 Open Access Article
Open Access ArticleRational molecular and doping strategies to obtain organic polymers with ultralong RTP†
Yuefa
Zhang
,
Shiguo
Zhang
,
Guanyu
Liu
,
Qikun
Sun
,
Shanfeng
Xue
 * and
Wenjun
Yang
* and
Wenjun
Yang
 *
*
Key Laboratory of Rubber-plastics of Ministry of Education/Shandong Provincial Key Laboratory of Rubber-plastics, School of Polymer Science & Engineering, Qingdao University of Science &Technology, Qingdao, China
First published on 19th April 2023
Abstract
Organic-doped polymers and room-temperature phosphorescence (RTP) mechanisms have been widely reported. However, RTP lifetimes >3 s are rare and RTP-enhancing strategies are incompletely understood. Herein, we demonstrate a rational molecular doping strategy to obtain ultralong-lived, yet bright RTP polymers. The n–π* transitions of boron- and nitrogen-containing heterocyclic compounds can promote a triplet-state population, and the grafting of boronic acid onto polyvinyl alcohol can inhibit molecular thermal deactivation. However, excellent RTP properties were achieved by grafting 1–0.1% (N-phenylcarbazol-2-yl)-boronic acid rather than (2-/3-/4-(carbazol-9-yl)phenyl)boronic acids to afford record-breaking ultralong RTP lifetimes up to 3.517–4.444 s. These results showed that regulation of the interacting position between the dopant and matrix molecules to directly confine the triplet chromophore could more effectively stabilize triplet excitons, disclosing a rational molecular-doping strategy for achieving polymers with ultralong RTP. Based on the energy-donor function of blue RTP, an ultralong red fluorescent afterglow was demonstrated by co-doping with an organic dye.
Introduction
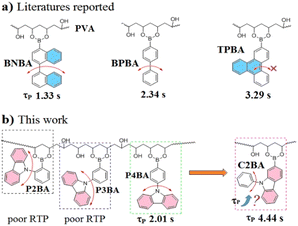
Ultralong and bright room-temperature phosphorescence (RTP) materials have potential applications in optoelectronic devices, bio-imaging, anti-counterfeiting, information storage, and glow-in-the-dark products. Among them, organic-doped RTP polymers are attracting much attention due to their economy, good processability, biocompatibility, and diverse mechanical properties.1–5 Polymers rich in hydrogen bonds such as polyvinyl alcohol (PVA) and poly-acrylamide (PAM) have been widely used as matrices in view of their high rigidity and strong interactions with the polar groups of organic dopant molecules.6–20 Utilizing PVA as a matrix, Huang et al. obtained a RTP lifetime of 3.16 s by doping carbazol-9-ylacetic acid,18 Yang et al. doped triphenylen-2-yl-boronic acid and realized a RTP lifetime of 3.29 s.21 Li et al. doped simple bi- and ter-phenylboronic acids and achieved RTP lifetimes of 2.34–2.43 s.6 Chen et al. co-polymerized 9-vinylcarbazole and acrylamide and realized a RTP lifetime of 4.2 s.19 These are the longest-lived RTP lifetimes obtained so far. Therefore, the number of organic-doped RTP polymers with ultralong lifetimes (>3 s) is limited. Moreover, the effect of comparable-structure dopants and material aggregates on organic RTP properties is incompletely understood.1,20,22–24We are interested in whether RTP lifetimes can be advanced to a higher level and the mechanism of such advancement. Considering the prominent roles played by aromatic boronic acids and carbazole units in ultralong organic-doped RTP polymers, we intended to develop boronic acid-containing N-phenyl-carbazoles as dopants because the coexistence of boron and nitrogen is conducive to inter-system crossing. Recently, we doped 4-(carbazol-9-yl)phenylboronic acid (P4BA) into PVA, and achieved a long component and averaged RTP lifetime of 3.21 and 2.01 s respectively.20 These values are not what we hoped for because P4BA has an extended π system compared with carbazol-9-yl-acetic acid and an additional hetero atom compared with aryl boronic acids (Fig. 1). We noted that carbazol-9-yl-acetic acid did not have an aryl internal rotation unit and, among aryl boronic acids, BNBA had a more distorted internal rotation unit than BPBA, whereas fused TPBA had eliminated aryl internal rotation. These results suggested that inhibiting the internal rotation of a triplet chromophore may be an effective way of achieving ultralong RTP. For N-phenylcarbazole, the boronic-acid unit can be introduced at the phenyl or carbazole rings, and the latter can inhibit the triplet chromophore directly and should improve the RTP properties. However, the RTP properties of N-arylcarbazoles with hetero-atom groups on the carbazole ring in polymer films have not been reported.
Herein, we isomerized boronic acid from phenyl to carbazole and prepared 9-phenylcarbazol-2-ylboronic acid (C2BA) as a dopant. C2BA was dispersed in a PVA matrix using solution-cast films, and then the films were thermoplastic-processed into sheets. Our organic-doped RTP polymers were prepared and characterized in the form of solution-cast films. This should not be the best aggregate state for inhibiting the thermal motion of doped molecules because most polymers usually aggregate together in loose coils according to the theory of how polymer solutions behave. Thermoplastic processing is a more extensive method of obtaining complex products for practical use. Moreover, polymers can be sheared and orientated to form denser stacking structures according to the rheology of polymers during processing, which is conducive to limiting thermal deactivation. Moreover, few conjugated organic dopants are soluble in water or well dispersed in PVA, whereas thermoplastic processing can promote the grafting and uniform dispersion of C2BA in PVA. As a result, trace doping may also achieve excellent RTP properties. Herein, we report that thermoplastic processing boosted RTP afterglows considerably, and that C2BA/PVA was superior to the PVA sheets doped by (2/3/4-(carbazol-9-yl)-phenyl)boronic acids. Notably, C2BA doping (1.0 wt%) in PVA could achieve a RTP lifetime of 3.517 s, and the longest RTP lifetime (up to 4.444 s) was optimized by tuning the doping amount. Our experimental results indicate that ultralong and bright organic RTP depends not only on the material system but also on the molecular and doping strategies, and that direct inhibition of triplet chromophores is conducive to ultralong RTP.
Results and discussion
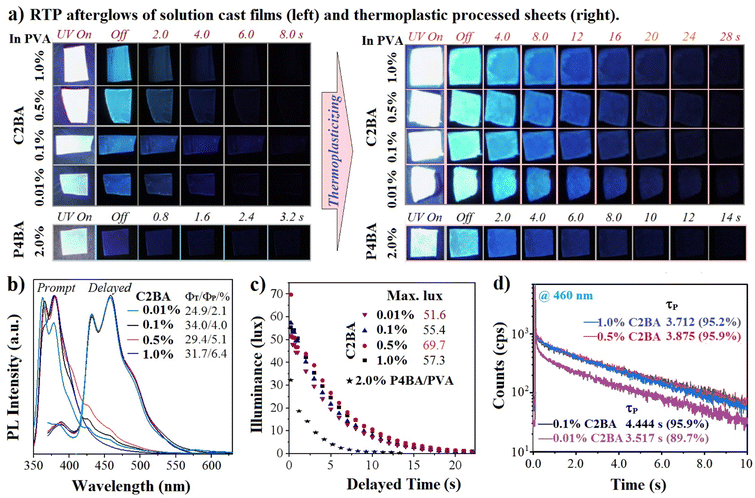
C2BA and (2/3/4-(carbazol-9-yl)phenyl)boronic acids (P2BA, P3BA, and P4BA) are commercially available. They were re-purified by silica-gel column chromatography and re-crystallized before use. These crystals emitted very inferior RTP (Fig. S1, ESI†), which could not be explained by inter-molecular interactions or chemical composition. However, they all emitted RTP in PVA due to changes in molecular-confined environments (Fig. S2†) by negating the deduction of RTP from each other in crystals and polymers. The thermoplastic-processed sheets showed an improved RTP afterglow. P4BA was the best among the three phenylboronic acids-doped PVA, and the RTP lifetime of 0.1 wt% P4BA/PVA sheet was 1.512 s (Fig. S3†). In sharp contrast, C2BA/PVA sheets exhibited significantly boosted RTP afterglows after UV excitation at 365 nm regardless of the large changes in doping weights (1.0–0.1% versus PVA) (Fig. 2a). The steady-state photoluminescence (PL) spectra showed that C2BA/PVA sheets emitted near-UV fluorescence and blue RTP with a maximal wavelength of 382 nm and 459 nm, respectively (Fig. 2b). Overall, the measured PL efficiency (ΦT) by an integrating sphere and the calculated RTP efficiency (ΦP) were reduced with a decrease in the amount of C2BA (inset in Fig. 2b and S4†). As a comparison of relative brightness, afterglow illuminance is measured under identical conditions of preparation and light excitation. C2BA/PVA sheets at 1.0, 0.5, 0.1, and 0.01 wt% showed maximal afterglow illuminance of 57.3, 69.7, 55.4, and 51.6 lux, respectively (Fig. 2c).In contrast, the P4BA/PVA sheet exhibited low afterglow illuminance (23.5 lux). The time-resolved RTP decay curves were monitored, and the fitted long-component RTP lifetimes (τP) were in the range 3.512–4.444 s with high ratios (89.7–95.9%) (Fig. 2d and Table S1†). Also, doping with 0.1% C2BA led to the longest RTP lifetime (up to 4.444 s), and doping at 1.0% could achieve an RTP afterglow with a lifetime of 3.512 s and illuminance of 51.6 lux. Overall, we boosted the RTP lifetime of organic-doped PVA materials to a much higher level.6–10 The relatively low ΦP but ultralong (yet bright) afterglow meant that abundant triplet excitons were mainly stabilized to emit a delayed RTP.
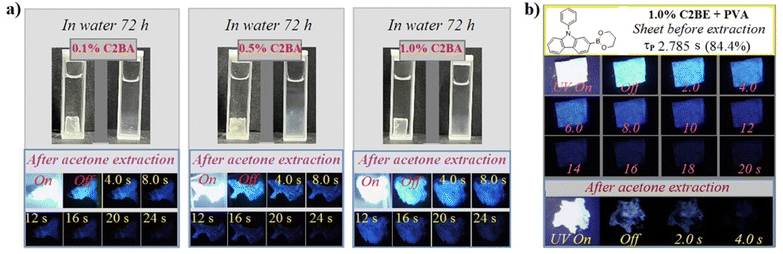
Arylboronic acid-doped PVA is a special system in which PVA can esterify arylboronic acid to form a graft polymer. To verify esterification, a conventional dissolution-extraction experiment was conducted because it should be simpler and more reliable than spectroscopy for trace doping and grafting. Thus, the C2BA/PVA sheet was redissolved in water and extracted with acetone (solvent for C2BA). Fig. 3a shows that C2BA/PVA showed excessive swelling rather than dissolution, possibly due to the heat-induced partial crosslinking of PVA. Importantly, RTP afterglows of dried residues continued to be ultralong, indicating that triplet dopant molecules had not been removed. To examine the effect of grafting on RTP properties further, C2BA was esterified by 1,3-propandiol in advance to form C2BE and then to dope PVA. C2BE/PVA sheets also emitted a commendable RTP afterglow but it was inferior to that of C2BA/PVA (Fig. 3b and S5†). However, the C2BE/PVA residue no longer showed ultralong RTP after dissolution-extraction under an identical procedure. Experimental results verified boron esterification between PVA and C2BA molecules as well as the positive effect of chemical bonding of triplet chromophores onto PVA chains by inhibiting triplet thermal deactivation.
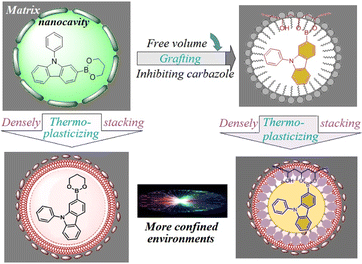
Based on the results stated above, we propose a stabilizing RTP mechanism (Fig. 4). Organic aromatic backbones are weakly compatible with a polymer matrix even if they are dispersed in the matrix at the molecular level by a co-solvent method. A nano cavity is formed and the free volume is increased, which is not conducive to inhibition of molecular thermal motion. Strong intermolecular interactions (especially covalent bonding) can reduce the free volume, and densely stacking the matrix by thermoplastic processing can also have a similar role. If polymers have strong intermolecular interactions with triplet chromophores, the free volume and thermal motions of aromatic backbones should be reduced conspicuously. As a consequence, triplet excitons are stabilized effectively and increasingly radiate decay, thereby enabling ultralong and bright RTP afterglows. In this context, for a conjugated organic molecule, the bonding positions of peripheral polar groups require an appropriate design, and their RTP properties in polymers cannot be evaluated by solution-cast films alone.
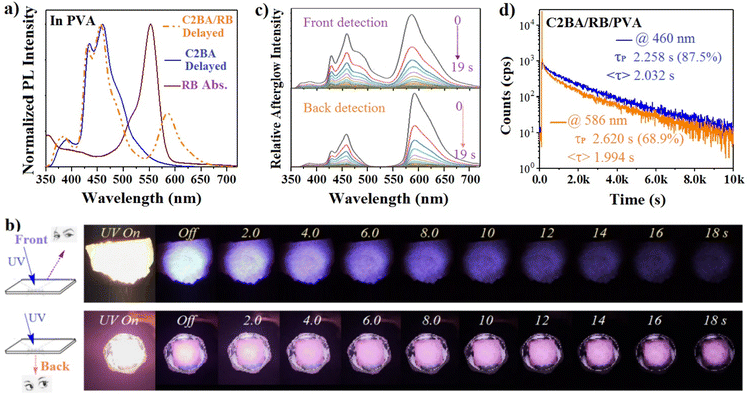
Finally, we demonstrated persistent ultralong blue RTP and red fluorescence dual emission with afterglow lifetimes of >2 s by co-doping C2BA and rhodamine B (RB) into PVA. A very broad absorption spectrum with a main absorption band at ∼550 nm was observed for RB in PVA (Fig. 5a), which overlapped with the RTP emission of C2BA/PVA only in the blue-green region. Therefore, persistent partial-energy transfer contributed to the ultralong mixed-color emission composed of blue RTP and orange fluorescence. When C2BA (1.0 wt%) and RB (0.2 wt%) were doped, the C2BA/RB/PVA sheet (thickness = 1.0 mm) was semi-transparent. The afterglow color viewed by the naked eye from the excitation back was redder than that viewed from the excitation front (Fig. 5b). Hence, more blue afterglow energy was transferred to RB during its propagation in the PVA matrix. The different colors and degree of energy transfer was confirmed by time-dependent charge-coupled device (CCD) spectroscopy (Fig. 5c). It is interesting and rarely reported that different color ultralong afterglows were observed from a sample by viewing from different sides. The measured PL efficiency for C2BA, C2BA/RB and RB in PVA was 31.7%, 32.1% and 23.5%, respectively (Fig. S6†). C2BA/RB/PVA showed much lower PL efficiency than the sum (55.2%) of C2BA/PVA and RB/PVA, which suggested inter-molecular interactions and energy transfer between C2BA and RB.
We measured the time-resolved PL decay curves of C2BA/RB/PVA sheets at 460 nm and 586 nm under light excitation of 365 nm (Fig. 5d). The fitted long-component afterglow lifetime was 2.258 s (87.5%) and 2.620 s (68.9%), respectively, but the average lifetime was almost identical (2.032 s and 1.994 s, respectively), which implied a persistent and proportional energy transfer from the triplet state of C2BA to the singlet state of RB. This triplet-to-singlet energy transfer is thought to follow the Förster theory.25–28 Thus, the efficiency of energy transfer could be calculated according to the equation ΦET = 1 – τD/τD0, where τD and τD0 are the RTP lifetimes of an energy donor (C2BA) in PVA in the presence and absence of an energy acceptor (RB), and introduction of an energy acceptor reduces the RTP lifetime of an energy donor. Based on C2BA (1.0 wt%), the average RTP lifetime of C2BA/PVA and C2BA/RB/PVA was 3.168 s and 2.032 s, respectively, and ΦET was calculated to be 35.8%. A high amount of RB doping could improve ΦET, but afterglow brightness and the lifetime could be reduced markedly due to the strong hiding power and self-absorption of RB.
Conclusions
In summary, we have demonstrated that the RTP properties of organic-doped polymers are determined by the organic dopant, polymer matrix and preparation method of the materials. PVA rich in hydrogen bonds has strong rigidity and cohesion, and can form covalent linkages with arylboronic acid to stabilize the triplet state and achieve ultralong RTP. However, for a given aromatic compound, the introduction positions of polar groups can significantly affect the RTP properties. Our experimental results show that the direct interactions between the triplet chromophore and polymer via strong hydrogen bonding and covalent bonding were conducive to the inhibition of triplet thermal deactivation. We also present that thermoplastic processing solution-cast films could densely stack the matrix to help stabilize ultralong RTP. RTP lifetimes of 3.517–4.444 s were achieved by tuning the position of boronic acid on N-phenylcarbazole. In this way, new ultralong organic RTP polymers not limited to a PVA matrix or carbazole derivative-dopants are being developed by our research team.Author contributions
S. X., W. Y. and Q. S. supervised the project. Y. Z. and W. Y. designed the experiments. Y. Z., S. Z. and G. L. carried out all experiments. Y. Z. and S. Z. undertook data analyses. Y. Z. and W. Y. contributed to experimental design. Y. Z. wrote the manuscript with the help of W. Y., Q. S. and S. X.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support for this research was provided by the National Natural Science Foundation of China (52273183), Natural Science Foundation of Shandong Province (ZR2020QE083), and Taishan Scholar Constructive Engineering Foundation of Shandong Province of China (tsqn202211164). We thank the open project of The State Key Laboratory of Supramolecular Structure and Materials of Jilin University (sklssm2023029).References
- Y. Zhang, Q. Sun, L. Yue, Y. Wang, S. Cui, H. Zhang, S. Xue and W. Yang, Adv. Sci., 2022, 9, 2103402 CrossRef CAS PubMed.
- R. Liu, B. Ding, D. Liu and X. Ma, Chem. Eng. J., 2021, 421, 129732 CrossRef CAS.
- J. Wang, X. Gu, H. Ma, Q. Peng, X. Huang, X. Zheng, S. Sung, G. Shan, J. Lam, Z. Shuai and B. Z. Tang, Nat. Commun., 2018, 9, 2963 CrossRef PubMed.
- W. Chen, Z. Chen, L. Zhang, B. Wang, Z. Lin, R. Cao, W. Wang, Y. Chen and Y. Wang, Chem. Eng. J., 2022, 432, 134411 CrossRef CAS.
- (a) Q. Cao, K.-K. Liu, Y.-C. Liang, S.-Y. Song, Y. Deng, X. Mao, Y. Wang, W.-B. Zhao, Q. Lou and Ch.-X. Shan, Nano Lett., 2022, 22, 4097 CrossRef CAS PubMed; (b) Z. Ma, Z. Yang, L. Mu, L. Deng, L. Chen, B. Wang, X. Qiao, D. Hu, B. Yang, D. Ma, J. Peng and Y. Ma, Chem. Sci., 2021, 12, 14808 RSC.
- D. Li, Y. Yang, J. Yang, M. Fang, B. Z. Tang and Z. Li, Nat. Commun., 2022, 13, 347 CrossRef CAS PubMed.
- (a) W. Huang, C. Fu, Z. Liang, K. Zhou and Z. He, Angew. Chem., Int. Ed., 2022, 61, e202202977 CAS; (b) H. Liu, D.-D. Ren, P.-F. Gao, K. Zhang, Y.-P. Wu, H.-R. Fu and L.-F. Ma, Chem. Sci., 2022, 13, 13922 RSC.
- M. Xu, X. Wu, Y. Yang, C. Ma, W. Li, H. Yu, Z. Chen, J. Li, K. Zhang and S. Liu, ACS Nano, 2020, 14, 11130 CrossRef CAS.
- D. Wang, H. Wu, J. Gong, Y. Xiong, Q. Wu, Z. Zhao, L. Wang, D. Wang and B. Z. Tang, Mater. Horiz., 2022, 9, 1081–1088 RSC.
- Y. Su, S. Z. F. Phua, Y. Li, X. Zhou, D. Jana, G. Liu, W. Q. Lim, W. K. Ong, C. Yang and Y. Zhao, Sci. Adv., 2018, 4, eaas9732 CrossRef PubMed.
- Y. Su, Y. Zhang, Z. Wang, W. Gao, P. Jia, D. Zhang, C. Yang, Y. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 9967 CrossRef CAS PubMed.
- L. Ma, S. Sun, B. Ding, X. Ma and H. Tian, Adv. Funct. Mater., 2021, 31, 2010659 CrossRef CAS.
- Z. Wang, Y. Zhang, C. Wang, X. Zheng, Y. Zheng, L. Gao, C. Yang, Y. Li, L. Qu and Y. Zhao, Adv. Mater., 2020, 32, 1907355 CrossRef CAS PubMed.
- R. Tian, S.-M. Xu, Q. Xu and C. Lu, Sci. Adv., 2020, 6, eaaz6107 CrossRef CAS PubMed.
- Y. Yang, Y. Liang, Y. Zheng, J. Li, S. Wu, H. Zhang, T. Huang, S. Luo, C. Liu, G. Shi, F. Sun, Z. Chi and B. Xu, Angew. Chem., Int. Ed., 2022, 61, e202201820 CAS.
- Y. Zhang, Y. Su, H. Wu, Z. Wang, C. Wang, Y. Zheng, X. Zheng, L. Gao, Q. Zhou, Y. Yang, X. Chen, C. Yang and Y. Zhao, J. Am. Chem. Soc., 2021, 143, 13675 CrossRef CAS PubMed.
- S. Kuila, S. Garain, S. Bandi and S. J. George, Adv. Funct. Mater., 2020, 30, 2003693 CrossRef CAS.
- X. Yao, H. Ma, X. Wang, H. Wang, Q. Wang, X. Zou, Z. Song, W. Jia, Y. Li, Y. Mao, M. Singh, W. Ye, J. Liang, Y. Zhang, Z. Liu, Y. He, J. Li, Z. Zhou, Z. Zhao, Y. Zhang, G. Niu, C. Yin, S. Zhang, H. Shi, W. Huang and Z. An, Nat. Commun., 2022, 13, 4890 CrossRef CAS PubMed.
- H. Peng, G. Xie, Y. Cao, L. Zhang, X. Ya, X. Zhang, S. Miao, Y. Tao, H. Li, C. Zheng, W. Huang and R. Chen, Sci. Adv., 2022, 8, eabk2925 CrossRef CAS PubMed.
- Y. Zhang, J. Chen, Q. Sun, H. Zhang, S. Xue and W. Yang, Chem. Eng. J., 2023, 452, 139385 CrossRef CAS.
- F. Lin, H. Wang, Y. Cao, R. Yu, G. Liang, H. Huang, Y. Mu, Z. Yang and Z. Chi, Adv. Mater., 2022, 34, 2108333 CrossRef CAS.
- Y. Zhang, Q. Sun, J. Chen, S. Cui, H. Zhang, S. Xue and W. Yang, Chem. Eng. J., 2022, 447, 137458 CrossRef CAS.
- W. Zhao, Z. He and B. Z. Tang, Nat. Rev. Mater., 2020, 5, 869 CrossRef CAS.
- H.-T. Feng, J. Zeng, P.-A. Yin, X.-D. Wang, Q. Peng, Z. Zhao, J. W. Y. Lam and B. Z. Tang, Nat. Commun., 2020, 11, 2617 CrossRef CAS PubMed.
- Q. X. Dang, Y. Y. Jiang, J. F. Wang, J. Q. Wang, Q. H. Zhang, M. K. Zhang, S. M. Luo, Y. J. Xie, K. Y. Pu, Q. Q. Li and Z. Li, Adv. Mater., 2020, 32, 2006752 CrossRef.
- Y. X. Mu, B. J. Xu, Z. Yang, H. H. Wen, Z. Y. Yang, S. K. B. Mane, J. Zhao, Y. Zhang, Z. G. Chi and B. Z. Tang, ACS Appl. Mater. Interfaces, 2020, 12, 5073 CrossRef CAS.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X.-P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110 RSC.
- X. Zhang, Y. Hu, X. Yang, Y. Tang, S. Han, A. Kang, H. Deng, Y. Chi, D. Zhu and Y. Lu, Biosens. Bioelectron., 2019, 138, 111314 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc01276j |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: