1.3 | ||
| 2 | 33 | Manβ1,4-S-GlcNAc-N334 | 20 | 3.8 |
| Manβ1,4-Manβ1,4-S-GlcNAc-N335 | 23 | 5.9 | ||
| Manβ1,4-Manβ1,4-Manβ1,4-S-GlcNAc-N336 | 11 | 3.4 |
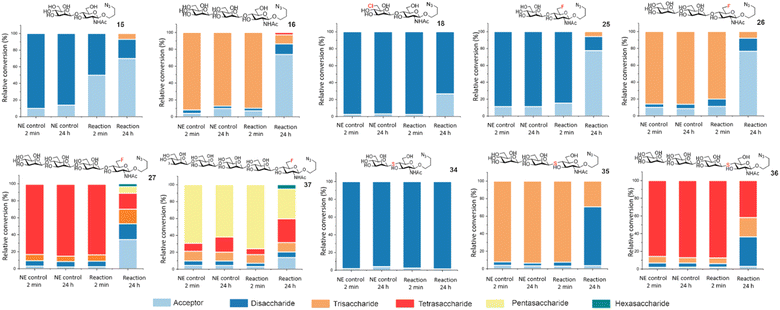 | ||
| Fig. 4 Phosphorolysis of Manβ1,4-GlcNAc-N3 analogues, with relative conversions determined by ITag LC-MS analysis. NE denotes: no enzyme control. | ||
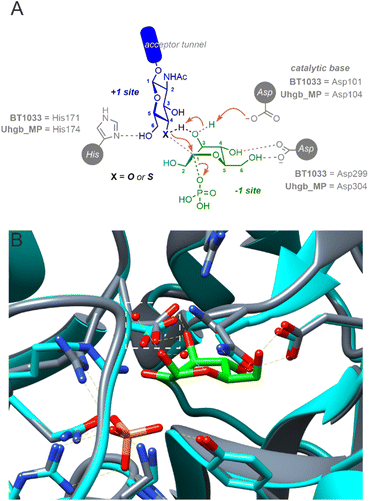
BT1033 belongs to the GH130 enzyme family, which includes β-mannoside phosphorylases MGP (4-O-β-D-mannosyl-D-glucose phosphorylase) and Uhgb_MP (β-1,4-mannosyl-N-glycan phosphorylase) from Bacteroides sp.13,23,24 Guided by structural studies of MGP and Uhgb_MP, GH130 catalysis is proposed to proceed through a “proton shuttle” mechanism (Fig. 5A). For the synthetic reaction, it is hypothesised that mannose in the −1 subsite, existing in an unstable B2,5 boat conformation, is deprotonated by a catalytic Asp residue (Asp131 in MGP or Asp104 in Uhgb_MP) at the 3-OH which subsequently deprotonates the incoming GlcNAc acceptor via its 3-OH group.23,25 Amino acid sequence alignment of BT1033 and Uhgb_MP, identified Asp101 as the putative catalytic residue in BT1033 (Fig. S36†). Superimposition of a BT1033 alphafold model with the structure of Uhgb_MP in complex with β-D-mannose and phosphate, showed that BT1033 Asp101 overlayed with Uhgb_MP Asp104, supporting this hypothesis (Fig. 5B). To reinforce this proposed role of Asp101 in BT1033 catalysis, we also produced a BT1033 D101A mutant and investigated the synthetic activity of the enzyme with both GlcNAc-N311 and SH-GlcNAc-N333, using the natural Man-1P 2 donor (Fig. S23†). As anticipated, BT1033 D101A displayed no activity towards GlcNAc-N311, confirming that this residue is required for catalysis. Interestingly, no activity towards SH-GlcNAc-N333 was observed either implying that despite the lower pKa of the thiol acceptor, deprotonation of the incoming nucleophile within the active site is still required. Previously the enzymatic synthesis of diverse thioglycosides using “thioglycoligases”, glycosidase mutants with their catalytic acid/base residues mutated to an alanine or glycine, have been achieved and extensively explored by the Withers group26 and others.20,27 In contrast to thioglycoligases, we demonstrate here that the reverse thiophosphorylase activity of BT1033 is abolished in in the absence of the catalytic base, thus suggesting that a deeper understanding of the proposed GH130 ‘proton shuttle’ mechanism may be required to aid the design of more efficient reverse thiophosphorylases.
In summary, we have demonstrated that BT1033 can be exploited to access diverse Manβ1,4-GlcNAc analogues, and longer β-mannan like glycans. We also establish novel reverse thiophosphorylase activity favouring the synthesis of longer glycans by initially catalysing the formation of a stable thioglycoside linkage. Following incorporation of unnatural functionality into the enzymatic building blocks through chemical synthesis, we systematically screened BT1033 for activity towards these unnatural donors and acceptors in a MS-based strategy using a “clickable” ITag to facilitate product ionisation and detection. BT1033 displayed activity towards C6-modified donors, most notably 6Cl-Man-1P 4. Fluorinated acceptors were also turned over by the enzyme, and interestingly the presence of the fluorine appears to also facilitate extension of Manβ1,4-GlcNAc with Man to produce longer β-mannan like glycans, likely through slowing the rate of phosphorolysis. Whilst enzymatic strategies for the synthesis of thioglycosides to date have focused on the exploitation of “thioglycoligases”,20,26,27 to our knowledge the use of a wildtype GP to synthesise thioglycosides has not been explored. If this “reverse thiophosphorylase” activity was generally applicable to other GH130 phosphorylases, it could provide simple yet dynamic access to a diverse range of thioglycosides. As Manβ1,4GlcNAc has shown potential as a immune activator,10 the thioglycoside products of the reverse thiophosphorylase activity of BT1033 could have potential as non-hydrolysable β-mannose containing activators for immunotherapy. Furthermore, extension of the reverse thiophosphorylase approach to thiol substituted sugar-1P donors, could have potential utility in the construction of thiooligosaccharide homopolymers.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
TK and NH screened donors and acceptors and performed chemoenzymatic synthesis. TK performed phosphorolysis experiments. TK, NH and JW performed protein production. JP, AW, SA, DW JBV and MG performed the chemical synthesis. MAF, GJM, CG and BL supervised the project. TK, NH, GM and MAF wrote the manuscript and designed the study. All authors analysed the data and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
MAF thanks UKRI (EP/X023680/1) for project grant funding. UK Research and Innovation (UKRI, Future Leaders Fellow-ship, MR/T019522/1) and the Engineering and Physical Sciences Research Council (EP/P000762/1) are thanked for project grant funding to GJM. Keele University are thanked for PhD studentship funding to AW and JP. BL thanks EPSRC core capability funding EP/K039466/1. MCG thanks Cancer Research UK (grant number C30758/A2979) and European Research Council (ERC-COG GLYCOTOOLS 648239). MAF, BL and MCG are grateful to the Industrial Biotechnology Catalyst (Innovate UK, BBSRC, EPSRC) to support the translation, development and commercialization of innovative Industrial Biotechnology processes: BB/M028941/1, BB/M02847X/1, BB/M028976/1. We thank Dr Ed Bergstrom and The York Center of Excellence in Mass Spectrometry was created thanks to a major capital investment through Science City York, supported by Yorkshire Forward with funds from the Northern Way Initiative, and subsequent support from EPSRC (EP/K039660/1; EP/M028127/1). We thank Dr Alex Heyam for discussions around the IPAP NMR experiments.References
- H. Nakai, M. Kitaoka, B. Svensson and K. i. Ohtsubo, Recent development of phosphorylases possessing large potential for oligosaccharide synthesis, Curr. Opin. Chem. Biol., 2013, 17(2), 301–309 CrossRef CAS PubMed.
- H. Yu, V. Thon, K. Lau, L. Cai, Y. Chen, S. Mu, Y. Li, P. G. Wang and X. Chen, Highly efficient chemoenzymatic synthesis of β1–3-linked galactosides, Chem. Commun., 2010, 46(40), 7507–7509 RSC; L. Li, Y. Liu, T. Li, W. Wang, Z. Yu, C. Ma, J. Qu, W. Zhao, X. Chen and P. G. Wang, Efficient chemoenzymatic synthesis of novel galacto-N-biose derivatives and their sialylated forms, Chem. Commun., 2015, 51(51), 10310–10313 RSC; J. Yan, X. Chen, F. Wang and H. Cao, Chemoenzymatic synthesis of mono-and di-fluorinated Thomsen–Friedenreich (T) antigens and their sialylated derivatives, Org. Biomol. Chem., 2013, 11(5), 842–848 RSC; P. de Andrade, J. C. Muñoz-García, G. Pergolizzi, V. Gabrielli, S. A. Nepogodiev, D. Iuga, L. Fabian, R. Nigmatullin, M. A. Johns and R. Harniman, Chemoenzymatic synthesis of fluorinated cellodextrins identifies a new allomorph for cellulose-like materials, Chem.–Eur. J., 2021, 27(4), 1374–1382 CrossRef CAS PubMed; S. S. Macdonald, Z. Armstrong, C. Morgan-Lang, M. Osowiecka, K. Robinson, S. J. Hallam and S. G. Withers, Development and application of a high-throughput functional metagenomic screen for glycoside phosphorylases, Cell Chem. Biol., 2019, 26(7), 1001–1012 CrossRef PubMed; S. S. Macdonald, J. H. Pereira, F. Liu, G. Tegl, A. DeGiovanni, J. F. Wardman, S. Deutsch, Y. Yoshikuni, P. D. Adams and S. G. Withers, A Synthetic Gene Library Yields a Previously Unknown Glycoside Phosphorylase That Degrades and Assembles Poly-β-1, 3-GlcNAc, Completing the Suite of β-Linked GlcNAc Polysaccharides, ACS Cent. Sci., 2022, 8(4), 430–440 CrossRef PubMed.
- S.-J. Richards, T. Keenan, J.-B. Vendeville, D. E. Wheatley, H. Chidwick, D. Budhadev, C. E. Council, C. S. Webster, H. Ledru and A. N. Baker, Introducing affinity and selectivity into galectin-targeting nanoparticles with fluorinated glycan ligands, Chem. Sci., 2021, 12(3), 905–910 RSC.
- S. Ladeveze, E. Laville, J. Despres, P. Mosoni and G. Potocki-Véronèse, Mannoside recognition and degradation by bacteria, Biol. Rev., 2017, 92(4), 1969–1990 CrossRef CAS PubMed; G. O. Aspinall, The polysaccharides, Academic Press, 2014 CrossRef PubMed; R. Schröder, R. G. Atkinson and R. J. Redgwell, Re-interpreting the role of endo-β-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall, Ann. Bot., 2009, 104(2), 197–204 CrossRef PubMed; E. Fabre, T. Hurtaux and C. Fradin, Mannosylation of fungal glycoconjugates in the Golgi apparatus, Curr. Opin. Microbiol., 2014, 20, 103–110 CrossRef PubMed.
- K. Zhang and S. M. Beverley, Mannogen-ing central carbon metabolism by Leishmania, Trends Parasitol., 2019, 35(12), 947–949 CrossRef CAS PubMed.
- E. Román, I. Correia, A. Salazin, C. Fradin, T. Jouault, D. Poulain, F.-T. Liu and J. Pla, The Cek1-mediated MAP kinase pathway regulates exposure of α-1, 2 and β-1, 2-mannosides in the cell wall of Candida albicans modulating immune recognition, Virulence, 2016, 7(5), 558–577 CrossRef CAS PubMed; N. Shibata, T. Ichikawa, M. Tojo, M. Takahashi, N. Ito, Y. Okubo and S. Suzuki, Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide, Arch. Biochem. Biophys., 1985, 243(2), 338–348 CrossRef PubMed; D. S. Domozych, M. Ciancia, J. U. Fangel, M. D. Mikkelsen, P. Ulvskov and W. G. Willats, The cell walls of green algae: a journey through evolution and diversity, Front. Plant Sci., 2012, 3, 82 Search PubMed; D. Passos da Silva, M. L. Matwichuk, D. O. Townsend, C. Reichhardt, D. Lamba, D. J. Wozniak and M. R. Parsek, The Pseudomonas aeruginosa lectin LecB binds to the exopolysaccharide Psl and stabilizes the biofilm matrix, Nat. Commun., 2019, 10(1), 1–11 CrossRef PubMed.
- P. Stanley, K. W. Moremen, N. E. Lewis, N. Taniguchi and M. Aebi, N-Glycans – Essentials of Glycobiology, 4th edn, 2022 Search PubMed.
- C. S. Fermaintt, K. Sano, Z. Liu, N. Ishii, J. Seino, N. Dobbs, T. Suzuki, Y.-X. Fu, M. A. Lehrman and I. Matsuo, A bioactive mammalian disaccharide associated with autoimmunity activates STING-TBK1-dependent immune response, Nat. Commun., 2019, 10(1), 1–12 CrossRef CAS PubMed.
- M. Hasan, C. S. Fermaintt, N. Gao, T. Sakai, T. Miyazaki, S. Jiang, Q.-Z. Li, J. P. Atkinson, H. C. Morse III and M. A. Lehrman, Cytosolic nuclease TREX1 regulates oligosaccharyltransferase activity independent of nuclease activity to suppress immune activation, Immunity, 2015, 43(3), 463–474 CrossRef CAS PubMed.
- H. Ishikawa and G. N. Barber, STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling, Nature, 2008, 455(7213), 674–678 CrossRef CAS PubMed; D. L. Burdette, K. M. Monroe, K. Sotelo-Troha, J. S. Iwig, B. Eckert, M. Hyodo, Y. Hayakawa and R. E. Vance, STING is a direct innate immune sensor of cyclic di-GMP, Nature, 2011, 478(7370), 515–518 CrossRef PubMed.
- M. Jiang, P. Chen, L. Wang, W. Li, B. Chen, Y. Liu, H. Wang, S. Zhao, L. Ye and Y. He, cGAS-STING, an important pathway in cancer immunotherapy, J. Hematol. Oncol., 2020, 13(1), 1–11 CrossRef CAS PubMed; M. Luo, H. Wang, Z. Wang, H. Cai, Z. Lu, Y. Li, M. Du, G. Huang, C. Wang and X. Chen, A STING-activating nanovaccine for cancer immunotherapy, Nat. Nanotechnol., 2017, 12(7), 648–654 CrossRef PubMed.
- I. Pongener, D. A. Pepe, J. J. Ruddy and E. M. McGarrigle, Stereoselective β-mannosylations and β-rhamnosylations from glycosyl hemiacetals mediated by lithium iodide, Chem. Sci., 2021, 12(29), 10070–10075 RSC; D. Crich and H. M. Li, Direct stereoselective synthesis of beta-thiomannoside, J. Org. Chem., 2000, 65(3), 801–805, DOI:10.1021/jo9914667; M. Heuckendorff, J. Bendix, C. M. Pedersen and M. Bols, beta-Selective Mannosylation with a 4,6-Silyiene-Tethered Thiomannosyl Donor, Org. Lett., 2014, 16(4), 1116–1119, DOI:10.1021/ol403722f; M. Heuckendorff, P. S. Bols, C. B. Barry, T. G. Frihed, C. M. Pedersen and M. Bols, beta-Mannosylation with 4,6-benzylidene protected mannosyl donors without preactivation, Chem. Commun., 2015, 51(68), 13283–13285, 10.1039/c5cc04716a; Y. Park, K. C. Harper, N. Kuhl, E. E. Kwan, R. Y. Liu and E. N. Jacobsen, Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions, Science, 2017, 355(6321), 162–166 CrossRef PubMed; Q. Li, S. M. Levi and E. N. Jacobsen, Highly selective β-mannosylations and β-rhamnosylations catalyzed by bis-thiourea, J. Am. Chem. Soc., 2020, 142(27), 11865–11872 CrossRef PubMed; A. Ishiwata, K. Tanaka, J. Ao, F. Ding and Y. Ito, Recent advances in stereoselective 1,2-cis-O-glycosylations, Front. Chem., 2022, 10, 972429 CrossRef PubMed; C. Alex and A. V. Demchenko, Recent Advances in Stereocontrolled Mannosylation: Focus on Glycans Comprising Acidic and/or Amino Sugars, Chem. Rec., 2021, 21(11), 3278–3294 CrossRef PubMed; D. Crich and O. Vinogradova, On the influence of the C2-O2 and C3-O3 bonds in 4,6-O-benzylidene-directed beta-mannopyranosylation and alpha-glucopyranosylation, J. Org. Chem., 2006, 71(22), 8473–8480, DOI:10.1021/jo061417b; D. Crich, M. de la Mora and A. U. Vinod, Influence of the 4,6-O-benzylidene, 4,6-O-phenylboronate, and 4,6-O-polystyrylboronate protecting groups on the stereochemical outcome of thioglycoside-based glycosylations mediated by 1-benzenesulfinyl piperidine/triflic anhydride and N-iodosuccinimide/trimethylsilyl triflate, J. Org. Chem., 2003, 68(21), 8142–8148, DOI:10.1021/jo0349882.
- T. Nihira, E. Suzuki, M. Kitaoka, M. Nishimoto, K. i. Ohtsubo and H. Nakai, Discovery of β-1, 4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase involved in the metabolism of N-glycans, J. Biol. Chem., 2013, 288(38), 27366–27374 CrossRef CAS PubMed.
- A. Li, M. Benkoulouche, S. Ladeveze, J. Durand, G. Cioci, E. Laville and G. Potocki-Veronese, Discovery and biotechnological exploitation of glycoside-phosphorylases, Int. J. Mol. Sci., 2022, 23(6), 3043 CrossRef CAS PubMed.
- S. Ahmadipour, G. Pergolizzi, M. Rejzek, R. A. Field and G. J. Miller, Chemoenzymatic Synthesis of C6-Modified Sugar Nucleotides to Probe the GDP-D-Mannose Dehydrogenase from Pseudomonas aeruginosa, Org. Lett., 2019, 21(12), 4415–4419 CrossRef CAS PubMed; S. Ahmadipour, A. J. Wahart, J. P. Dolan, L. Beswick, C. S. Hawes, R. A. Field and G. J. Miller, Synthesis of C6-modified mannose 1-phosphates and evaluation of derived sugar nucleotides against GDP-mannose dehydrogenase, Beilstein J. Org. Chem., 2022, 18(1), 1379–1384 CrossRef PubMed; L. Beswick, E. Dimitriou, S. Ahmadipour, A. Zafar, M. Rejzek, J. Reynisson, R. A. Field and G. J. Miller, Inhibition of the GDP-d-Mannose Dehydrogenase from Pseudomonas aeruginosa Using Targeted Sugar Nucleotide Probes, ACS Chem. Biol., 2020, 15(12), 3086–3092 CrossRef PubMed; L. Beswick, S. Ahmadipour, G.-J. Hofman, H. Wootton, E. Dimitriou, J. Reynisson, R. A. Field, B. Linclau and G. J. Miller, Exploring anomeric glycosylation of phosphoric acid: Optimisation and scope for non-native substrates, Carbohydr. Res., 2020, 488 Search PubMed.
- M. C. Galan, A. T. Tran and C. Bernard, Ionic-liquid-based catch and release mass spectroscopy tags for enzyme monitoring, Chem. Commun., 2010, 46(47), 8968–8970 RSC; M. Ghirardello, Y.-Y. Zhang, J. Voglmeir and M. C. Galan, Recent applications of ionic liquid-based tags in glycoscience, Carbohydr. Res., 2022, 520, 108643 CrossRef CAS PubMed; Y.-Y. Zhang, M. Ghirardello, T. Wang, A.-M. Lu, L. Liu, J. Voglmeir and M. C. Galan, Imidazolium labelling permits the sensitive mass-spectrometric detection of N-glycosides directly from serum, Chem. Commun., 2021, 57(57), 7003–7006 RSC; B. Calle, G. Bineva-Todd, A. Marchesi, H. Flynn, M. Ghirardello, O. Y. Tastan, C. Roustan, J. Choi, M. C. Galan and B. Schumann, Benefits of chemical sugar modifications introduced by click chemistry for glycoproteomic analyses, J. Am. Soc. Mass Spectrom., 2021, 32(9), 2366–2375 CrossRef PubMed.
- S. G. Withers, I. P. Street and S. J. Rettig, The preferred conformation of 2-fluoro-2-deoxy β-D-mannopyranosyl fluoride. An X-ray crystallographic and 2-dimensional proton nuclear magnetic resonance study, Can. J. Chem., 1986, 64(2), 232–236 CrossRef CAS; B. Linclau, S. Golten, M. Light, M. Sebban and H. Oulyadi, The conformation of tetrafluorinated methyl galactoside anomers: crystallographic and NMR studies, Carbohydr. Res., 2011, 346(9), 1129–1139 CrossRef PubMed.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, Applications of fluorine in medicinal chemistry, J. Med. Chem., 2015, 58(21), 8315–8359 CrossRef CAS PubMed; E. Durantie, C. Bucher and R. Gilmour, Fluorine-Directed β-Galactosylation: Chemical Glycosylation Development by Molecular Editing, Chem.–Eur. J., 2012, 18(26), 8208–8215 CrossRef PubMed; C. E. Council, K. J. Kilpin, J. S. Gusthart, S. A. Allman, B. Linclau and S. S. Lee, Enzymatic glycosylation involving fluorinated carbohydrates, Org. Biomol. Chem., 2020, 18(18), 3423–3451 RSC; B. Linclau, A. Ardá, N.-C. Reichardt, M. Sollogoub, L. Unione, S. P. Vincent and J. Jiménez-Barbero, Fluorinated carbohydrates as chemical probes for molecular recognition studies. Current status and perspectives, Chem. Soc. Rev., 2020, 49(12), 3863–3888 RSC.
- T. Keenan, F. Parmeggiani, J. Malassis, C. Q. Fontenelle, J.-B. Vendeville, W. Offen, P. Both, K. Huang, A. Marchesi and A. Heyam, Profiling substrate promiscuity of wild-type sugar kinases for multi-fluorinated monosaccharides, Cell Chem. Biol., 2020, 27(9), 1199–1206 CrossRef CAS PubMed; J.-S. Zhu, N. E. McCormick, S. C. Timmons and D. L. Jakeman, Synthesis of α-deoxymono and difluorohexopyranosyl 1-phosphates and kinetic evaluation with thymidylyl-and guanidylyltransferases, J. Org. Chem., 2016, 81(19), 8816–8825 CrossRef PubMed; S. S. Lee, S. Y. Hong, J. C. Errey, A. Izumi, G. J. Davies and B. G. Davis, Mechanistic evidence for a front-side, SNi-type reaction in a retaining glycosyltransferase, Nat. Chem. Biol., 2011, 7(9), 631–638 CrossRef PubMed.
- C. Peyrot, B. Didak, L. Guillotin, L. Landemarre, P. Lafite, L. Lemiegre and R. Daniellou, Enzymatic synthesis of a series of thioglycosides: Analogs of arbutin with efficient antipigmentation properties, Eur. J. Org Chem., 2021, 2021(27), 3812–3818 CrossRef CAS.
- O. Norberg, B. Wu, N. Thota, J.-T. Ge, G. Fauquet, A.-K. Saur, T. Aastrup, H. Dong, M. Yan and O. Ramström, Synthesis and binding affinity analysis of α1-2-and α1-6-O/S-linked dimannosides for the elucidation of sulfur in glycosidic bonds using quartz crystal microbalance sensors, Carbohydr. Res., 2017, 452, 35–42 CrossRef CAS PubMed; A. J. Thompson, G. Speciale, J. Iglesias-Fernández, Z. Hakki, T. Belz, A. Cartmell, R. J. Spears, E. Chandler, M. J. Temple and J. Stepper, Evidence for a Boat Conformation at the Transition State of GH76 α-1, 6-Mannanases—Key Enzymes in Bacterial and Fungal Mannoprotein Metabolism, Angew. Chem., Int. Ed., 2015, 54(18), 5378–5382 CrossRef PubMed; C.-X. Huo, X.-J. Zheng, A. Xiao, C.-C. Liu, S. Sun, Z. Lv and X.-S. Ye, Synthetic and immunological studies of N-acyl modified S-linked STn derivatives as anticancer vaccine candidates, Org. Biomol. Chem., 2015, 13(12), 3677–3690 RSC; L. Zhang, C. Mc Carthy and X. Zhu, Synthesis of a glucosylated α-S-galactosylceramide as potential immunostimulant, Carbohydr. Res., 2017, 448, 43–47 CrossRef PubMed; C. V. Garcia De Gonzalo, L. Zhu, T. J. Oman and W. A. Van Der Donk, NMR structure of the S-linked glycopeptide sublancin 168, ACS Chem. Biol., 2014, 9(3), 796–801 CrossRef PubMed; J. Rodrigue, G. Ganne, B. Blanchard, C. Saucier, D. Giguère, T. C. Shiao, A. Varrot, A. Imberty and R. Roy, Aromatic thioglycoside inhibitors against the virulence factor LecA from Pseudomonas aeruginosa, Org. Biomol. Chem., 2013, 11(40), 6906–6918 RSC; M. C. Meneghetti, L. Naughton, C. O'Shea, D. S.-E. Koffi Teki, V. Chagnault, H. B. Nader, T. R. Rudd, E. A. Yates, J. Kovensky and G. J. Miller, Using NMR to Dissect the Chemical Space and O-Sulfation Effects within the O-and S-Glycoside Analogues of Heparan Sulfate, ACS Omega, 2022, 7(28), 24461–24467 CrossRef PubMed.
- I. Timári, L. Kaltschnee, A. Kolmer, R. W. Adams, M. Nilsson, C. M. Thiele, G. A. Morris and K. E. Kövér, Accurate determination of one-bond heteronuclear coupling constants with “pure shift” broadband proton-decoupled CLIP/CLAP-HSQC experiments, J. Magn. Reson., 2014, 239, 130–138 CrossRef PubMed.
- S. Nakae, S. Ito, M. Higa, T. Senoura, J. Wasaki, A. Hijikata, M. Shionyu, S. Ito and T. Shirai, Structure of novel enzyme in mannan biodegradation process 4-O-β-D-mannosyl-D-glucose phosphorylase MGP, J. Mol. Biol., 2013, 425(22), 4468–4478 CrossRef CAS PubMed.
- S. Ladeveze, L. Tarquis, D. A. Cecchini, J. Bercovici, I. André, C. M. Topham, S. Morel, E. Laville, P. Monsan and V. Lombard, Role of glycoside phosphorylases in mannose foraging by human gut bacteria, J. Biol. Chem., 2013, 288(45), 32370–32383 CrossRef CAS PubMed.
- S. Ladeveze, G. Cioci, P. Roblin, L. Mourey, S. Tranier and G. Potocki-Véronèse, Structural bases for N-glycan processing by mannoside phosphorylase, Acta Crystallographica Section D: Biological Crystallography, 2015, 71(6), 1335–1346 CrossRef CAS PubMed.
- G. Tegl, J. Hanson, H. M. Chen, D. H. Kwan, A. G. Santana and S. G. Withers, Facile Formation of β-thioGlcNAc Linkages to Thiol-Containing Sugars, Peptides, and Proteins using a Mutant GH20 Hexosaminidase, Angew. Chem., 2019, 131(6), 1646–1651 CrossRef CAS; M. Jahn, J. Marles, R. A. J. Warren and S. G. Withers, Thioglycoligases: mutant glycosidases for thioglycoside synthesis, Angew. Chem., Int. Ed., 2003, 42(3), 352–354 CrossRef PubMed; Y. W. Kim, H. M. Chen, J. H. Kim, J. Müllegger, D. Mahuran and S. G. Withers, Thioglycoligase-Based Assembly of Thiodisaccharides: Screening as β-Galactosidase Inhibitors, ChemBioChem, 2007, 8(13), 1495–1499 CrossRef PubMed; Y.-W. Kim, A. L. Lovering, H. Chen, T. Kantner, L. P. McIntosh, N. C. Strynadka and S. G. Withers, Expanding the thioglycoligase strategy to the synthesis of α-linked thioglycosides allows structural investigation of the parent enzyme/substrate complex, J. Am. Chem. Soc., 2006, 128(7), 2202–2203 CrossRef PubMed.
- C. Li, J.-H. Kim and Y.-W. Kim, α-Thioglycoligase-based synthesis of O-aryl α-glycosides as chromogenic substrates for α-glycosidases, J. Mol. Catal. B: Enzym., 2013, 87, 24–29 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, chemical synthesis, chemoenzymatic synthesis, LC-MS and NMR data (PDF). See DOI: https://doi.org/10.1039/d3sc04169g |
| ‡ TK and NH contributed equally. |
| § Present address: Department of Organic and Macromolecular Chemistry, Ghent University, Campus Sterre, Krijgslaan 281-S4, Ghent, 9000, Belgium. |
| This journal is © The Royal Society of Chemistry 2023 |