Open Access Article
Open Access ArticleSelf-healing polymers for surface scratch regeneration
Sana Ahmed
a,
Ji-Eun Jeong
b,
Jin Chul Kim
*b,
Saifullah Lone
 *c and
In Woo Cheong
*c and
In Woo Cheong
 *a
*a
aDepartment of Applied Chemistry, Kyungpook National University, Daegu, 41566, Republic of Korea. E-mail: inwoo@knu.ac.kr
bResearch Center for Green Fine Chemicals, Korea Research Institute of Chemical Technology, Ulsan 44412, Republic of Korea. E-mail: jckim81@krict.re.kr
cDepartment of Chemistry, iDREAM (Interdisciplinary Division for Renewable Energy & Advanced Materials), NIT, Srinagar, 190006, India. E-mail: saifullah.lone@nitsri.net
First published on 1st December 2023
Abstract
Recently, there has been a significant increase in academic and industrial interest in self-healing polymers (SHPs) due to their remarkable ability to regenerate scratched surfaces and materials of astronomical significance. Scientists have been inspired by the magical repairing mechanism of the living world. They transformed the fiction of self-healing into reality by designing engrossing polymeric materials that could self-repair mechanical abrasions repeatedly. As a result, the durability of the materials is remarkably improved. Thus, the idea of studying SHPs passively upholds economic and environmental sustainability. However, the critical areas of self-healing (including healing efficiency, healing mechanism, and thermo-mechanical property changes during healing) are under continuous scientific improvisation. This review highlights recent notable advances of SHPs for application in regenerating scratched surfaces with various distinctive underlying mechanisms. The primary focus of the work is aimed at discussing the impact of SHPs on scratch-healing technology. Beyond that, insights regarding scratch testing, methods of investigating polymer surfaces, wound depths, the addition of healing fillers, and the environmental conditions maintained during the healing process are reviewed thoroughly. Finally, broader future perspectives on the challenges and prospects of SHPs in healing surface scratches are discussed.
1. Introduction
Self-healing is a ubiquitous surviving prowess of the living world to repair physical damage routinely. The intrinsic repairing ability in nature has inspired scientists to develop self-healing polymers (SHPs) with recent application in vital areas, including electronic gadgets,1 sensors,2 e-skin,3 adhesives,4 supercapacitors,5 wearable devices,6 automobiles, and steel coatings.7,8 The self-repairing properties in synthetic materials have improved the final design's lifetime, strength, and safety. This can substantially decrease per capita purchases, industrial waste, and environmental pollution.9 Thus, SHPs can exquisitely reverse climate change.10In the 1970s, as interfacial macromolecular interpenetration began to be studied as one of the repair mechanisms for damage occurring in thermoplastic resins, the concept of self-healing emerged as a research topic for polymeric materials.11 However, it is not an exaggeration to say that the systematic research on full-scale self-healing began with the study of the microcapsule polymer complex by White's group in 2001.12 This is the first extrinsic self-healing polymer composite, and this concept was expanded to microvascular network in 2007 by the same group.13 In 2002, Chen et al. presented a polymer with a cross-linked structure that can be re-mendable by heat using the Diels–Alder/retro-Diels–Alder (DA/rDA) reaction, and which is the first intrinsic self-healing polymer based on reversible covalent bonding.14 In the same year, research on shape memory effects and self-healing began.15 In 2008, a self-healing polymeric material based on a supramolecular assembly relying on hydrogen bonds was presented by Leibler et al., pioneering as the first self-healing rubber using non-covalent bonds.16 In 2011, the Leibler group first reported the concept of ‘vitrimer’, which can maintain a constant crosslinking density of polymers that can be reformed by heating based on transesterification reaction of epoxy self-healing polymers.17 Since 2011, studies on endogenous self-healing mechanisms for various dynamic covalent and non-covalent bonds have increased intensively and extensively.
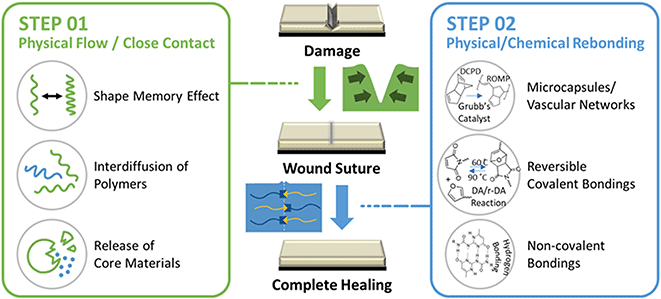
It has been well-known that polymers with self-healing mechanisms are broadly classified into two types: extrinsic and intrinsic.18 Depending on the specific mechanism, self-healing polymers can be differentiated into several different types. However, most self-healing polymers involve two important steps in the wound-healing process.19 As illustrated in Fig. 1, when polymers are damaged by external impact (e.g., scratches, cracks, cuts, etc.), the wound should be sutured through physical flow or diffusion of the polymer or by the elastic shape memory effect towards the injured area.11 In the case of intrinsic self-healing, one of these mechanisms is required, whereas in the case of extrinsic self-healing, the release of substances such as monomers that can be polymerized autonomously included in microcapsules or microvascular network structures.13 In the next step, substantial self-healing occurs through molecular-level recombination of the damaged polymer owing to physical or chemical bonding. Intrinsic self-healing includes both physical and chemical rebonding. Physical bonds include hydrogen bonding, ionic bonding, metal–ligand complexes, etc. Chemical bonds include Diels–Alder,20 disulfides,21 imines,22 hindered urea,23 thiourethane,24 etc. Extrinsic self-healing, on the other hand, is exemplified by polymer formation through methods such as ROMP that complete the self-healing process.25 Recently, research on hybrid self-healing materials has been actively conducted, which can operate through various mechanisms triggered by external stimuli such as light, pH, specific chemicals, and magnetic fields, as well as simple external stimuli such as temperature.26
 | ||
| Fig. 1 Schematic illustration of the self-healing repairing mechanisms and types of SHPs when the scratch is induced on the surface of a film or coating. | ||
In reference to the surface scratches on display panels and automobiles, the SHPs are critically important to recover physical abrasions quickly.27 Additionally, since damage or large cracks that can affect the lifespan of all products always start from scratches or small cracks, the problem of formation and recovery of scratches on polymer surfaces is an important issue. Therefore, this review focuses on the formation of scratch wounds and their self-healing and deals with the most recent developments of SHPs with application in self-repairing surface scratches by describing various underlying mechanisms. Moreover, the advancements made in designing such polymers to perform under hostile environmental changes such as extreme temperature, radiation, electric field, corrosion, and acidic and alkaline conditions have been analysed. The review will give a detailed account of the factors influencing polymer scratch and recovery. Besides, scratch testing (such as sharp/blunt tip scratching or abrasive scratching), methods of investing polymer surfaces, wound depths, the addition of healing fillers, and conditions maintained during the healing process are reviewed thoroughly. Further, various progressive surface examinations (including sliding friction, micro to nano-scratch, nanoindentation, and evaluation of penetration depth, residual depth, residual scratch width, contact scratch width, and elastic recovery rates) are also included. Finally, a broader future perspective on the challenges and prospects of SHPs in healing surface scratches is presented.
2. Various generations of SHPs
Initially, the goal of self-healing research was focused on developing materials that could repair surface wounds such as cracks or scratches. However, further investigation revealed that the healing process often resulted in compromising the mechanical or chemical properties of the polymer during the healing process. To address this challenge, researchers began exploring different categories of SHPs that could embed the polymer's intrinsic properties with self-healing capabilities, thereby making them more useful for practical applications. Notably, some examples of these categories include intrinsic self-healing materials, which rely on reversible chemical bonds to facilitate healing; extrinsic self-healing materials, which use external stimuli (such as heat or light) to trigger the healing process, and hybrid self-healing materials, which combine multiple self-healing mechanisms to achieve enhanced healing properties. Overall, the development of various categories of SHPs has allowed researchers to craft materials that are both mechanically robust and capable of self-repair, making them promising candidates for a wide range of applications in the industry including aerospace, automobiles, and biomedical engineering.The SHPs within the first type category operate under the extrinsic repairing mechanism of delivering self-repairing microcapsules to the wounded spot to prolong the shelf-life of host materials.28 The development of similar types of capsules led to the concept of expanding their application by forming vascular-shaped SHPs. For instance, the incorporation of resin-filled embedded hollow glass fibers (HGF) within a carbon fiber-reinforced epoxy (CFRP) laminate proved to reduce the damage to aerospace materials.29 However, the limitation of the one-time repairing capacity of self-healing capsules led to the development of a second type of SHPs with multiple healing abilities by adjusting covalent bonds such as disulfide21 or hindered urea bond (HUB)30 within acrylate polymer. A few prominent examples include the work of Zechel and his team31 producing a 25 MPa rigid polymer based on a dynamic urea bond. Besides healing performance, the polymer offered remarkable mechanical strength. Zhang with his team32 synthesized a waterborne polyurethane polymer (WPUR) consisting of aromatic disulfide groups. The polymer demonstrates excellent mechanical properties, heals at room temperature, and proves to be recyclable. Incorporating HUB into hetero-nanostructured block copolymer micelles is a strategy introduced by Cheong et al.,33 which aims to add phase-separated polymers with both soft and hard domains while maintaining mechanical properties. The resulting material exhibits mechanical robustness, high transparency, and room-temperature self-recovery features. A continuation of this brought the third type of SHPs which consisted of non-covalent interactions involving hydrogen bond34 or ionic interactions.3 Park's team demonstrated the significance of non-covalent hydrogen bonds formed in a hydrogel which resulted in the repair of wounds when triggered by NIR.2 An example of ionic interactions was shown by Ko's group by fabricating an ionic biogel which could heal by the electrostatic interactions between the polymer chain and the ionic liquid.35 The main focus of both systems constituted healing from numerous scratches via bonding/debonding features of reversible bonds.
To include more versatile properties in the healing polymers, an advanced class of the family of SHPs was developed, which included combining several healing strategies into one hybrid polymer.36 These fascinating polymers include ion clusters made from imide acrylates doped with ionic liquid offering ultra-fast wound recovery (for instance, one-minute recovery at laboratory temperature). This work demonstrates the new deformable electronics platforms, such as area-adjustable iono-skin sensors and user-reconfigurable AC electroluminescent displays (ACEDs).1 Another study intrigues the innovation ahead by using HUB-based polymer as an automobile clearcoat that healed under sunlight within 30 s.27
3. Underlying mechanisms
3.1. SHPs with extrinsic mechanism
Extrinsic self-healing materials are designed to regain their thermo-mechanical properties after sustaining mechanical damage by using a separate healing agent that is stored in the material.37,38 The healing agent undergoes a chemical reaction triggered by the damage to restore the material's properties. One effective chemical reaction system in extrinsic self-healing materials is using UF or MF polymer as a shell material to encapsulate dicyclopentadiene (DCPD) healing agents by the process of in situ polymerization in an O/W emulsion. The polymer shell, on rupture, releases DCPD which undergoes ROMP catalysed by Grubb's catalyst and fills the wounded area. This type of self-healing mechanism operates automatically, without the need for human intervention, making the process more efficient and less prone to error. Additionally, the mechanism can treat large areas of damage, making it a suitable solution for large-scale repair applications, such as coatings and adhesives.4,39 However, a few drawbacks such as one-time wound healing and light scattering (or opacity) issues of the self-healing agents, can limit these types of polymers to a very narrow range of applications. Recently, while facing the challenge of one-time self-healing Chung's group developed vegetable oil-loaded self-healing microcapsules that can heal twice on repeat after inflicting a wound.403.2. SHPs with intrinsic mechanism
The SHPs with intrinsic crosslinking networks can repair themselves without the need for external intervention, making them useful in a variety of applications. The intrinsic self-healing mechanism operates by two routes after physical flow or close contact around the scratch area: (a) chemical bonding and (b) physical bonding. Chemical bonding involves the use of molecules that can chemically react with each other when they come in contact. Once the material is damaged, molecular inter-diffusion or suture of the wound allows the exposed debonded functional groups to start to react, forming new chemical bonds. As a result, the damage is repaired via chemical bonding, which results in substantial healing, as the chemical bonds formed during the reaction are stronger than physical interaction. The suture of a wound can be faster by the shape memory effect than by physical flow that can be accelerated by heat or other stimuli.41–43 However, we will not discuss shape memory SHP in detail as it is beyond the scope of our current review.On the other hand, the physical bonding self-healing mechanism relies on physical bonds, such as hydrogen bonds, ionic interaction, van der Waals, elastic recovery, and so on. In this process, the material can stretch and return to its original shape when stress is applied due to the presence of physical bonds. If the material is damaged, the physical bonds are broken, but they can reform when the stress is removed, allowing the material to recover its original shape. This process is reversible and can be repeated multiple times, making it useful for materials that experience repeated stress cycles. However, these mechanisms will depend upon the conditions under which specific functional groups within these networks interact.44,45 All the latest two types of SHPs offering multiple cycles of wound healing are based on intrinsic repair mechanisms. These networks form reversible bond dissociation and reassociation and can be further classified into two types, i.e., covalent adaptable networks (CANs) and supramolecular networks (non-CANs).
The next type of CAN involves an associative type of bonding, also known as exchangeable bonding, which is generated in response to changes in the environment. One example of this type of interaction occurs when a reactive alcohol participates in a transesterification reaction with the ester component of a self-healing agent.46 Another example is when a reactive acid participates in a transcarbonation reaction with the carbonate component of a self-healing agent.25 In trans-ureafication, the urea linkages in the polymer chains react with free amines to form reversible urea adducts.30 In the transamidation self-healing mechanism, the reaction between amide linkages and free amines forms reversible amide adducts.47 These adducts hence act as reversible crosslinks that can be broken and reformed, allowing the material to heal itself.
Both dissociative and associative covalent adaptive networks have unique advantages and disadvantages, making them suitable for different applications. Dissociative networks are well suited for materials that are subjected to high levels of stress, as the ability to break and re-form bonds provides a high level of flexibility and durability. On the other hand, associative networks are well suited for materials that require stability and consistency, as they maintain their original properties even after repair. However, dissociative networks are generally more advantageous than associative networks due to their ability to undergo more significant changes in structure and properties in response to external stimuli.
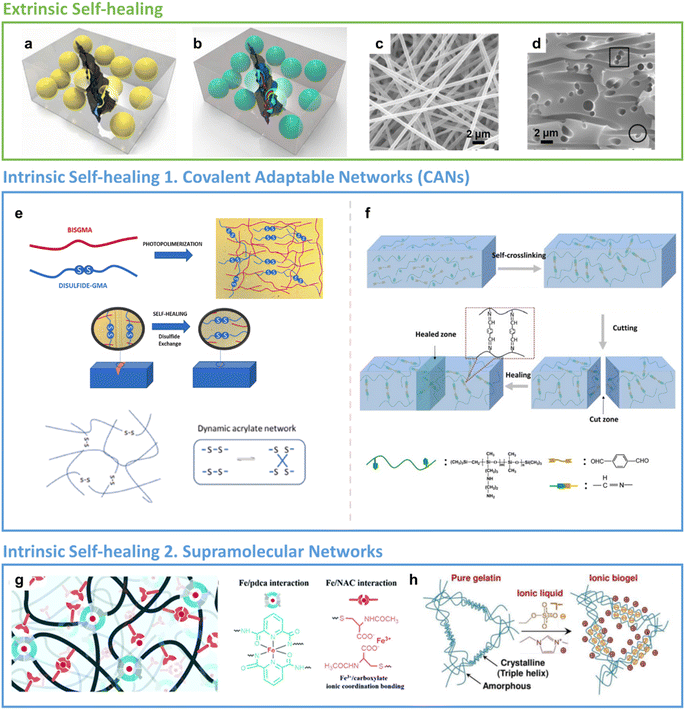 | ||
| Fig. 2 Healing mechanisms. Extrinsic SH: (a) single and (b) all-in-one capsule-based self-healing composites. Reproduced with permission25 Copyright 2015, Elsevier. (c) The side and (d) cross-sectional view of the epoxy-based nano-vascular network. Reproduced with permission39 Copyright 2016, Elsevier. Intrinsic SH 1 – Covalent Adaptable Networks (CANs): (e) disulphide based dynamic acrylate networks. Reproduced with permission21 Copyright 2023, Elsevier. (f) Reversible imine bond formation during self-healing process. Reproduced with permission101 Copyright 2020, Elsevier. Intrinsic SH 2 – supramolecular networks: (g) the metal ion coordination complexes formed by weak and strong interaction of PDMS network with Fe metal. Reproduced with permission48 Copyright 2020, RSC. (h) Ionic cross-linking to the gelatin polymer chain forming a supra-molecular hydrogen bonding network. Reproduced with permission35 Copyright 2022, Elsevier. | ||
4. SHPs designed for surface applications
In today's industrial landscape, the integrity of polymer surfaces is of paramount importance. Polymer materials find extensive use in a wide range of industrial applications, particularly when fabricated as thin films or coatings. However, maintaining the pristine condition of these surfaces is often challenging, given their exposure to various environmental factors and mechanical stresses. Surface scratch healing is often more efficient and quicker than bulk mechanical healing since it addresses localized surface damage, the energy required for repair is lower, and the process is highly focused. However, since the surface material is usually adhered to a hard substrate (e.g., plastic, steel, glass, etc.), the spontaneous inflow of material into the wound area or the wound suture must occur spontaneously for the scratch healing on the coating surfaces. In case of damage to bulk polymers, on the other hand, wound healing can generally be achieved by bonding and applying pressure to the wound area. In response to these challenges, self-healing technology has emerged as a crucial aspect of preserving and enhancing the performance of polymer materials. This discussion aims to shed light on the pivotal role of surface scratch healing from an industrial perspective and showcase examples that demonstrate how polymer materials can reap significant advantages through this remarkable capability. Polymer materials serve as versatile solutions in numerous industrial applications. Thin polymer films and coatings are employed in a wide spectrum of fields, including optical devices, mirrors, semiconductor components, magnetic media, food packaging, and more.51–53 However, the unique requirements of these applications often necessitate surface modifications to augment properties such as adhesion and wettability. For instance, surface treatments are vital for providing antistatic properties, corrosion resistance, wear reduction, and promoting adhesion, thereby enhancing the functionality and longevity of these materials.Besides surface features, the industrial landscape places a significant emphasis on biocompatibility, especially in biomedical and environmental applications.54 To tailor polymers with different surface features, researchers have applied numerous modification steps. Among the exquisite examples of combining surface properties and reprocessing include De Vos et al.55 work of producing recyclable barrier material for flexible food packaging materials utilizing polyethylenimine (PEI) and poly(acrylic acid) (PAA) having excellent oxygen barrier properties. The self-healing nature of these coatings ensures prolonged food freshness and reduces packaging waste. Additionally, Ude and his co-workers56 presented detailed insights into the recent developments, which highlighted the transition from conventional plastics to recyclable poly(lactic acid)-based materials while addressing their impact on the environment as food packaging materials.
In addition, a unique application includes super-hydrophobicity, chemical stability, and self-healing nature through coatings of PDMS-grafted cotton fibers presented by Zhang et al.57 These coatings find applications in various industries, including electronics and marine, where water-resistance and surface protection are critical. Another remarkable work by Hao and his team58 includes underwater engineering application of agarose/polyvinyl alcohol (PVA) double network utilizing a dynamic borate bond with outstanding self-healing underwater performance. However, repairing spliced films is quicker and much easier than coatings that completely rely on the polymer's mobility or flow toward the scratched areas. Spectacular advances were also made in biomedical applications from polymeric surface tailoring. Jiang and his team59 prepared a self-healing elastomer composed of polyurethanes based on dynamic dimethylglyoxime–urethane groups to treat certain clinical conditions, such as aneurysms, peripheral nerve amputation, and bone immobilization, while Chen et al.4 prepared an osteogenic glue by inorganic–inorganic integration between amine-modified mesoporous bioactive glass nanoparticles (AMBGN) and bioadhesive gelatin-dextran network (GelDex) for adhering bone fragments and healing fractures. However, more such attempts are needed for surface treatment and the integration of self-healing capabilities in various industries which could result in innovative solutions to real-world challenges. Nevertheless, these examples stand as compelling evidence of the transformative capacity of surface scratch healing in polymer materials, ranging from extending product lifespan and reducing maintenance costs to advancing eco-friendly practices and improving healthcare outcomes. The figures of surface-treated SHPs included in Fig. 3 exemplify the versatile applications of self-healing polymers in different industrial contexts.
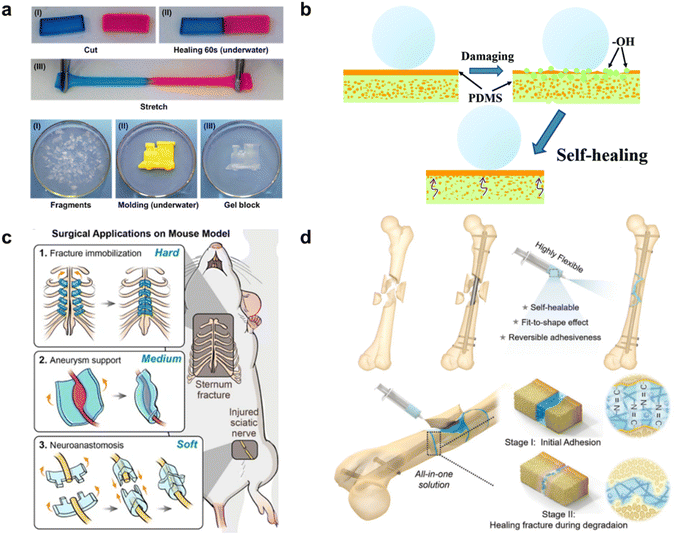 | ||
| Fig. 3 Surface-specific SHPs. (a) Agarose/PVA DN hydrogel stained, cut, and healed underwater after contacting for 60 s at room temperature without external intervention. Reproduced with permission58 Copyright 2018, American Chemical Society. (b) Intelligent self-healing process for PDMS@cotton fabric. Reproduced with permission57 Copyright 2020, RSC. (c) Three applications of SHEs in a biomedical area in vivo (from up to down). Fractured sternum bone immobilization, wrapping aneurysm for limiting the progress, and LEGO-playing peripheral nerve coaptation.59 (d) GelDex-based adhesive as an assistant tool for fixing bone fragments during surgical treatment (top), and as a bone glue to adhere the spliced fragments promoting fracture healing. Reproduced with permission4 Copyright 2021, John Wiley and Sons. | ||
5. Advances in surface scratch-repairing technology
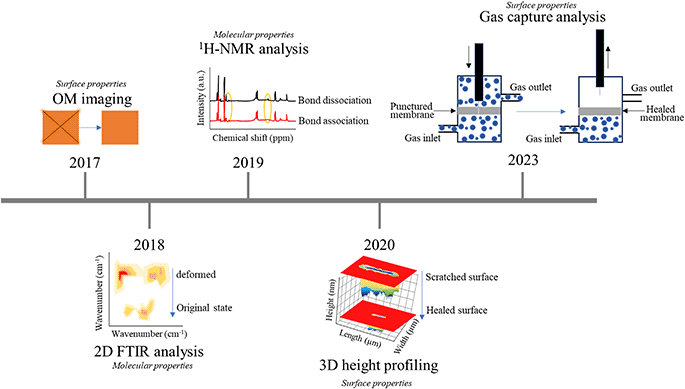
In the last few years, polymer surfaces have undergone notable modifications to cater to particular self-healing applications. These methods offer insights into the mechanisms governing self-healing, with a focus on quantitative analysis. Such quantitative analysis involves tracking specific changes that occur during the healing process, and various techniques have been employed to achieve this. For example, healing may rely entirely on changes in elasticity or other recovery properties, which can be observed in detail by tracking bond changes using techniques such as FTIR, NMR, Raman, XRD, and rheology.60 In this attempt, Urban's team performed a 2D-FTIR analysis for identifying molecular events during self-healing and shape memory cycles. The study gave insightful information regarding the relationship between shape memory and self-healing events in synthetic polymers resulting from the polymer network's ability to store entropic energy during deformation, allowing for shape recovery and damage closure.61 In a more comprehensive analysis, using 2D-FTIR correlation analysis, the opposite response of hydrogen bonding and non-hydrogen bonding gave crucial information regarding the damage repair cycle in thermoplastic polyurethanes.62In particular, 1H- and 13C-NMR are employed to study the degree of hydrogen bonding within different segments of polymer materials. Xia's group investigated the self-healing behavior utilizing NMR studies to understand the dissociation and association of rDA bond. The chemical shifts for furan and melamine groups with lowering of peak intensity when the elastomer was subjected to 80 °C temperature for 6 h and finally the retro DA reaction could be observed when dipped in DMF solution at 140 °C.63 Studying other than the domain differences within the SHPs, in other cases, intrinsic dynamic equilibrium and the behavior of reformable hydrogen bonds was studied in detail by Picchioni and his team using rheology. In this system, the dynamicity of reformable hydrogen bonds and reversible electrostatic repulsion between the aldehyde and the amine reactants marked the self-healing behavior in these Schiff base hydrogels and this was closely monitored through a series of rheological loop tests by shifts in storage (G′) and loss moduli (G′′).64
Conversely, it must be rationalized that the discussed studies have used instruments which cannot be efficiently employed for the extensive time-lapse self-healing detailed studies. Hence, quantitative analysis methods are essential, simpler, and faster techniques for tracking surface changes have gained prominence. These methods include OM, SEM, micro-CT, AFM, and integrated instruments combining multiple techniques. Recent studies have showcased the use of optical microscopy to observe the retardation of biofouling of poly(ethyl acrylate) type (PMEDSAH) microsphere coating by the reformation of ionic bonds between the protonated ammonium group and sulfonic group in aqueous salt solutions.65 In another study, similarly using an OM, the self-healing efficiency of a transparent thermoplastic polyurethane (TPU) was evaluated at room temperature. With the current analysis, the elastomer was deduced to possess remarkable self-healing capabilities with adequate toughness and rapid repair.66 More detailed examinations were made using SEM to monitor surface recoveries at a higher spanning from 200 μm down to 10 μm.67,68 Another group prepared healable oil-repellent antifogging films and with AFM highly detailed surface transformations were captured. The fabrication included layer-by-layer assembly of hyaluronic acid (HA) and branched poly(ethylenimine) (bPEI), followed by immersion in the aqueous solutions of perfluorooctanesulfonic acid potassium salt (PFOS). AFM revealed the surface of the scratched F-(HA/bPEI) × 50 film which was initially covered with shallow grooves but was eliminated on healing due to autonomous restoration with scratch-free transparency and oil repellency when exposed to moisture. Furthermore, AFM provided the RMS roughness details of the healed film which decreased from 20.7 to 1.4 nm.69 For instance, Hager and his team employed 3D profiling to assess the self-healing behavior of zwitterionic polymers. In a more comprehensive investigation, the same research group extended their analysis by applying laser scanning microscopy (LSM) to accurately quantify the incremental healing process of the polymer under examination.70,71
Yet to mend the gaps and enhance the detailing of the recovery process, hybridization of two techniques resulted with brilliant quantification as in one example optical pictures of the crack-healing of sandwich and porous CNT-based methacrylic acid composite (CNT/EMAA) served to give details regarding delamination-healing cycles with their corresponding infrared temperature fields after healing 30 and 45 min by electrical heating.72 Real-time monitoring of damage repair was observed in polyurethane/carbon nanotube composites using a basic tool like an optical microscope, which is indispensable for conducting experiments, such as corrosion protection, with direct observations of surface changes. The low current densities post-healing indicates the efficacy of the process.8 Among the real-time monitoring examples, Martín-Martínez fabricated an innovative device for in situ quantification of self-healing ability of different polyurethanes. The idea included observation of the amount of gas flowing out of the polymer material when poked and after healing.73 Nevertheless, only a limited number of attempts have been made at hybridization, indicating that there is still much unexplored territory in this field. Fig. 4 shows the timeline-based advancements made in scratch testing of polymeric surfaces.
6. Exceptional SHPs
The advancements made in instrumental aspects were quickly followed by developments in SHPs, leading to exceptional SHPs. Since external stimuli-responsive dissociation or association plays an essential role in the self-repairing mechanism of polymers with both covalent and non-covalent interactions, however, an inappropriate stimulus can degrade or completely alter the fundamental properties of polymers. Thus, while designing such SHPs, the uniqueness lies in their resilience and capacity to perform under conditions that conventional materials often fail to endure. The extraordinary conditions include extreme temperature ranges (−40 to 125 °C), pressure variations (vacuum or 200 bar), intense radiation, shock or vibration, high electric, or magnetic fields, high alkalinity or acidity (pH ≈<2 or >11), corrosive environments, and repeated wound inflictions.74 Adapting to such harsh conditions would not only mean that these polymers can be readily used, thereby significantly reducing operational costs and saving energy.Henceforth, a few exceptional SHPs will be discussed in this section with some of them presented in the pictorial format in Fig. 5. For instance, Lau et al.75 designed aramid, basalt, and carbon fiber reinforced composite bonded concrete that could operate under exceptionally high temperatures (300 °C) and making it invaluable in fire-risk environments, an achievement that conventional fiber reinforced polymers (FRPs) cannot surpass. It is worthy to point out that this study does not focus on the discussion of the chemical morphology that maintains the structural integrity at such elevated temperature. Addressing the challenging temperature conditions through a comprehensive follow-up on the chemical structure, Zhang and his co-workers76 synthesized a polyurethane enlisting a comprehensive clarification of the exceptional molecular chain mobility enabling it to recover from damages at extremely low temperature (−80 °C). Another example of a remarkable SHP includes the recyclable crosslinked polyurethane polymer consisting of disulfide in the main chain prepared by Zhang's group.77 The polymer on the infliction of the wound could recover within a mere five minutes of exposure to sunlight (>290 nm). Even so, repeating the wound infliction in a crosswise manner, as opposed to scratching the same surface repeatedly, raises concerns about the reliability of studies involving repeated scratches. Weder and his team78 further enhanced this concept, achieving a self-healing time even lower to twenty seconds while showing recovery process via atomic force micrographs. As a methodology optimization the group achieved this quick recovery by increasing the radiation intensity to UV light and incorporating telechelic poly(ethylene-co-butylene) functionalized with ureidopyrimidone (UPy) and cellulose nanocrystals (CNCs) via hydrogen bonding.
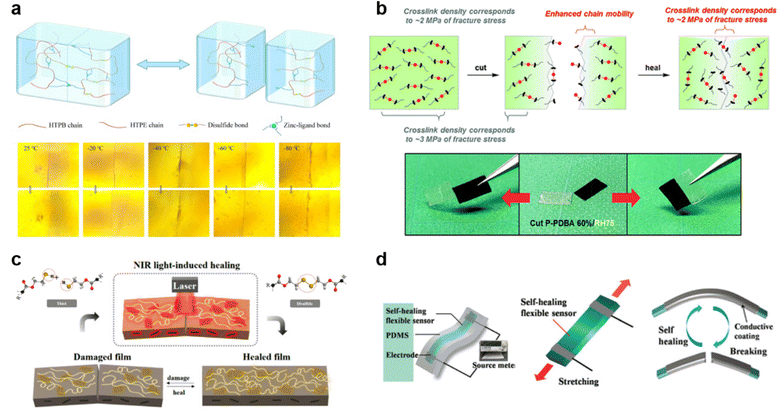 | ||
| Fig. 5 Exceptional SHPs. (a) Schematic of the healing mechanism and optical microscopy images of zinc-based polyurethane coatings at very low temperatures of −80 °C to 25 °C for 100 s. Reproduced with permission76 Copyright 2022, Elsevier. (b) Self-healing mechanism and film cut-rejoin images of phenyldiboronic acid polymer at ambient humidity of RH75 for 3 days. Reproduced with permission79 Copyright 2018, RSC. (c) Illustration of a self-healing system using photothermal polydopamine-coated graphene oxide polymer triggered by NIR laser irradiation at a power density of 0.1 W cm−2.84 (d) Illustrations of fabrication (top left), stretching of the strain sensor (top right), and the electrical self-healing process(bottom) of cellulose nanocrystal-based polymer within a mere 15 s. Reproduced with permission85 Copyright 2017, John Wiley and Sons. | ||
Furthermore, Yoshie's group79 prepared a polymeric network consisting of tetrahedral boronate-ester bonds, which were incredibly stable against hydrolysis leading to remarkable moisture-induced self-healing capabilities. The study clearly elucidates the reaction mechanisms involved when maintaining moisture conditions that occur during the healing process. Proceeding with similar motives, Tee, et al.3 synthesized a self-healing electronic skin-like polymer that mimics the attributes of a jellyfish. This material includes fluorocarbon elastomer, demonstrating electro-mechanical self-healing through interaction with ionic liquid via ion–dipole interactions. The reported healing ability was versatile, extendable to diverse environments such as dry, wet, acidic, and alkaline conditions and applicable to wide range of sensing devices. In search for narrowing the gap between naturally healing organisms and SHPs, Sumerlin, et al.80 worked out to make vitrimers that underwent the associative exchange of vinylogous urethanes utilizing methacrylic monomers and a trifunctional amine without the need for a catalyst. These vitrimers offered an environmentally sustainable approach to self-healing materials by being reprocessed up to five times without compromising their properties, as shown via FTIR and various other techniques.
Concentrating on super-toughness Liu81 and co-workers fabricated a spider silk inspired recyclable supramolecular poly(urethane-urea) (Supra-PU) elastomer with high fracture energy (215.2 kJ m−2) and highest tensile strength (75.6 MPa) while Wang's82 team developed a supramolecular elastomer that incorporates aromatic amide and acylsemicarbazide moieties, showcasing exceptional characteristics, including remarkable crack tolerance (fracture energy 282.5 kJ m−2), ultrahigh true stress at the point of rupture (2.3 GPa), excellent elasticity and self-healing capability. These types of polymers are a great promise for advancing aerospace applications. Zhang's group83 extended the work from engineering to biological applications by fabricating an ultra-robust self-healing material inspired by biological cartilage. The resultant nanocomposite material interweaving a noncovalent network between dendritic tannic acid-modified tungsten disulfide nanosheets and polyurethane matrix. It showed outstanding tensile strength (52.3 MPa) with high stretchability (1020.8%) and excellent room temperature self-healing. Adding further to unique class of SHPs, Jung's team84 took the concept of introducing new functionality to the next level by synthesizing a combination of inorganic–in-organic polyurethane graphene nanocomposites which offered rapid self-healing when using the thermal energy generated by the NIR laser, introducing a novel approach to self-healing materials. Nevertheless, the study falls short in terms of cross verifying the self-healing efficiency observed through multiple techniques.
Additionally, in the pursuit of preparing distinct SHPs, one example is of a supramolecular multiple hydrogen bonding elastomer developed by Yu and his co-workers85 to obtain a self-healing sensor for human–machine interactions. The researchers used bio-derived carboxyl cellulose nanocrystals (C-CNC) to construct multiple hydrogen bonding interactions with chitosan-decorated epoxy natural rubber latex. Along with high healing ability and efficiency, the polymer offered high sensitivity for tiny bio-motion detection. Thus, making it applicable for promising applications in healthcare systems, particularly for facial expression control in human–machine interaction scenarios. These exceptional SHPs break new ground in materials science by pushing the boundaries of conventional self-healing materials. Their unique capabilities and adaptability to harsh environments underscore their exceptional nature, setting them apart from conventional literature and expanding their potential applications across various industrial and technological domains.
7. Factors impacting the polymer scratch, and recovery
7.1. Impact of polymer surface
Numerous prominent works proposed unique models, different instruments, and the best techniques for investigating scratched polymer surfaces. However, it is also worthwhile to know the effect of various parameters (polymer structure, composition, fabrication, and amount or type of fillers, etc.) which can influence the impact damage on the polymer surface and its recoverability. In this direction, Bijwe's group86 put together a library for the scratch behavior of various polyamides (PAs). The report presented condensation-type PAs that have higher scratch hardness values than additional PAs. Additionally, PA containing highly flexible polyether groups in the backbone shows low scratch hardness values.In a similar attempt, Mai et al.87 presented a detailed review of the influence of nano-additives in polymer nanocomposites. Further, the impact of fillers was also explored in a study that demonstrated that hard fillers increased the scratch visibility while soft reduced the same improving the recovery characteristics of the polymer.88 Moreso, additives such as ionic liquid and a graphene component were worked on by Bermúdez and his team.89 This work aimed to develop the thermal stability and stiffness of epoxy resin causing improved wear resistance. However, besides different types of additives, the quantity of additives equally plays a significant role in the wear properties of the polymers. For this, Kharrat and co-workers90 demonstrated an increase in scratch resistance observed by an increased content of graphene oxide filler on a polyester matrix. While components such as fluorinated groups and their transient effect on the scratch and tribological properties of epoxy polymers were explored by Brostow91 in his numerous years of work. Through these studies, it can be stated that the samples with high fluorine concentrations exhibit elevated elastic modules and are highly ductile. As a result, compared to the pure epoxy polymer, an enhanced recovery process is achieved. Fig. 6 shows a few scratched polymeric surfaces when different additives were added within the polymer matrix.
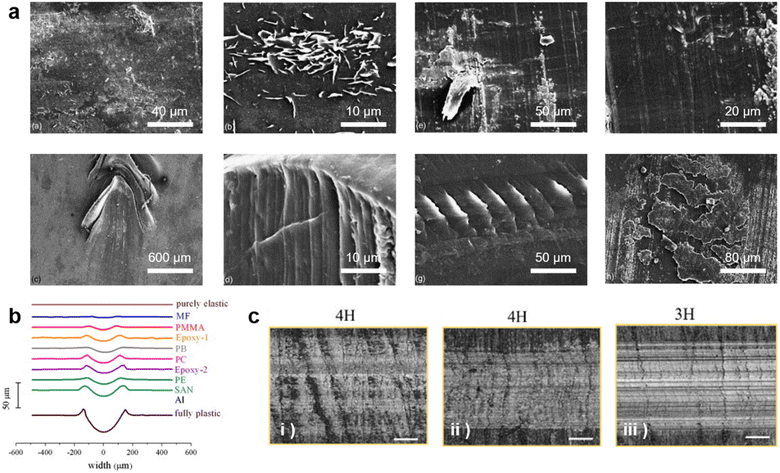 | ||
| Fig. 6 Impact of polymer surface on scratch recovery. (a) Surface topography shows different deformation patterns caused by scratching differently modified polyamide surfaces. Reproduced with permission86 Copyright 2005, Elsevier. (b) Profiles of the transverse sections of the scratch tracks for a range of polymers from fully plastic to elastic surface. Reproduced with permission88 Copyright 2012, Elsevier. (c) Pencil scratching shows a decrease in scratch resistance of epoxy resin surface by increasing crosslinking networks ((i) → (iii)). Reproduced with permission96 Copyright 2019, Elsevier. | ||
Another factor to consider is assembling the polymer coating into layers which can greatly alter the polymer's anti-wear capability. A multi-layered shish-kebab structure of polylactide (PLA) material introduced by Shen, et al.92 showed to greatly reduce the sacrificial cracking and deformation during the scratch process. The same team93 extended the work by making a layer-by-layer system of thermoplastic polyurethane (TPU) and co-continuous poly (butylene succinate) (PBS)/polycaprolactone (PCL) consisting of 128 layers capable of shape fixing and recovery. The interfacial shearing effect between the multilayer system offered temporary shape fixation and triggered recovery to the original state.
Other changes include the addition of certain nanoparticles such as in the work of Li94 designed for the application in automobiles and home appliances. The epoxy group-based silica nanoparticles were dispersed in polysiloxane coating and the resultant coating due to nanoparticles improved the scratch resistance and increased difficulty in surface penetration of the coatings as a result of the hardening effect.
Besides nanoparticles, Sue's group95 introduced necklace-like supramolecular structured polyrotaxane to poly(methyl methacrylate) (PMMA) which greatly reduced the scratch depth and scratch coefficient of friction (SCOF) with longer range molecular relaxation within the polymeric chains of the matrix. The same group96 also gave insights into crosslinking density changes in epoxy resins to investigate the scratch behavior. In this study, the compressive yield stress was found to increase as the molecular weight between the crosslinks (Mc) decreased, which greatly improved the scratch resistance of polymers by delaying the onset of crack formation. Similar intermolecular interactions were also studied97 by observing the structural effect of long chain branching of polypropylene (PP) polymer. The branching induced superior mechanical properties, however, there was a minor improvement in the scratch resistance of PP. Though all the cited investigations explore the impact of different parameters on the polymer's recoverability, however, while optimizing polymer properties, exploration of novel additives, that can address sustainability arises great concerns for improving the quality control measures in the industry.
7.2. Impact of environmental conditions
The recovery process during scratch healing is significantly affected by environmental conditions, prompting researchers to develop stimuli-responsive self-healing polymers. Initially, researchers focused on thermally driven recovery-based studies, such as those conducted by Yagci and Li's team.98,99 The former group anticipated healing through S–S bond cleavage-reformation in a polybenzoxazine-based SHP while the temperature required for the process was 185 °C for 30 min. Yet, the healing conditions outlined in this study appear challenging to uphold. Henceforth, the latter study reduced the healing time and temperature to 90 °C and 5 min by incorporating a motif containing hierarchical hydrogen bonds (urethane, urea, and UPy) to achieve complete recovery in a super tough elastomer. However, given that it is very hard to keep up with such high temperatures in everyday applications, researchers have recently utilized the advantages of NIR wavelengths, such as simplicity and deep penetration, to develop self-healable elastomers that respond to NIR light as featured in Fig. 7. One study demonstrated the ability of the PNIPAAm chain to move and the hydrogen bond from PDA to rearrange, enabling NIR-assisted self-healing at both acidic and basic conditions. In just 3 min the damaged site's temperature was increased to assist in this process.100 Continuing with the NIR-based healing, the subsequent work includes the use of NIR in a carbonized polydopamine-loaded conductive network containing zwitterionic polymer dot (cPDA@ZPD) reinforced hydrogel as a self-powered electronic skin sensor. Self-healing is achieved by the dynamic formation of hydrogen bonds, which are triggered by the NIR for 3 min and tuned further by regulating the pH.2 Inspired by skin-like materials, both discussed works focus on using wireless technology to monitor the self-healing process using a smart hydrogel. However, maintaining the appropriate environmental conditions, including pH levels and NIR treatment, is a significant concern. Thus, in the subsequent study, Zhu's group elaborated on a healing process that relies on the contact between two cut parts, thereby eliminating the temperature requirement. The transparent PDMS elastomer consisted of imine bonds which required only 1 h at room temperature to invoke the dynamic nature of the bond.101 The healing process discussed in the research mentioned is undoubtedly valuable, especially under typical environmental conditions. However, it is crucial to note that the reproducibility of scratch healing has not been addressed, and this aspect is vital considering the daily wear and tear experienced by various materials in our surroundings. Hence, the development of stimuli-responsive self-healing polymers has simplified the process of repairing scratches and has also facilitated the use of scratch-monitoring technology. Indeed, these advancements represent valuable steps toward making self-healing materials more practical and effective, addressing the real-world demands for resilient and sustainable materials.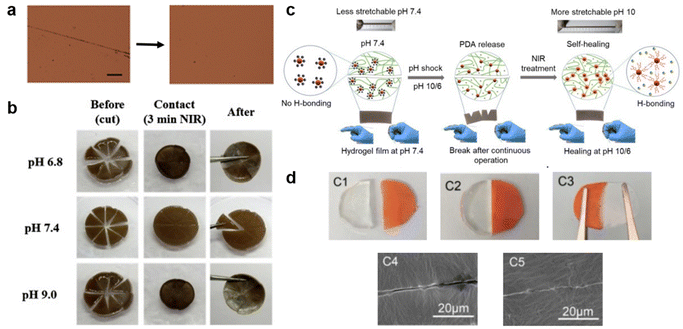 | ||
| Fig. 7 Impact of environmental conditions on scratch recovery. (a) Optical microscopy images of the scratched titin-mimicking polymer film before and after self-healing at 90 °C for 5 min. Scale bar: 100 μm. Reproduced with permission98 Copyright 2018, John Wiley and Sons. (b) Photograph of NIR-induced self-healing ability of pizza-like cut on zwitterionic-PNIPAAm hydrogel in varying pH followed by 3 min NIR treatment. Reproduced with permission100 Copyright 2019, Elsevier. (c) Schematic illustration of the real-time healing mechanism of the zwitterionic hydrogel after breaking and after pH and 3 min NIR treatments. Reproduced with permission2 Copyright 2021, Elsevier. (d) Photographs and SEM images of cut and healed polydimethylsiloxane elastomer samples healed at room temperature after 5 min contact. Reproduced with permission101 Copyright 2020, Elsevier. | ||
8. Summary and future outlook
The curiosity in and demand for advanced SHPs is growing considerably. In particular, three sectors, automobiles, display panels, and biomedical engineering, are shaping the current market and scientific trends in SHPs. In this review, we have focused on the impact of SHPs on scratch-repairing surfaces and the potential industry. Self-healing is based on various underlying mechanisms, and impressive advancements have been made in designing such polymers to perform under hostile environmental changes of temperature, radiation, electric field, corrosion, and acidic or alkaline conditions. The demand for an environmentally and economically sustainable future prompted researchers to upgrade the surface science applications of SHPs. Along with visualization of bond formation through FTIR, 1H-NMR, 13C-NMR and rheology, various advanced surface instruments, such as OM, AFM and SEM analysis have been utilized to investigate the self-healing mechanisms of scratched surfaces. Additionally, various advanced surface examination techniques, including sliding friction, micro to nano-scratch, nanoindentation, and evaluation of penetration depth, residual depth, residual scratch width, contact scratch width, and elastic recovery rates, have been thoroughly examined to prepare SHPs that can be more efficiently used in outdoor or indoor applications.In addition, SHPs have the potential to revolutionize various industries, including construction, automotive, and electronics. However, to fully understand and utilize their potential, several challenges should be taken into consideration. A key challenge is to accurately characterize the surface properties of SHPs, including surface energy, roughness, and topography to understand the relationship between these properties and the self-healing behavior. Another important improvement can be the development of a standardized and reproducible scratch-testing methodology to evaluate the self-healing properties of these materials. A deeper understanding of the mechanisms of self-healing at the surface and scratch level is required to optimize the self-healing behavior of these materials. Moreover, ensuring durability, and considering wear and tear resistance alongside self-healing behavior could potentially give rise to a wide range of real-world applications.
Furthermore, SHPs can be affected by various environmental conditions, such as temperature, light, pH, and physical contact can influence their self-healing behavior, highlighting the healing based on the stimuli-responsiveness of the polymer. Further research is needed to understand the impact of environmental conditions on the surface and scratch properties of SHPs. Overall, the challenges highlight the need for interdisciplinary approaches and the collaboration of experts across fields like materials science, chemistry, and engineering to tackle the drawbacks in the application of SHPs in surface scratch technology. Designing new methods for testing scratch resistance and self-healing properties of polymers in hostile environments will help to improve the durability and functionality of SHPs, which will be critical to suit different applications.
Abbreviations
| AFM | Atomic force microscopy |
| CAN | Covalent adaptive network |
| C-CNC | Carboxyl cellulose nanocrystal |
| CNT/EMAA | CNT-based poly[ethylene-co-(methacrylic acid)] composite |
| DA | Diels–Alder |
| DCPD | Dicyclopentadiene |
| DMF | N,N-Dimethylformamide |
| Fe | Iron metal |
| FRP | Fiber reinforced polymer |
| FTIR | Fourier-transform infrared spectroscopy |
| HUB | Hindered urea bond |
| LSM | Laser scanning microscopy |
| MF | Melamine-formaldehyde |
| Micro-CT | Micro-computed tomography |
| NMR | Nuclear magnetic resonance |
| NIR | Near-infrared light |
| OM | Optical microscopy |
| O/W | Oil-in-water |
| PA | Polyamide |
| PBS | Poly(butylene succinate) |
| PCL | Polycaprolactone |
| PDA | Polydopamine |
| PDMS | Poly(dimethylsiloxane) |
| PFOS | Perfluorooctanesulfonic acid potassium salt |
| PLA | Polylactide |
| PMEDSAH | (PEA)-b-poly-2-[(methacryloyloxy)ethyl] dimethyl-(3-sulfopropyl)ammonium hydroxide |
| PNIPAAm | Poly(N-isopropylacrylamide) |
| PVA | Poly(vinyl alcohol) |
| rDA | Retro-Diels–Alder |
| RMS | Root mean square |
| ROMP | Ring-opening metathesis polymerization |
| SCOF | Scratch coefficient of friction |
| SEM | Scanning electron microscopy |
| SHP | Self-healing polymer |
| TEM | Transmission electron microscopy |
| TPU | Thermoplastic polyurethane |
| UF | Urea-formaldehyde |
| UPy | Ureidopyrimidinone |
| XRD | X-ray diffraction |
Conflicts of interest
The authors declare no conflict of interest.Note added after first publication
This article replaces the version published on 1st December 2023, which contained errors in Fig. 1 and 4.Acknowledgements
The authors are grateful for the financial support from the National Research Foundation of Korea (Grant No. 20201I1A304491) and the Korea Research Institute of Chemical Technology (Grant No. SS2141-10-03). Also, the work is supported by SERB (Science & Technology Research Board)—a statutory body under the Department of Science & Technology, Government of India; under the Research grant of the Ramanujan Fellow Award (File number: SB/S2/RJN-013/2018).References
- Y. M. Kim, J. H. Kwon, S. Kim, U. H. Choi and H. C. Moon, Nat. Commun., 2022, 13, 3769 CrossRef CAS PubMed
.
- A. Shit, S. G. Kim, I. In and S. Y. Park, Chem. Eng. J., 2021, 426, 131846 CrossRef CAS
.
- Y. Cao, Y. J. Tan, S. Li, W. W. Lee, H. Guo, Y. Cai, C. Wang and B. C. K. Tee, Nat. Electron., 2019, 2, 75–82 CrossRef
.
- J. Tang, K. Xi, H. Chen, L. Wang, D. Li, Y. Xu, T. Xin, L. Wu, Y. Zhou and J. Bian, Adv. Funct. Mater., 2021, 31, 2102465 CrossRef CAS
.
- A. Taj, R. Zia, S. Younis, H. Hayat, W. S. Khan, and S. Z. Bajwa, Electrodes for Flexible Self-Healable Supercapacitors, Flex. Supercapacitor Nanoarchitectonics, Wiley online library, 2021, pp. 461–483 Search PubMed
.
- J. H. Yoon, S. M. Kim, Y. Eom, J. M. Koo, H. W. Cho, T. J. Lee, K. G. Lee, H. J. Park, Y. K. Kim and H. J. Yoo, ACS Appl. Mater. Interfaces, 2019, 11, 46165–46175 CrossRef CAS PubMed
.
- M. Goyal, S. N. Agarwal and N. Bhatnagar, J. Appl. Polym. Sci., 2022, 139, e52816 CrossRef CAS
.
- H. Kong, X. Luo, P. Zhang, J. Feng, P. Li, W. Hu, X. Wang and X. Liu, Nanomaterials, 2022, 13, 124 CrossRef PubMed
.
- L. Guadagno, M. Raimondo, M. Catauro, A. Sorrentino and E. Calabrese, J. Therm. Anal. Calorim., 2022, 147, 5463–5472 CrossRef CAS
.
- J. C. Cremaldi and B. Bhushan, Bioinspired self-healing materials: lessons from nature, Beilstein J. Nanotechnol., 2018, 9, 907–935 CrossRef CAS PubMed
.
- J. I. Park, A. Choe, M. P. Kim, H. Ko, T. H. Lee, S. M. Noh, J. C. Kim and I. W. Cheong, Polym. Chem., 2018, 9, 11–19 RSC
.
- S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2001, 409, 794–797 CrossRef CAS PubMed
.
- K. S. Toohey, N. R. Sottos, J. A. Lewis, J. S. Moore and S. R. White, Nat. Mater., 2007, 6, 581–585 CrossRef CAS PubMed
.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS PubMed
.
- C. C. Hornat and M. W. Urban, Prog. Polym. Sci., 2020, 102, 101208 CrossRef CAS
.
- P. Cordier, F. Tournilhac, C. Soulié-Ziakovic and L. Leibler, Nature, 2008, 451, 977–980 CrossRef CAS PubMed
.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed
.
- S. Utrera-Barrios, R. Verdejo, M. A. López-Manchado and M. H. Santana, Mater. Horiz., 2020, 7, 2882–2902 RSC
.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446–7467 RSC
.
- G. Dhanaraju, B. S. Ben and R. K. Pittala, Polymer, 2022, 245, 124734 CrossRef CAS
.
- L. M. Sáiz, M. G. Prolongo, V. Bonache, A. Jiménez-Suárez and S. G. Prolongo, Polym. Test., 2023, 117, 107832 CrossRef
.
- P. Mo, Z. Hu, Z. Mo, X. Chen, J. Yu, M. S. Selim, R. Sun, J. Xu, X. Zeng and Z. Hao, ACS Appl. Polym. Mater., 2022, 4, 4709–4718 CrossRef CAS
.
- L. Jiang, Y. Lei, Y. Xiao, X. Fu, W. Kong, Y. Wang and J. Lei, J. Mater. Chem. A, 2020, 8, 22369–22378 RSC
.
- S. Huang, M. Podgórski, X. Han and C. N. Bowman, Polym. Chem., 2020, 11, 6879–6883 RSC
.
- D. Y. Zhu, M. Z. Rong and M. Q. Zhang, Prog. Polym. Sci., 2015, 49, 175–220 CrossRef
.
- Y. Li, Y. Wang, S. Wang, Z. Ye, C. Bian, X. Xing, T. Hong and X. Jing, Macromolecules, 2022, 55, 9091–9102 CrossRef CAS
.
- D. H. Son, H. E. Bae, M. J. Bae, S. H. Lee, I. W. Cheong, Y. Il Park, J. E. Jeong and J. C. Kim, ACS Appl. Polym. Mater., 2022, 4, 3802–3810 CrossRef CAS
.
- E. N. Brown, M. R. Kessler, N. R. Sottos and S. R. White, J. Microencapsulation, 2003, 20, 719–730 CrossRef CAS PubMed
.
- G. Williams, R. Trask and I. Bond, Composites, Part A, 2007, 38, 1525–1532 CrossRef
.
- H. Ying, Y. Zhang and J. Cheng, Nat. Commun., 2014, 5, 3218 CrossRef PubMed
.
- S. Zechel, R. Geitner, M. Abend, M. Siegmann, M. Enke, N. Kuhl, M. Klein, J. Vitz, S. Gräfe and B. Dietzek, NPG Asia Mater., 2017, 9, e420 CrossRef CAS
.
- L. Zhang, T. Qiu, X. Sun, L. Guo, L. He, J. Ye and X. Li, Polymers, 2020, 12, 989 CrossRef CAS PubMed
.
- H. M. Lee, S. Perumal, G. Y. Kim, J. C. Kim, Y. R. Kim, M. P. Kim, H. Ko, Y. Rho and I. W. Cheong, Polym. Chem., 2020, 11, 3701–3708 RSC
.
- Z. Xie, B. L. Hu, R. W. Li and Q. Zhang, ACS Omega, 2021, 6, 9319–9333 CrossRef CAS PubMed
.
- S. K. Ghosh, M. P. Kim, S. Na, Y. Lee, J. Park, S. Cho, J. Cho, J. J. Kim and H. Ko, Nano Energy, 2022, 100, 107438 CrossRef CAS
.
- M. Cheng, Q. Fu, B. Tan, Y. Ma, L. Fang, C. Lu and Z. Xu, Prog. Org. Coat., 2022, 167, 106790 CrossRef CAS
.
- V. K. Thakur and M. R. Kessler, Polymer, 2015, 69, 369–383 CrossRef CAS
.
- S. H. Pezzin, Mechanism of Extrinsic and Intrinsic Self-healing in Polymer Systems, Springer Nature, Singapore, 2023, pp. 107–138 Search PubMed
.
- A. Torre-Muruzabal, L. Daelemans, G. Van Assche, K. De Clerck and H. Rahier, Polym. Test., 2016, 54, 78–83 CrossRef CAS
.
- Y. K. Song, H. W. Kim and C. M. Chung, Polymers, 2022, 14, 2013 CrossRef CAS PubMed
.
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2011, 10, 14–27 CrossRef CAS PubMed
.
- S. J. Garcia, Eur. Polym. J., 2014, 53, 118–125 CrossRef CAS
.
- N. F. M. Sani, H. J. Yee, N. Othman, A. Abd Talib and R. K. Shuib, Polym. Test., 2022, 111, 107598 CrossRef
.
- Y. Zhang, J. Zheng, W. Ma, X. Zhang, Y. Du, K. Li, Y. Liu, G. Yu and Y. Jia, React. Funct. Polym., 2022, 178, 105364 CrossRef CAS
.
- A. Liguori and M. Hakkarainen, Macromol. Rapid Commun., 2022, 43, 2100816 CrossRef CAS PubMed
.
- P. Wu, L. Liu and Z. Wu, Composites, Part A, 2022, 162, 107170 CrossRef CAS
.
- L. Pettazzoni, F. Leonelli, A. Martinelli, L. M. Migneco, S. Alfano, D. Di Luca, L. Celio and V. Di Lisio, J. Appl. Polym. Sci., 2022, 139, e52408 CrossRef CAS
.
- M. Tian, H. Zuo, J. Wang, N. Ning, B. Yu and L. Zhang, Polym. Chem., 2020, 11, 4047–4057 RSC
.
- K. Sugane, Y. Maruoka and M. Shibata, J. Polym. Res., 2021, 28, 1–10 CrossRef
.
- A. Khan, N. Ahmed and M. Rabnawaz, Polymers, 2020, 12, 2027 CrossRef CAS PubMed
.
- N. S. Kasalkova, P. Slepicka, Z. Kolska and V. Svorcik, Wetting Wettability, 2015, 12, 323–356 Search PubMed
.
- F. C. Birjandi and J. Sargolzaei, Colloids Surf., A, 2014, 448, 93–106 CrossRef
.
- M. Z. Khan, J. Militky, M. Petru, B. Tomková, A. Ali, E. Tören and S. Perveen, Eur. Polym. J., 2022, 111481 CrossRef
.
- S. Wang, K. Liu, X. Yao and L. Jiang, Chem. Rev., 2015, 115, 8230–8293 CrossRef CAS PubMed
.
- J. Li, G. Van Ewijk, D. J. Van Dijken, J. Van Der Gucht and W. M. De Vos, ACS Appl. Mater. Interfaces, 2021, 13, 21844–21853 CrossRef CAS PubMed
.
- L. K. Ncube, A. U. Ude, E. N. Ogunmuyiwa, R. Zulkifli and I. N. Beas, Materials, 2020, 13, 4994 CrossRef CAS PubMed
.
- M. Ge, C. Cao, F. Liang, R. Liu, Y. Zhang, W. Zhang, T. Zhu, B. Yi, Y. Tang and Y. Lai, Nanoscale Horiz., 2020, 5, 65–73 RSC
.
- W. P. Chen, D. Z. Hao, W. J. Hao, X. L. Guo and L. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 1258–1265 CrossRef CAS PubMed
.
- C. Jiang, L. Zhang, Q. Yang, S. Huang, H. Shi, Q. Long, B. Qian, Z. Liu, Q. Guan and M. Liu, Nat. Commun., 2021, 12, 4395 CrossRef CAS PubMed
.
- S. Bode, M. Enke, M. Hernandez, R. K. Bose, A. M. Grande, S. van der Zwaag, U. S. Schubert, S. J. Garcia and M. D. Hager, Self-Healing Mater., 2016, 113–142 CAS
.
- Y. Yang, D. Davydovich, C. C. Hornat, X. Liu and M. W. Urban, Chem, 2018, 4, 1928–1936 CAS
.
- C. C. Hornat and M. W. Urban, Nat. Commun., 2020, 11, 1028 CrossRef CAS PubMed
.
- J. Zhao, R. Xu, G. Luo, J. Wu and H. J. Xia, J. Mater. Chem. B, 2016, 4, 982–989 RSC
.
- A. P. Putri, R. K. Bose, M. Chalid and F. Picchioni, Polymers, 2023, 15, 1010 CrossRef CAS PubMed
.
- Z. Wang, G. Fei, H. Xia and H. Zuilhof, J. Mater. Chem. B, 2018, 6, 6930–6935 RSC
.
- S. M. Kim, H. Jeon, S. H. Shin, S. A. Park, J. Jegal, S. Y. Hwang, D. X. Oh and J. Park, Adv. Mater., 2018, 30, 1705145 CrossRef PubMed
.
- A. V. Menon, G. Madras and S. Bose, ACS Omega, 2018, 3, 1137–1146 CrossRef CAS PubMed
.
- M. S. Koochaki, S. N. Khorasani, R. E. Neisiany, A. Ashrafi, S. P. Trasatti and M. Magni, J. Mater. Sci., 2021, 56, 1794–1813 CrossRef
.
- F. Xu, X. Li, Y. Li and J. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 27955–27963 CrossRef CAS PubMed
.
- J. Dahlke, J. Kimmig, M. Abend, S. Zechel, J. Vitz, U. S. Schubert and M. D. Hager, NPG Asia Mater., 2020, 12, 13 CrossRef CAS
.
- M. Abend, L. Tianis, C. Kunz, S. Zechel, S. Gräf, F. A. Müller, U. S. Schubert and M. D. Hager, Polym. Test., 2020, 90, 106699 CrossRef CAS
.
- Q. Ouyang, L. Liu and Z. Wu, Polymers, 2022, 14, 4313 CrossRef CAS PubMed
.
- Y. Paez-Amieva, J. Carpena-Montesinos and J. M. Martín-Martínez, Polymers, 2023, 15, 2152 CrossRef CAS PubMed
.
- J. Ekeocha, C. Ellingford, M. Pan, A. M. Wemyss, C. Bowen and C. Wan, Adv. Mater., 2021, 33, 2008052 CrossRef CAS PubMed
.
- A. Zhou, Q. Qiu, C. L. Chow and D. Lau, Composites, Part A, 2020, 131, 105802 CrossRef CAS
.
- Y. Zhang, J. Zheng, W. Ma, X. Zhang, Y. Du, K. Li, Y. Liu, G. Yu and Y. Jia, Eur. Polym. J., 2022, 175, 111394 CrossRef CAS
.
- W. M. Xu, M. Z. Rong and M. Q. Zhang, J. Mater. Chem. A, 2016, 4, 10683–10690 RSC
.
- M. V. Biyani, E. J. Foster and C. Weder, ACS Macro Lett., 2013, 2, 236–240 CrossRef CAS PubMed
.
- C. Kim, H. Ejima and N. Yoshie, J. Mater. Chem. A, 2018, 6, 19643–19652 RSC
.
- J. J. Lessard, L. F. Garcia, C. P. Easterling, M. B. Sims, K. C. Bentz, S. Arencibia, D. A. Savin and B. S. Sumerlin, Macromolecules, 2019, 52, 2105–2111 CrossRef CAS
.
- Z. Li, Y. L. Zhu, W. Niu, X. Yang, Z. Jiang, Z. Y. Lu, X. Liu and J. Sun, Adv. Mater., 2021, 33, 2101498 CrossRef CAS PubMed
.
- L. Wang, L. Guo, K. Zhang, Y. Xia, J. Hao and X. Wang, Angew. Chem., Int. Ed., 2023, 202301762 Search PubMed
.
- Y. Wang, X. Huang and X. Zhang, Nat. Commun., 2021, 12, 1291 CrossRef CAS PubMed
.
- Y. M. Ha, Y. N. Kim and Y. C. Jung, Polymers, 2021, 13, 1274 CrossRef CAS PubMed
.
- J. Cao, C. Lu, J. Zhuang, M. Liu, X. Zhang, Y. Yu and Q. Tao, Angew. Chem., 2017, 129, 8921–8926 CrossRef
.
- J. J. Rajesh and J. Bijwe, Wear, 2005, 259, 661–668 CrossRef CAS
.
- A. Dasari, Z. Z. Yu and Y. W. Mai, Mater. Sci. Eng., R, 2009, 63, 31–80 CrossRef
.
- P. Kurkcu, L. Andena and A. Pavan, Wear, 2012, 290, 86–93 CrossRef
.
- N. Saurín, J. Sanes and M. D. Bermúdez, Tribol. Lett., 2014, 56, 133–142 CrossRef
.
- N. Gafsi, R. Verdejo, M. Kharrat, M. Barletta, M. Á. López-Manchado and M. Dammak, J. Coat. Technol. Res., 2021, 18, 1269–1280 CrossRef CAS
.
- W. Brostow, P. E. Cassidy, J. Macossay, D. Pietkiewicz and S. Venumbaka, Polym. Int., 2003, 52, 1498–1505 CrossRef CAS
.
- L. Yi, Y. Xu, D. Li, J. Shen, S. Guo and H. J. Sue, Ind. Eng. Chem. Res., 2018, 57, 4320–4328 CrossRef CAS
.
- Y. Zheng, X. Ji, M. Yin, J. Shen and S. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 32270–32279 CrossRef CAS PubMed
.
- Y. Li, L. Zhang and C. Li, J. Colloid Interface Sci., 2020, 559, 273–281 CrossRef CAS PubMed
.
- G. Molero, C. Tsai, C. Liu, H. Sue, S. Uenuma, K. Mayumi and K. Ito, J. Appl. Polym. Sci., 2021, 138, 51237 CrossRef CAS
.
- G. Molero and H. J. Sue, Mater. Des., 2019, 182, 107965 CrossRef CAS
.
- C. Tsai, C. Chang, M. Zhao and H. Sue, J. Appl. Polym. Sci., 2021, 138, 50993 CrossRef CAS
.
- M. Arslan, B. Kiskan and Y. Yagci, Sci. Rep., 2017, 7, 1–11 CrossRef CAS PubMed
.
- Y. Song, Y. Liu, T. Qi and G. L. Li, Angew. Chem., Int. Ed., 2018, 57, 13838–13842 CrossRef CAS PubMed
.
- A. I. Robby, G. Lee and S. Y. Park, Sens. Actuators, B, 2019, 297, 126783 CrossRef CAS
.
- P. Wang, L. Yang, B. Dai, Z. Yang, S. Guo, G. Gao, L. Xu, M. Sun, K. Yao and J. Zhu, Eur. Polym. J., 2020, 123, 109382 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |