Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Using internal strain and mass to modulate Dy⋯Dy coupling and relaxation of magnetization in heterobimetallic metallofullerenes DyM2N@C80 and Dy2MN@C80 (M = Sc, Y, La, Lu)†
Yajuan
Hao
ab,
Georgios
Velkos
a,
Sandra
Schiemenz
a,
Marco
Rosenkranz
a,
Yaofeng
Wang
a,
Bernd
Büchner
a,
Stanislav M.
Avdoshenko
 *a,
Alexey A.
Popov
*a,
Alexey A.
Popov
 *a and
Fupin
Liu
*a and
Fupin
Liu
 *a
*a
aLeibniz Institute for Solid State and Materials Research (IFW Dresden), 01069 Dresden, Germany. E-mail: s.avdoshenko@ifw-dresden.de; a.popov@ifw-dresden.de; f.liu@ifw-dresden.de
bSchool of Electrical and Mechanical Engineering, Pingdingshan University, Pingdingshan 467000, China
First published on 17th November 2022
Abstract
Endohedral clusters inside metallofullerenes experience considerable inner strain when the size of the hosting cage is comparably small. This strain can be tuned in mixed-metal metallofullerenes by combining metals of different sizes. Here we demonstrate that the internal strain and mass can be used as variables to control Dy⋯Dy coupling and relaxation of magnetization in Dy-metallofullerenes. Mixed-metal nitride clusterfullerenes DyxY3−xN@Ih-C80 (x = 0–3) and Dy2LaN@Ih-C80 combining Dy with diamagnetic rare-earth elements, Y and La, were synthesized and characterized by single-crystal X-ray diffraction, SQUID magnetometry, ab initio calculations, and spectroscopic techniques. DyxY3−xN clusters showed a planar structure, but the slightly larger size of Dy3+ in comparison with that of Y3+ resulted in increased elongation of the nitrogen thermal ellipsoid, showing enhancement of the out-of-plane vibrational amplitude. When Dy was combined with larger La, the Dy2LaN cluster appeared strongly pyramidal with the distance between two nitrogen sites of 1.15(1) Å, whereas DyLa2N@C80 could not be obtained in a separable yield. Magnetic studies revealed that the relaxation of magnetization and blocking temperature of magnetization in the DyM2N@C80 series (M = Sc, Y, Lu) correlated with the mass of M, with DySc2N@C80 showing the fastest and DyLu2N@C80 the slowest relaxation. Ab initio calculations predicted very similar g-tensors for Dy3+ ground state pseudospin in all studied DyM2N@C80 molecules, suggesting that the variation in relaxation is caused by different vibrational spectra of these compounds. In the Dy2MN@C80 series (M = Sc, Y, La, Lu), the magnetic and hysteretic behavior was found to correlate with Dy⋯Dy coupling, which in turn appears to depend on the size of M3+. Across the Dy2MN@C80 series, the energy difference between ferromagnetic and antiferromagnetic states changes from 5.6 cm−1 in Dy2ScN@C80 to 3.0 cm−1 in Dy2LuN@C80, 1.0 cm−1 in Dy2YN@C80, and −0.8 cm−1 in Dy2LaN@C80. The coupling of Dy ions suppresses the zero-field quantum tunnelling of magnetization but opens new relaxation channels, making the relaxation rate dependent on the coupling strengths. DyY2N@C80 and Dy2YN@C80 were found to be non-luminescent, while the luminescence reported for DyY2N@C80 was caused by traces of Y3N@C80 and Y2ScN@C80.
Introduction
Nitride clusterfullerenes with the molecular composition M3N@C2n is one of the most versatile classes of endohedral metallofullerenes (EMFs).1–6 The cage size ranges from C68 to C98 and even larger; however the highly symmetric Ih-C80 fullerene is usually most abundantly produced in the synthesis. The M3N cluster has a triangular shape with a nitride ion in its center and three M(III) rare-earth metals located at the apexes of the triangle. Of particular interest is the possibility to combine two or even three different metals in the M3N cluster in the arc-discharge process and the ability of chromatographic techniques to separate mixtures of such heterometallic (mixed-metal) EMFs into individual components.7–20 When specific interactions between metal ions are important, the mixed-metal approach provides the possibility to study such interactions by systematically varying the composition of the M3N cluster. For instance, the magnetic properties of EMFs depend on the dipolar and exchange interactions between endohedral lanthanide ions,21,22 and these interactions can be addressed by changing the number and type of magnetic ions in the heterobimetallic M′xM′′3−xN@C80 system (x = 0–3, M′ and M′′ are different rare-earth metals, including non-magnetic Sc, Y, La, or Lu, and magnetic 4f lanthanides).22–31 Such a precise composition control in a virtually identical chemical environment is quite unique and rarely available for heterometallic systems in lanthanide chemistry.32–38In this work, we employ the M′xM′′3−xN@C80 platform to study the influence of the internal strain on the static and dynamic magnetic properties of Dy-containing nitride clusterfullerenes. The use of external stimuli to modulate the properties of single molecule magnets has attracted significant attention as it opens the way for multifunctional magnetic systems.39,40 Mechanical pressure was found to change both the ligand field and magnetic interactions in several transition-metal single-molecule magnets (SMMs),41–48 but the studies of pressure-induced variations in the SMM behavior remain very scarce for lanthanide SMMs.49–52 One of the main effects of mechanical pressure on molecular systems is the internal strain, which not necessarily requires the use of external pressure as it can be addressed by a judicious molecular design. Endohedral fullerenes provide an opportunity to engineer the internal strain through varying the size of the endohedral cluster. A fullerene cage is rather rigid and its size is usually not changed much when the internal species are varied. As a result, the endohedral M3N cluster experiences a strain of different magnitude depending on the size of constituting metals.53 This strain can be attested for via variation of different structural and physicochemical properties. For instance, the C80-Ih fullerene cage is the most preferred for the metal-nitride cluster, and M3N@Ih-C80 species are usually obtained with the highest yield among other cages, but when the metal ions are too large, the yield of EMFs decreases and the M3N cluster changes its geometry from planar to pyramidal (M = Tb, Gd).54,55 With a further increase of the metal size, the distribution shifts from C80 to larger cages, and for La and Ce, M3N@C80 is not formed at all.56–58 Likewise, the preferable fullerene cage isomers can be changed depending on the size and shape of the endohedral cluster.9,59–61 The size of the cluster and the associated internal strain were also shown to influence NMR and vibrational spectroscopic properties,7,13,62 dynamics of the M3N cluster inside the cage,63,64 preferable cycloaddition sites,65–68 and even electrochemical potentials of endohedral Ce(IV/III)14,15 and Ti(IV/III)69,70 redox couples. An earlier study demonstrated that the replacement of Sc by Lu in both DySc2N@C80 and Dy2ScN@C80 metallofullerenes resulted in the noticeable variation of their dynamic magnetic properties and Dy⋯Dy coupling.24 The exact reasons why diamagnetic metals can influence the magnetic behavior of Dy remained unclear as both the mass and size of the diamagnetic ion could play a certain role. Here we extend the DyM2N@C80 and Dy2MN@C80 series with further diamagnetic rare-earth metals, Y and La, and analyze the changes in internal strain and its relationship with magnetic properties when the size of the diamagnetic metal M is varied from Sc to La. Thanks to the different variations of the ionic radius and atomic mass in the Sc3+–Y3+–La3+–Lu3+ series, we could distinguish two factors playing the main roles in the relaxation of magnetization of nitride clusterfullerenes, that is, the mass of M in the DyM2N@C80 series and the size-related strain in the Dy2MN@C80 series.
Synthesis and separation
Nitride clusterfullerenes were synthesized using a previously developed approach.71 Dy–Sc and Dy–Lu nitride clusterfullerenes were obtained by us earlier,13,24,71,72 and in this work the synthesis was performed for Dy–Y and Dy–La systems. A mixture of M2O3 (M = Y or La), Dy2O3, guanidinium thiocyanate and graphite powder with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 was filled in core-drilled graphite rods. The rods were evaporated by the direct current arc-discharge process in the chamber filled with He as a cooling gas (180 mbar). The produced soot was washed with acetone for 2 hours to remove the polyaromatic hydrocarbons, and then Soxhlet extracted with CS2 overnight to dissolve the fullerenes. The CS2 extract was separated by high-performance liquid chromatography (HPLC).
15 was filled in core-drilled graphite rods. The rods were evaporated by the direct current arc-discharge process in the chamber filled with He as a cooling gas (180 mbar). The produced soot was washed with acetone for 2 hours to remove the polyaromatic hydrocarbons, and then Soxhlet extracted with CS2 overnight to dissolve the fullerenes. The CS2 extract was separated by high-performance liquid chromatography (HPLC).
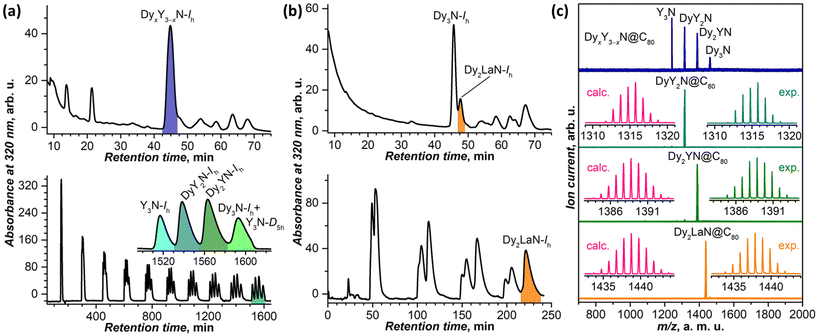
Fig. 1a and b show typical HPLC profiles of the extracts obtained in the Dy–Y and Dy–La EMF syntheses. The use of the organic nitrogen source suppresses the formation of empty fullerenes, and nitride clusterfullerenes become the main fullerene products.73 The most abundant fraction in the Dy–Y system is the DyxY3−xN@Ih-C80 mixture. According to mass-spectrometry analysis (Fig. 1c) and recycling HPLC (Fig. 1a), it contains components with all x from 0 to 3. This agrees with the similarity of the ionic radii of Dy3+ and Y3+, which largely determines the probability of the EMF formation with a given cage size. The metals with substantially different ionic radii give a strong variation of the composition in the mixed-metal EMF synthesis,13 and the Dy–La system is a good example of this behaviour. The overall yield of Dy–La EMFs is considerably lower than that of Dy–Y EMFs, and the main HPLC fraction is Dy3N@Ih-C80 (Fig. 1b). Dy2LaN@C80 is found in the adjacent fraction with much lower abundance, whereas DyLa2N@C80 and La3N@C80 were not formed at all, at least in the amounts detectable by LDI-TOF MS. Apparently, the La3+ ion is too large, and the formation of La2MN@C80 was not detected even in the La–Sc system, with rather small Sc3+ ions.74,75 As to La3N-clusterfullerenes, the smallest cage able to accommodate the La3N cluster is C88.57
Individual target compounds were obtained by subjecting the respective fractions to recycling HPLC (Fig. 1a and b). The DyxY3−xN@Ih-C80 mixture is well resolved after 10 cycles, allowing collection of pure Y3N@Ih-C80, DyY2N@Ih-C80, and Dy2YN@Ih-C80 in subsequent cycles, whereas Dy3N@Ih-C80 still has an admixture of another isomer of Y3N@C80 with a D5h cage, which has longer elution time than the Ih isomer. Dy2LaN@Ih-C80 could be obtained after 4 cycles, the main admixtures being the tail of Dy3N@Ih-C80 from the preceding fraction and sulphide clusterfullerene Dy2S@C82 formed due to the presence of sulfur in the nitrogen source.76 The purity of isolated Dy–Y and Dy–La nitride clusterfullerenes was evaluated by laser desorption/ionization time-of-flight mass spectrometry (LDI-TOF MS) as shown in Fig. 1c. UV-vis-NIR and FT-IR absorption spectra of DyY2N@C80, Dy2YN@C80, and Dy2LaN@C80 (Fig. S1 and S2†) exhibit the characteristic pattern of M3N@Ih-C80 EMFs.
Single-crystal X-ray diffraction
Molecular structures of DyxY3−xN@Ih-C80 (x = 0–3) and Dy2LaN@Ih-C80 were studied by single-crystal X-ray diffraction (SC-XRD). Crystals were obtained by co-crystallization of fullerenes with nickel(II) octaethylporphyrin (NiOEP). For this, benzene or CS2 solutions of EMFs were layered with NiOEP solution in benzene in glass tubes and left to diffuse over 2–4 weeks, resulting in the formation of black block crystals on the walls. X-ray diffraction data collection was carried out at the BESSY storage ring (BL14.2, Berlin-Adlershof, Germany).77 XDSAPP2.0 suite was employed for data processing.78,79 The structure was solved by direct methods and refined using SHELXL-2018.80 Hydrogen atoms were added geometrically and refined with a riding model. The crystal data are presented in Tables S1 and S2.†DyxY3−xN@Ih-C80 (x = 0–3)
M3N@C80 molecules usually co-crystallize with NiOEP in one of the three space groups, C2/c with 8 molecules, C2/m with 4 molecules, and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with 2 molecules in the unit cell (Table S3†). C2/c is found in this work for Y3N@C80, DyY2N@C80, and Dy3N@C80. Their asymmetric unit contains one intact NiOEP molecule, one intact fullerene, one intact solvent benzene, and two halves of solvent benzene. Dy2YN@C80 crystallized in the P
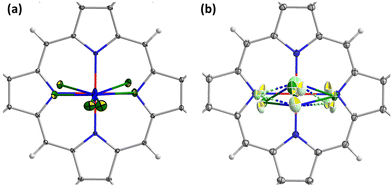
with 2 molecules in the unit cell (Table S3†). C2/c is found in this work for Y3N@C80, DyY2N@C80, and Dy3N@C80. Their asymmetric unit contains one intact NiOEP molecule, one intact fullerene, one intact solvent benzene, and two halves of solvent benzene. Dy2YN@C80 crystallized in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and its asymmetric unit contains one intact NiOEP molecule, one intact fullerene, and two intact solvent benzene molecules. The fullerene cage is ordered and unambiguously assigned to the Ih(7) isomer in all four crystals, and the symmetry notation of Ih(7)-C80 will be omitted hereafter. The packing in the crystals, with one fullerene molecule supported by one NiOEP molecule, is also typical of EMF·NiOEP co-crystals.72 An identical orientation of the fullerene cage versus NiOEP is found in all three crystals with the C2/c space group, whereas the cage orientation of Dy2YN@C80 is somewhat different (Fig. 2).
space group, and its asymmetric unit contains one intact NiOEP molecule, one intact fullerene, and two intact solvent benzene molecules. The fullerene cage is ordered and unambiguously assigned to the Ih(7) isomer in all four crystals, and the symmetry notation of Ih(7)-C80 will be omitted hereafter. The packing in the crystals, with one fullerene molecule supported by one NiOEP molecule, is also typical of EMF·NiOEP co-crystals.72 An identical orientation of the fullerene cage versus NiOEP is found in all three crystals with the C2/c space group, whereas the cage orientation of Dy2YN@C80 is somewhat different (Fig. 2).
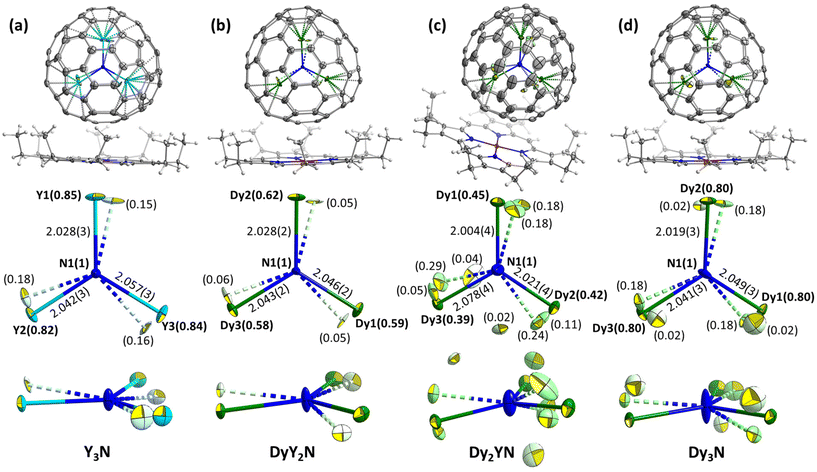
Analysis of all reported M3N@Ih-C80·NiOEP crystal structures (see Table S3 in the ESI†) shows that unless the M3N cluster is severely disordered, it usually has two M3N sites, of which the major one has an occupancy of 0.7–0.9. The two sites share a common nitrogen position and are related by a rotation of 10–20° around an axis located nearly perpendicular to their M3N planes so that the two sites appear roughly in one plane. This common pattern was found earlier for Gd3N,54 Dy3N,62 Ho3N,81 Er3N,81 Tm3N,82 Lu3N,64 DyEr2N,13 and DyGd2N,13 and in this work for Y3N, DyY2N and Dy3N (Fig. 2a, b and d). Dy2YN shows a higher degree of disorder in the cluster (Fig. 2c).
The ionic radii of Y3+ (0.90 Å) and Dy3+ (0.91 Å) are rather similar, and it is therefore hard to expect that these metals will have different positions in Y2DyN@C80 and YDy2N@C80. The more plausible scenario is the uniform distribution of Y and Dy in all three positions in the M3N cluster. Indeed, we could not differentiate between Dy and Y in the crystal structures of these fullerenes, and decided to apply free occupancy refinement on all possible metal positions with the relatively heavier Dy to identify the metal positions of DyY2 and Dy2Y. Since Y has fewer electrons than Dy, the maximal total occupancy of such “Dy3+” sites should be 0.68 in DyY2N and 0.84 in Dy2YN. Despite the similarity of ionic radii, the influence of the metal size on the cluster is still discernible. With the stepwise replacement of Y3+ with Dy3+, the shape of the nitrogen thermal ellipsoid gradually elongates in the direction perpendicular to the M3 plane. This points to the increasing amplitude of the out-of-plane nitrogen vibration, which is associated with the trend towards M3N pyramidalization with the increasing radius of M3+. At the threshold between the planar and pyramidal clusters, the amplitude is the highest as shown in ref. 13, and this is the reason for a particular strong ellipsoid elongation found in Dy3N here and also in an earlier report62 (our new structure has different Dy3N site occupancies but otherwise is very similar). We therefore refined the data with one position of N and the elongated ellipsoid, rather than splitting the nitrogen into two sites.
For Y3N@C80, a slightly pyramidal Y3N cluster with a displacement of nitrogen from the Y3 plane by 0.129(6) Å was reported in the pyrrolidine adduct,83 while a planar Y3N cluster was found in a phenyl-C81-butyric acid methyl ester derivative.84 In the co-crystal of pristine Y3N@C80 with NiOEP reported here for the first time, the Y3N cluster is planar.
Dy2LaN@Ih-C80
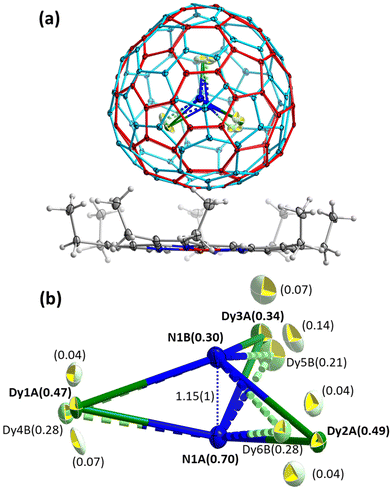
Dy2LaN@C80 also co-crystallized with NiOEP in the C2/c space group. The asymmetric unit contains one intact NiOEP molecule, one intact fullerene with two overlapping orientations, one intact solvent benzene, and two halves of solvent benzene. The fullerene cage is disordered between two orientations (site occupancy ratio of 0.61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.39) as shown in Fig. 4a and S3,† which are correlated with a mirror plane passing through N–Ni–N bonds and perpendicular to the NiOEP molecule.
0.39) as shown in Fig. 4a and S3,† which are correlated with a mirror plane passing through N–Ni–N bonds and perpendicular to the NiOEP molecule.
The Dy2LaN cluster has considerable disorder with four metal sites (Fig. 4b), which hampers the reliable differentiation between Dy and La. We applied free occupancy refinement with the heavier Dy to identify the metal positions of Dy2La. Note that for La uniformly distributed between three sites, the site occupancy of such “Dy” should be 0.94. The Dy2LaN cluster is pyramidal and has two nitrogen sites (N1A/N1B with occupancies of 0.70/0.30), which is common for the M3N cluster in M3N@C80 molecules with large metals, such as DyGd2N,13 CeLu2N,85 Tb3N,55 or Gd3N.54 The Dy2LaN cluster has the highest sum of ionic radii of three M3+ ions among all M3N@C80 molecules studied by SC-XRD and shows the highest pyramidalization with an N1A/N1B out of M3-plane displacement of 0.619(4)/0.530(9) Å determined for Dy1A/Dy2A/Dy3A sites with the largest occupancy, and an N1A⋯N1B distance of 1.15(1) Å. This can be compared to 0.522(8)/0.46(2) Å in Gd3N (N⋯N distance: 0.991 Å),54 0.46(2)/0.45(2) Å in DyGd2N (0.91(3) Å),13 0.453(4)/0.405(7) Å in Tb3N (0.858(8) Å),55 and 0.349(8)/0.325(8) Å in CeLu2N (0.685(9) Å).85 The M–N1A distances in the most abundant sites of Dy2LaN are 2.095(4) (Dy1A), 2.082(3) (Dy2A), and 2.118(5) Å (Dy3A). For comparison, DFT predicts the Dy–N and La–N bonds in Dy2LaN@C80 to be 2.072 Å and 2.156 Å long, and the lack of a clear differentiation between Dy–N and La–N bond lengths in the crystal structure indicates that La is equally distributed between all metal positions. The same conclusion can also be made based on the occupancies of metal sites in Fig. 4b.
NiOEP has a considerable influence on the position of metal atoms inside the fullerene. In a vast majority of M3N@C80·NiOEP co-crystals, two metal atoms of the M3N cluster are located above the N–Ni–N bonds.63,64,81,86,87 The recently reported Tb2@C80(CF3) co-crystallized with NiOEP also shows such a tendency.88 This effect seems to have an electrostatic nature as endohedral metals tend to be located close to negatively charged nitrogens of the porphyrin,87 and this alignment was observed in the absence of Ni, when the LaSc2N@C80 fullerene was co-crystallized with H2OEP.75Fig. 3 and S4† show that this pattern is also realized in DyxY3−xN@Ih-C80 (x = 0–3) and Dy2LaN@Ih-C80. Even when the metals are disordered, the sites with the highest occupancy are located above NiOEP nitrogens.
Magnetic properties
Structure of the M3N cluster and single-ion magnetic anisotropy of Dy3+
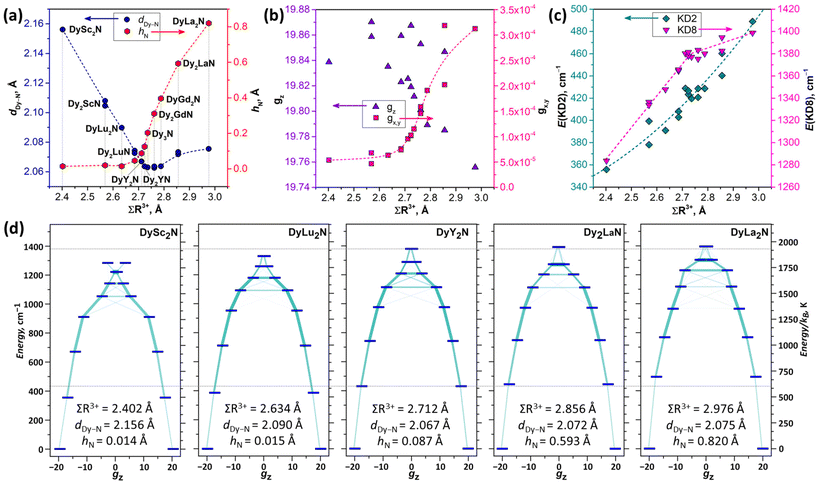
Confinement of the M3N cluster inside the rigid fullerene cage results in a strain, which can be assessed through geometrical parameters of the cluster, such as the unusually short M–N bond lengths. For instance, the Dy–N bond length distribution in molecular compounds has a maximum near 2.5 Å, whereas Dy–N bonds in M3N@C80 are shorter than 2.1 Å (see Fig. 2 for examples from this work and ref. 13 for more details). This shortening has important consequences on magnetic properties since the negatively charged nitride ion (formal charge −3, Bader charge of −1.729,89) produces a strong uniaxial ligand field and hence leads to the large magnetic anisotropy of Dy3+.The structural parameters of the M3N cluster can be further adjusted by the variation of its composition, and in particular by combining metals of different sizes. For instance, Y3+ is larger than Sc3+, and the Dy–N bond in DyY2N@C80 (Fig. 2b) is shorter than that in DySc2N@C80 (2.096(6) Å according to SC-XRD72). Thus, combining Dy in M3N with large metals might be considered as a strategy to shorten Dy–N bonds and thereby increase the magnetic anisotropy. However, this compression effect has its limits, and after a certain threshold, the M–N bonds will not be shortened anymore. Instead, the cluster changes its shape from planar to pyramidal with the nitride ion elevated above the metal plane.13,54,55,81,85 Experimental examples for Dy-containing M3N@C80 characterized by SC-XRD are DyGd2N@C80 from ref. 13 and Dy2LaN@C80 described in this work (Fig. 4). As the ligand field is not purely an electrostatic phenomenon,90 the influence of this geometrical change on the magnetic anisotropy is a priori not clear. To better understand it, we performed a systematic ab initio computational study for a series of DyM2N@C80 and Dy2MN@C80 molecules. A combination of Dy (R3+ = 0.912 Å) with Sc (0.745 Å), Lu (0.861 Å), Y (0.900 Å), Gd (0.938 Å), or La (1.032 Å) allows for a smooth variation of the cluster size. We used DFT-optimized structures from ref. 13 for this survey because not all SC-XRD structures are available, and the available ones often suffer from a significant degree of disorder, which prevents the analysis of a subtle variation of the M–N bond lengths (see Fig. 2 and 4). As the parameter describing the net size of metals in the M3N cluster, the sum of their Shannon's ionic radii91 (∑R3+) is used.
Fig. 5a illustrates the bond shortening and cluster pyramidalization trends in DFT-optimized structures. The Dy–N bond length (dDy–N) decreases fast with the increase of ∑R3+ from 2.4 Å (DySc2N) to 2.7 Å (DyY2N), but then the shortening of dDy–N stagnates and even reverses to a slight elongation in Dy2LaN and DyLa2N when ∑R3+ exceeds 2.8 Å. In parallel, the cluster pyramidalization (hN) starts to grow when ∑R3+ exceeds 2.7 Å, and the growth continues all the way up to hN = 0.82 Å in the hypothetical DyLa2N@C80 with a ∑R3+ of 2.98 Å.
Single-ion magnetic anisotropy of Dy3+ ions was then studied ab initio at the CASSCF(9,7)/RASSI-SO level in OpenMOLCAS.92,93 The Dy atoms in M3N@C80 molecules were treated one at a time, while other magnetic ions (Dy and Gd) were replaced with Y. The results of ab initio computations are summarized in Fig. 5b–d, and full data are given in Tables S4–S6.† The ground magnetic state of Dy3+ in all studied molecules is the Kramers doublet (KD) with essentially a pure mJ = ±15/2 character. For the rigorous mJ = ±15/2 KD, the principal values of the pseudospin g-tensor should be (0, 0, 20). In M3N@C80 molecules, gz values are spread between 19.78 and 19.88 without any obvious regularity except for a vague trend to decrease for larger metals, and in particular for DyLa2N@C80 (Fig. 5b). The transversal components, quantified as 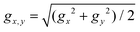 , exhibit a more regular variation with ∑R3+. The gx,y value increases with the size of metals and closely follows hN dependence. In fact, there is a good linear correlation between gx,y and hN (Fig. S5†). Since the transversal components of the ground-state g-tensors define the tunnelling gap and thus determine the efficiency of the quantum tunnelling of magnetization (QTM) in zero magnetic field, the cluster pyramidalization may be considered an important factor for the QTM. But in the DyM2N@C80 series, for which this kind of QTM is crucial, the strong cluster pyramidalization is achieved only in the experimentally non-available DyLa2N@C80, whereas in DyM2N@C80 with Sc, Lu, and Y the cluster is nearly planar.
, exhibit a more regular variation with ∑R3+. The gx,y value increases with the size of metals and closely follows hN dependence. In fact, there is a good linear correlation between gx,y and hN (Fig. S5†). Since the transversal components of the ground-state g-tensors define the tunnelling gap and thus determine the efficiency of the quantum tunnelling of magnetization (QTM) in zero magnetic field, the cluster pyramidalization may be considered an important factor for the QTM. But in the DyM2N@C80 series, for which this kind of QTM is crucial, the strong cluster pyramidalization is achieved only in the experimentally non-available DyLa2N@C80, whereas in DyM2N@C80 with Sc, Lu, and Y the cluster is nearly planar.
Fig. 5c compares the size of the ligand-field splitting in the studied molecules focusing on the energy of the first excited KD (KD2) and on the whole splitting of the ground-state 6H15/2 multiplet (which is equal to the energy of the eighth KD, KD8). Selected examples of the LF splitting in individual DyM2N@C80 and Dy2MN@C80 molecules are shown in Fig. 5d. Both the overall LF splitting and the KD2 energy increase with ∑R3+, but in a different fashion. E(KD8) shows two distinct segments in its growth, which are related to the nature of the structural changes. While dDy–N decreases (that is, for ∑R3+ < 2.7 Å), the growth of E(KD8) is relatively fast, but beyond the cluster pyramidalization threshold, the growth of E(KD8) is slowed down. In contrast, E(KD2) grows continuously in the whole ∑R3+ range, which can be well described by a quadratic function (Fig. 5c).
To summarize, our computations showed that the ground magnetic state of Dy3+ in M3N@C80 molecules is weakly affected by the size of the metals forming the cluster and the variation of the cluster geometry. The transversal components of the g-tensors do increase with the cluster pyramidalization, but there is a lack of experimentally available molecules, where this effect may have a pronounced influence on magnetic behavior. Ligand field splitting is more susceptible to the cluster shape variations. The total splitting can be indeed related to the Dy–N bond length, and therefore it experiences only marginal changes when the metal size increase leads to cluster pyramidalization. But the energy of the first excited KD appears to be sensitive to the size-dependent strain itself, irrespective of the changes in the cluster geometry it causes.
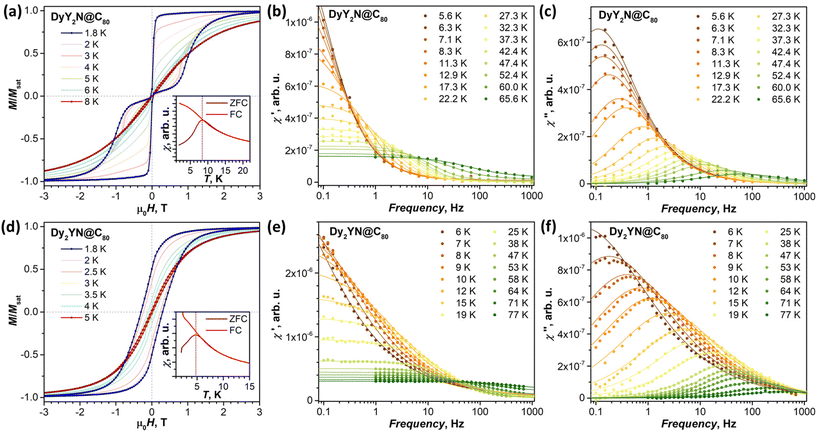
Magnetic hysteresis
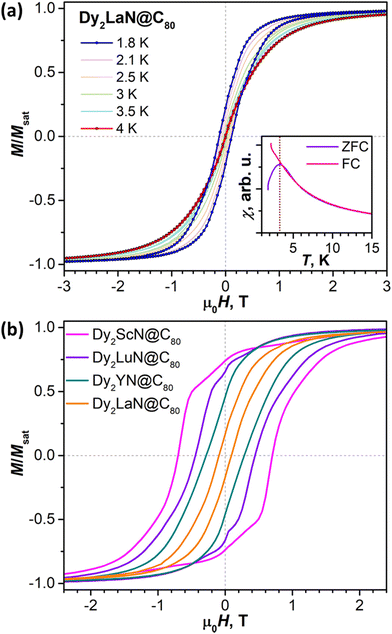
We next turn to the experimental studies of the magnetic properties of DyY2N@C80, Dy2YN@C80, and Dy2LaN@C80 by SQUID magnetometry. DyY2N@C80 shows waist-restricted (butterfly) magnetic hysteresis with an abrupt drop of magnetization near zero field (Fig. 6a). This feature is caused by the quantum tunnelling of magnetization (QTM) and is typical of single-ion Dy SMMs.27,30,94–98 The hysteresis remains open up to 8 K when measured at a sweep rate of 2.9 mT s−1. Determination of the blocking temperature TB from the zero-field cooled/in-field cooled (ZFC/FC) temperature dependence of magnetic susceptibility gives a value of 8.4 K (Fig. 6a). Similar characteristics of magnetic hysteresis were obtained for DyY2N@C80 in a recent report by Wang et al.30Magnetic hysteresis of Dy2YN@C80 remains open up to 5 K. Likewise, the hysteresis without distinct QTM features is measured for Dy2LaN@C80 up to 4 K (Fig. 7a). TB values in Dy2YN@C80 and Dy2LaN@C80 are 4.7 and 3.3 K, respectively. The zero-field QTM feature in the hysteresis curves of both EMFs is absent, as is common for dinuclear EMF-SMMs.22,24,99–101 The coupling of two magnetic moments prevents an independent QTM-flip of one of the moments in zero field,22 whereas the simultaneous flip of both moments is a rare-probability process detectable only at very low temperatures.102
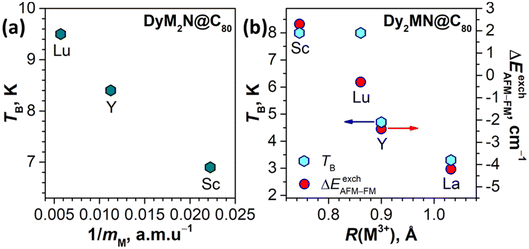
With the data from this work, we can compare hysteretic behaviour in complete sets of DyM2N@C80 and Dy2MN@C80 with the diamagnetic rare-earth M. Earlier we found that the TB of DyLu2N@C80, 9.5 K, is higher than that of DySc2N@C80 by 2.6 K.24 The value of 8.4 K determined here for DyY2N@C80 lies in between. Different from DySc2N@C80 and DyY2N@C80, DyLu2N@C80 showed some remanence and less efficient zero-field QTM.24 Overall, the TB in the DyM2N@C80 series increases from Sc to Y to Lu and thus tends to correlate with the increase of the atomic mass rather than the ionic radius in this row (Fig. 8a).
A different situation is found in the binuclear Dy2MN@C80 series. Dy2ScN@C80 and Dy2LuN@C80 showed similar TB and hysteresis closing temperatures of 8 K, but with a narrower hysteresis in Dy2LuN@C80.24 The new data from this work reveal a decrease of the blocking temperature on going from Dy2LuN to Dy2YN to Dy2LaN (Fig. 8b), as well as a decrease of the 2 K coercive field in the Dy2MN series, Sc (0.70 T) > Lu (0.44 T) > Y (0.28 T) > La (0.09 T). The latter effect is illustrated in Fig. 7b, which compares the hysteresis curves of four EMFs. Thus, the hysteretic behaviour in the Dy2MN@C80 series changes systematically with the M3+ size.
Importantly, when the metal M in DyM2N@C80 or Dy2MN@C80 is paramagnetic, the hysteretic behaviour is quite different from those of its diamagnetic analogs. At 1.8 K, Dy3N@C80 shows only a narrow hysteresis with fast QTM in zero field, which is caused by the multiply degenerate frustrated ground state.22 In Dy2GdN@C80, the presence of Gd accelerates the relaxation of magnetization when compared to Dy2ScN@C80 and Dy2YN@C80, so that only a very narrow hysteresis is open at 1.8 K.23 In DyGd2N@C80, DyEr2N@C80, and Dy2ErN@C80, we could not find an open hysteresis down to 1.8 K (see Fig. S6† for magnetization curves; the synthesis is described in ref. 13).
Dy⋯Dy coupling in Dy2MN@C80
A considerable difference between the hysteresis curves of DyY2N@C80 and Dy2YN@C80 emphasizes that the interaction between the magnetic moments of Dy ions plays a crucial role in the static and dynamic magnetic behaviour, especially at low temperatures. To determine the sign and size of the Dy⋯Dy coupling, we employed the effective spin Hamiltonian (eqn (1)) successfully used earlier for other {Dy2} EMFs:24,99,100,102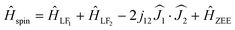 | (1) |
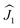 and
and 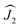 ) scaled by the effective coupling constant j12, and the interaction with the magnetic field is given in the Zeeman term ĤZEE. In essence, the ligand-field terms split the ground-state multiplet of each Dy ion into 8 KDs, whereas the interaction term couples individual KDs of different ions. In the low-energy part of the spectrum, the Hamiltonian gives two quasi-doublets formed by coupling of the ground state KDs of two ions (KD1 and KD1′) with ferromagnetic (FM) and antiferromagnetic (AFM) alignment of magnetic moments (Fig. S7†). The energy difference between the AFM and FM states is:
) scaled by the effective coupling constant j12, and the interaction with the magnetic field is given in the Zeeman term ĤZEE. In essence, the ligand-field terms split the ground-state multiplet of each Dy ion into 8 KDs, whereas the interaction term couples individual KDs of different ions. In the low-energy part of the spectrum, the Hamiltonian gives two quasi-doublets formed by coupling of the ground state KDs of two ions (KD1 and KD1′) with ferromagnetic (FM) and antiferromagnetic (AFM) alignment of magnetic moments (Fig. S7†). The energy difference between the AFM and FM states is: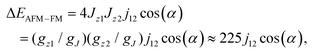 | (2) |
To determine j12 and thereby ΔEAFM–FM for Dy2YN@C80 and Dy2LaN@C80, we measured their magnetization curves at different temperatures between 1.8 and 200 K and fitted them using the Hamiltonian (1) with j12 as a free parameter. The ab initio ligand-field splitting is already discussed above (Fig. 5 and Tables S4–S6†); the angles between the magnetic moments of 60.6° (Dy2YN) and 63.0° (Dy2LaN) were obtained from the same ab initio calculations and are approximately equal to the geometrical angle: α° ≈ 180 − ∠(Dy–N–Dy). The fits performed employing the PHI code106 with powder averaging gave the ΔEAFM–FM values of 1.0 ± 0.1 cm−1 (j12 = 0.009 ± 0.001 cm−1) in Dy2YN@C80 and −0.8 ± 0.2 cm−1 (j12 = −0.008 ± 0.002 cm−1) in Dy2LaN@C80 (see Fig. S8 and S9† for comparison of experimental points and fitted curves). These values can be compared to ΔEAFM–FM = 5.6 cm−1 in Dy2ScN@C80 and ΔEAFM–FM = 3.0 cm−1 in Dy2LuN@C80.24 Thus, we conclude that the ΔEAFM–FM value in the Dy2MN@C80 series systematically decreases and even changes its sign with the increase of the M3+ size. Furthermore, the contribution to ΔEAFM–FM from dipole–dipole interactions can be calculated using geometry parameters and α angles, giving similar values of ΔEdipAFM–FM = 3.3–3.4 cm−1 in all Dy2MN@C80 molecules. Therefore, the metal size dependence is mainly caused by the exchange contribution (likely Dy–N–Dy superexchange), which is ferromagnetic in Dy2ScN@C80 (ΔEexchAFM–FM = +2.3 cm−1), but becomes progressively antiferromagnetic in other Dy2MN@C80 molecules with the increase of M (−0.3 cm−1 for Lu, −2.4 cm−1 for Y, and −4.2 cm−1 for La). Interestingly, this systematic variation cannot be well correlated with the cluster geometrical parameters such as the Dy–N bond length (the values are similar for Dy2LuN and Dy2LaN), or the Dy–N–Dy angle (the values remain similar for all Dy2MN), or the degree of cluster pyramidalization (Dy2ScN and Dy2LuN are just planar and Dy2YN is nearly planar). Only the M3+ ionic radius appears to be a suitable parameter to correlate with ΔEexchAFM–FM (Fig. 8b).
Relaxation of magnetization
The magnetic hysteresis indicates that the studied EMFs feature single-molecule magnetic behaviour, which calls for a study of the relaxation of magnetization. Relaxation times, τM, were measured at different temperatures employing both DC and AC techniques. In the former, the samples were saturated at 7 Tesla, then the field was ramped at 70 mT s−1 to 0 T or to 0.2 T, and then a decay of magnetization was measured and fitted with stretched exponential function. This technique allows reliable measurement of τM not shorter than 50–100 s; the values longer than 104 s are also hard to determine since one would need to measure magnetization decay for a very long time. In AC measurements, the field was oscillated around 0 T with the amplitude of 5 Oe, giving in-phase and out-of-phase magnetic susceptibilities, χ′ and χ′′. At each temperature, the frequency dependence of χ′ and χ′′ was measured and the curves were fitted with a generalized Debye model. Fig. 6(b, c, e and f) shows χ′ and χ′′ data for DyY2N@C80 and Dy2YN@C80. The values of relaxation times are listed in Tables S7 and S8 in the ESI.† The amount of synthesized Dy2LaN@C80 was not sufficient for its studies by AC magnetometry.τM−1(T) = τQTM−1 + CTn + τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/T) exp(−Ueff/T) | (3) |
The QTM regime with τQTM = 2.2 ± 0.1 s dominates below 7 K, the Raman regime with C = (3.1 ± 0.3) × 10−3 s−1 K−n and n = 2.53 ± 0.03 has the main contribution up to 55 K, and the Orbach mechanism with an attempt time of τ0 = (2.5 ± 1.8) × 10−9 s−1 and an effective barrier of Ueff = 929 ± 47 K outweighs the Raman process above 60 K. The Orbach mechanism involving excited ligand-field states is naturally expected for DyY2N@C80, and most probably it is its onset that we detect above 60 K. But since this process can only be attested by a few highest-temperature τM points measured at the accuracy limits of our magnetometer, the reliability of the Ueff value does not seem sufficient for a discussion of particular KDs involved.
In the finite field of 0.2 T, applied to suppress the QTM, the relaxation times of DyY2N@C80 increased dramatically and could be measured by the DC technique between 2.8 and 7 K. The values showed a steady decrease with temperature and could be well described by the Raman process with C = (1.2 ± 0.1) × 10−6 s−1 K−n and n = 5.05 ± 0.08 (dashed blue line in Fig. 9). An alternative fit with the Orbach mechanism gave τ0 = 3.0 ± 0.5 s−1 and Ueff = 21.3 ± 0.6 K, similar to the values reported by Wang et al.,30 but the agreement with the experimental data is worse than that for the Raman mechanism. Note that the removal of the QTM part from eqn (2) and keeping the fitted zero-field parameters for Raman and Orbach mechanisms cannot produce the long relaxation times measured at 0.2 T, indicating that there may be more processes involved in the relaxation and that the mechanism identified at zero field as Raman should be also coupled to the QTM, despite its obvious temperature dependence. Whether the QTM shows unconventional temperature dependence,72,107 or there may be another relaxation process, which evades detection by AC measurements due to its lower rate, is hard to determine at this moment.
τM−1(T) = CTn + τ0,1−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff1/T) + τ0,2−1 exp(−Ueff1/T) + τ0,2−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff2/T) exp(−Ueff2/T) | (4) |
The Raman mechanism with C = (1.11 ± 0.07) × 10−4 s−1 K−n and n = 3.34 ± 0.06 describes the low-T part (1.8–4 K) measured by DC magnetometry. The first Orbach regime with τ0,1 = (1.84 ± 0.06) × 10−3 s−1 and a barrier of Ueff1 = 43.8 ± 0.3 K is well established between 6 and 50 K, whereas the second Orbach regime with τ0,2 = (8 ± 4) × 10−8 s−1 and a barrier of Ueff2 = 680 ± 40 K dominates above 60 K. Similar to the measurements for DyY2N@C80, the parameters of the high-T regime are determined with limited accuracy because of the low signal intensity at these temperatures near the sensitivity limits. Thus, we can hypothesize that this process corresponds to the relaxation via ligand-field excited states, but the precise determination of the involved states is hardly possible.
The Orbach regime with a barrier of 44 K (30 cm−1) is quite remarkable. It cannot be assigned to ligand-field excited states since the latter have energies at least an order of magnitude higher (Fig. 5). Thus, Ueff1 most likely corresponds to a vibrational mode with a strong spin–phonon coupling. The Arrhenius behavior with the energy equal vibrational frequency was predicted for spin–lattice relaxation coupled to local or optical phonons already back in 1960s108,109 and re-introduced in more recent theoretical studies.110 The fullerene cages are rather rigid, and their vibrations occur above 200 cm−1, but the endohedral clusters are composed of heavy atoms and have several modes at low frequencies.24,62,101 In Dy2YN@C80, the lowest-frequency modes predicted by DFT in harmonic approximation for an isolated molecule are rotational motions (librations) of the cluster at 40.6, 41.1, and 48.3 cm−1. A simple harmonic approximation is most probably not fully suitable for these modes; besides, such intramolecular vibrations can strongly mix with lattice modes,24 which altogether would affect their frequencies and facilitate the momentum transfer.
In the low-temperature range, the Raman process with a small exponent is only weakly affected by a magnetic field of 0.2 T, presumably via the direct mechanism. Note that the relaxation times of Dy2YN@C80 at low temperatures are much longer than those in DyY2N@C80 in zero field, but considerably shorter than the latter in a field of 0.2 T. Thus, the coupling of Dy moments quenches the zero-field QTM, but adds new relaxation pathways, which makes the relaxation of Dy2YN@C80 faster once the QTM in DyY2N@C80 is suppressed. The difference in relaxation times between mono- and dinuclear systems is considerable at higher temperatures as well and remains detectable even when the relaxation starts to be dominated by ligand-field excited states.
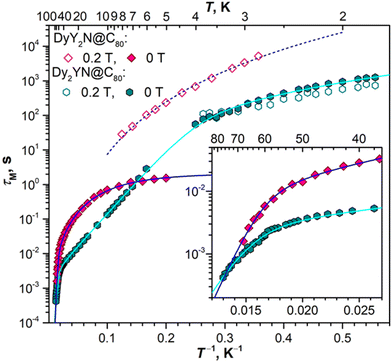
Fig. 10 compares magnetization relaxation times in DyM2N@C80 and Dy2MN@C80 series. At low temperatures, all three mono-Dy EMFs show similar relaxation behaviour, including the very long relaxation in a field of 0.2 T and the fast temperature-independent QTM in zero field. The longest time both in zero field and in a finite field is found for DyLu2N@C80, followed by DyY2N@C80, and then DySc2N@C80. These data are in line with the row of TB values for these EMFs. At higher temperatures, DyY2N@C80 and DySc2N@C80 both show a power-law temperature dependence, DySc2N@C80 being again somewhat faster. The data for DyLu2N@C80 in that temperature range are not available as the sample amount in ref. 24 was not sufficient for AC measurements.
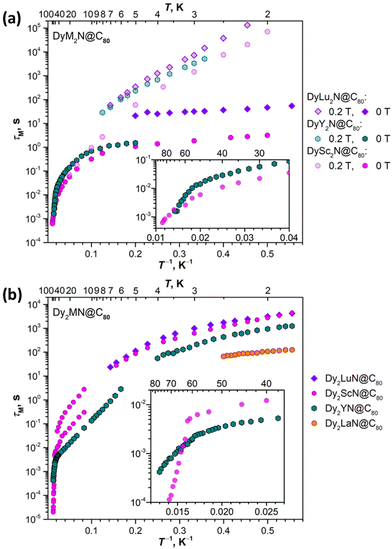 | ||
| Fig. 10 Magnetization relaxation times of the DyM2N@C80 series (a; M = Sc, Lu, Y; field 0 T and 0.2 T) and Dy2MN@C80 series (b; M = Sc, Lu, Y, La; field 0 T). Insets show enlargement of high-temperature parts. The data for M = Sc are from ref. 72 (DySc2N) and ref. 71 (Dy2ScN), for M = Lu – from ref. 24. | ||
The differences in relaxation rates in single-ion magnets can be either attributed to the different single-ion magnetic anisotropy or to the aforementioned spin–phonon interactions. As our computational study showed that the ground-state KD properties of Dy3+ are virtually identical (Fig. 5), it makes the differences in the spin–phonon coupling a primary factor. The correlation with the mass of M rather than its size is also a strong indication toward the phonon-based difference in relaxation rates in the DyM2N@C80 series. But the found sequence of relaxation rates (DySc2N is faster than DyY2N, and the latter is faster than DyLu2N) is rather counter-intuitive. One could expect a reversed order of relaxation rates because heavier clusters have larger vibrational DOS at lower energies, which facilitates the spin–lattice relaxation at low temperature.
In the Dy2MN@C80 series, the rates determined at low temperature for Dy2ScN and Dy2LuN are very similar, while Dy2YN and Dy2LaN show progressively faster relaxation. The fast decrease of τM falls in line with the decrease of the ΔEAFM–FM difference in this row and hence correlates with the size of M3+. At higher temperatures, the relaxation in Dy2YN@C80 remains faster than that in Dy2ScN@C80 until the switch to the Orbach regime via single-ion LF-excitations. At that moment, Dy2ScN@C80 starts to show a faster relaxation as it also has a higher barrier and thus its relaxation accelerates faster with temperature. We conclude that while the relaxation of magnetization in Dy2MN@C80 is governed by a Dy⋯Dy coupled state, the ΔEAFM–FM value seems to be the main factor in play, and variations of the phonon DOS caused by a different mass of the M ion are less critical.
Photoluminescence of DyY2N@C80
Our earlier finding of thermally activated delayed fluorescence (TADF) in the series of YxSc3−xN@C80 EMFs (x = 0–3)111,112 suggested that similar phenomena may be present in other M3N@C80 molecules. Indeed, TADF was recently reported in DyY2N@C80.30 This result was very intriguing since the presence of Dy in the endohedral cluster was expected to increase the spin–orbit coupling and quench the fullerene-based radiative process. As ref. 30 lacked the characterization of the triplet state of DyY2N@C80 below 80 K, we decided to study the photoluminescence (PL) of DyY2N@C80 at helium temperatures. The measurements appeared complicated by a PL signal of the Y3N@C80 trace (which is also identifiable in ref. 30), and additional HPLC cycles were performed to minimize its contribution. After more thorough removal of Y3N@C80 (note that its content was already below the sensitivity of HPLC and MALDI-TOF and could only be assessed by PL measurements), we realized that the spectral features and PL lifetimes of purified DyY2N@C80 are virtually identical to those of Y2ScN@C80 (Fig. S10†).111 This surprising finding was then confirmed by LDI-TOF mass-spectrometry analysis, which revealed a small signal of Y2ScN@C80 when the measurements were performed with strongly increased laser power; under our standard LDI-TOF measurement conditions, this signal was not detectable (Fig. S11†). Based on these studies, we conclude that DyY2N@C80 is non-luminescent, whereas the PL signals that we and others30 detected in DyY2N@C80 samples were caused by tiny traces of Y3N@C80 and Y2ScN@C80, which could not be detected by other techniques, but were still measurable in PL spectra. The contamination with Y2ScN@C80 is presumably caused by traces of Sc in the Y2O3 oxide used as the Y source in the arc-discharge synthesis. Analogous measurements for Dy2YN@C80 also did not show PL activity.Conclusions
In this work, we reported the synthesis, isolation, and single-crystal X-ray diffraction study of Dy2LaN@Ih-C80 and complete DyxY3−xN@Ih-C80 series (x = 0–3). The M3N cluster is found to be planar in all DyxY3−xN EMFs, albeit with a systematic increase of the nitrogen out-of-plane amplitude from Y3N to Dy3N. The Dy2LaN cluster is strongly pyramidal with a nitrogen out-of-plane displacement of 0.619(4)/0.530(9) Å. The studies of the magnetic properties of DyY2N@C80, Dy2YN@C80, and Dy2LaN@C80 revealed SMM behavior with magnetic hysteresis in all EMFs but with strongly metal-dependent parameters. Comparison of the relaxation behavior to other DyM2N@C80 and Dy2MN@C80 EMFs (M = Sc, Lu) and extended ab initio calculations revealed that the SMM properties in the DyM2N@C80 series can be correlated with the mass of M, whereas the variation of magnetic properties in the Dy2MN@C80 row correlates with the ionic radius of M3+. The mass dependence of spin–lattice relaxation points to a clear connection with the phonon degrees of freedom, whereas the size dependence appears to be related to the variation of the Dy⋯Dy coupling. In particular, the energy of the exchange Dy⋯Dy interaction changes from a positive (ferromagnetic) value of +2.3 cm−1 in Dy2ScN@C80 to an antiferromagnetic coupling of −4.2 cm−1 in Dy2LaN@C80 with intermediate values of −0.3 cm−1 in Dy2LuN@C80 and −2.4 cm−1 in Dy2YN@C80. These magneto-structural correlations demonstrate that the variation of the endohedral cluster size and engineering of the inner strain in EMFs can be used as control parameters for tuning the static and dynamic magnetic properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support by Deutsche Forschungsgemeinschaft (grants LI 3055/3-1, PO 1602/6-1, PO 1602/7-1, and AV 169/3-1) and the China Scholarship Council (Fellowship to Y. H.). Diffraction data have been collected on BL14.2 at the BESSY II electron storage ring operated by the Helmholtz-Zentrum Berlin; we would particularly like to acknowledge the help and support of Manfred Weiss and his group members during the experiments at BESSY II. Computational resources were provided by the Center for High Performance Computing at the TU Dresden. We appreciate the technical support with computational resources in IFW Dresden by Ulrike Nitzsche. Dr Anja Wolter-Giraud and Sebastian Gaß are acknowledged for their help with magnetic measurements in IFW Dresden.References
- L. Dunsch and S. Yang, Metal Nitride Cluster Fullerenes: Their Current State and Future Prospects, Small, 2007, 3(8), 1298–1320 CrossRef CAS PubMed.
- J. Zhang, S. Stevenson and H. C. Dorn, Trimetallic Nitride Template Endohedral Metallofullerenes: Discovery, Structural Characterization, Reactivity, and Applications, Acc. Chem. Res., 2013, 46(7), 1548–1557 CrossRef CAS.
- M. N. Chaur, F. Melin, A. L. Ortiz and L. Echegoyen, Chemical, Electrochemical, and Structural Properties of Endohedral Metallofullerenes, Angew. Chem., Int. Ed., 2009, 48, 7514–7538 CrossRef CAS PubMed.
- A. A. Popov, S. Yang and L. Dunsch, Endohedral Fullerenes, Chem. Rev., 2013, 113(8), 5989–6113 CrossRef CAS PubMed.
- X. Lu, W. Shen and S. Hu, Endohedral Metallofullerenes: New Structures and Unseen Phenomena, Chem. – Eur. J., 2020, 26(26), 5748–5757 CrossRef PubMed.
- S. Yang, T. Wei and F. Jin, When metal clusters meet carbon cages: endohedral clusterfullerenes, Chem. Soc. Rev., 2017, 46(16), 5005–5058 RSC.
- S. Yang, A. A. Popov and L. Dunsch, Carbon Pyramidalization in Fullerene Cages Induced by the Endohedral Cluster: Non-Scandium Mixed Metal Nitride Clusterfullerenes, Angew. Chem., Int. Ed., 2008, 47, 8196–8200 CrossRef CAS PubMed.
- S. F. Yang, M. Kalbac, A. Popov and L. Dunsch, Gadolinium-based mixed metal nitride clusterfullerenes GdxSc3−xN@C80 (x = 1, 2), ChemPhysChem, 2006, 7(9), 1990–1995 CrossRef CAS PubMed.
- A. L. Svitova, A. A. Popov and L. Dunsch, Gd/Sc-based Mixed Metal Nitride Cluster Fullerenes: The Mutual Influence of the Cage and Cluster Size and the Role of Sc in the Electronic Structure, Inorg. Chem., 2013, 52(6), 3368–3380 CrossRef CAS PubMed.
- S. Yang, A. A. Popov, C. Chen and L. Dunsch, Mixed Metal Nitride Clusterfullerenes in Cage Isomers: LuxSc3−xN@C80 (x = 1, 2) As Compared with MxSc3−xN@C80 (M = Er, Dy, Gd, Nd), J. Phys. Chem. C, 2009, 113(18), 7616–7623 CrossRef CAS.
- T. Wei, F. Liu, S. Wang, X. Zhu, A. A. Popov and S. Yang, An Expanded Family of Dysprosium–Scandium Mixed-Metal Nitride Clusterfullerenes: The Role of Lanthanide Metal on the Carbon Cage Size Distribution, Chem. – Eur. J., 2015, 21(15), 5750–5759 CrossRef CAS PubMed.
- Z. Zhang, Y. Liu, P. Han, S. Zhuang, T. Wang, S. Luo and B. Xu, Metallofullerenes Encaging Mixed-Metal Clusters: Synthesis and Structural Studies of GdxHo3−xN@C80 and GdxLu3−xN@C80, ChemPhysChem, 2015, 16, 295–298 CrossRef CAS PubMed.
- C. Schlesier, F. Liu, V. Dubrovin, L. Spree, B. Büchner, S. Avdoshenko and A. A. Popov, Mixed dysprosium-lanthanide nitride clusterfullerenes DyM2N@C80-Ih and Dy2MN@C80-Ih (M = Gd, Er, Tm, and Lu): synthesis, molecular structure, and quantum motion of the endohedral nitrogen atom, Nanoscale, 2019, 11, 13139–13153 RSC.
- Y. Zhang, A. A. Popov and L. Dunsch, Endohedral Metal or a Fullerene Cage Based Oxidation? Redox Duality of Nitride Clusterfullerenes CexM3−xN@C78–88 (x = 1, 2; M = Sc and Y) Dictated by the Encaged Metals and the Carbon Cage Size, Nanoscale, 2014, 6, 1038–1048 RSC.
- Y. Zhang, S. Schiemenz, A. A. Popov and L. Dunsch, Strain-Driven Endohedral Redox Couple CeIV/CeIII in Nitride Clusterfullerenes CeM2N@C80 (M = Sc, Y, Lu), J. Phys. Chem. Lett., 2013, 4, 2404–2409 CrossRef CAS.
- C. Chen, F. Liu, S. Li, N. Wang, A. A. Popov, M. Jiao, T. Wei, Q. Li, L. Dunsch and S. Yang, Titanium/Yttrium Mixed Metal Nitride Clusterfullerene TiY2N@C80: Synthesis, Isolation, and Effect of the Group-III Metal, Inorg. Chem., 2012, 51(5), 3039–3045 CrossRef CAS.
- S. Yang, C. Chen, A. Popov, W. Zhang, F. Liu and L. Dunsch, An endohedral titanium(III) in a clusterfullerene: putting a non-group-III metal nitride into the C80-Ih fullerene cage, Chem. Commun., 2009, 6391–6393 RSC.
- T. Wei, S. Wang, X. Lu, Y. Tan, J. Huang, F. Liu, Q. Li, S. Xie and S. Yang, Entrapping a Group-VB Transition Metal, Vanadium, within an Endohedral Metallofullerene: VxSc3−xN@Ih-C80 (x = 1, 2), J. Am. Chem. Soc., 2016, 138(1), 207–214 CrossRef CAS PubMed.
- M. Nie, J. Xiong, C. Zhao, H. Meng, K. Zhang, Y. Han, J. Li, B. Wang, L. Feng, C. Wang and T. Wang, Luminescent single-molecule magnet of metallofullerene DyErScN@Ih-C80, Nano Res., 2019, 12(7), 1727–1731 CrossRef CAS.
- M. M. Olmstead, A. de Bettencourt-Dias, J. C. Duchamp, S. Stevenson, H. C. Dorn and A. L. Balch, Isolation and crystallographic characterization of ErSc2N@C80: an endohedral fullerene which crystallizes with remarkable internal order, J. Am. Chem. Soc., 2000, 122(49), 12220–12226 CrossRef CAS.
- L. Spree and A. A. Popov, Recent advances in single molecule magnetism of dysprosium-metallofullerenes, Dalton Trans., 2019, 48(9), 2861–2871 RSC.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, K. Krämer, S.-X. Liu, S. Decurtins, A. Popov, S. Yang, L. Dunsch and T. Greber, Tunneling, remanence, and frustration in dysprosium-based endohedral single-molecule magnets, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89(6), 060406 CrossRef.
- A. Kostanyan, C. Schlesier, R. Westerström, J. Dreiser, F. Fritz, B. Büchner, A. A. Popov, C. Piamonteze and T. Greber, Gadolinium as an accelerator for reaching thermal equilibrium and its influence on the ground state of Dy2GdN@C80 single-molecule magnets, Phys. Rev. B, 2021, 103(1), 014404 CrossRef CAS.
- L. Spree, C. Schlesier, A. Kostanyan, R. Westerström, T. Greber, B. Büchner, S. Avdoshenko and A. A. Popov, Single molecule magnets DyM2N@C80 and Dy2MN@C80 (M = Sc, Lu): The impact of diamagnetic metals on the Dy3+ magnetic anisotropy, Dy⋯Dy coupling, and mixing of molecular and lattice vibrations, Chem. – Eur. J., 2020, 26(11), 2436–2449 CrossRef CAS PubMed.
- A. Kostanyan, R. Westerström, D. Kunhardt, B. Büchner, A. A. Popov and T. Greber, Sub-Kelvin hysteresis of the dilanthanide single-molecule magnet Tb2ScN@C80, Phys. Rev. B, 2020, 101(13), 134429 CrossRef CAS.
- A. Kostanyan, R. Westerström, Y. Zhang, D. Kunhardt, R. Stania, B. Büchner, A. A. Popov and T. Greber, Switching Molecular Conformation with the Torque on a Single Magnetic Moment, Phys. Rev. Lett., 2017, 119(23), 237202 CrossRef.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch and T. Greber, An Endohedral Single-Molecule Magnet with Long Relaxation Times: DySc2N@C80, J. Am. Chem. Soc., 2012, 134(24), 9840–9843 CrossRef PubMed.
- A. L. Svitova, Y. Krupskaya, N. Samoylova, R. Kraus, J. Geck, L. Dunsch and A. A. Popov, Magnetic moments and exchange coupling in nitride clusterfullerenes GdxSc3−xN@C80 (x = 1–3), Dalton Trans., 2014, 43, 7387–7390 RSC.
- Y. Zhang, D. Krylov, S. Schiemenz, M. Rosenkranz, R. Westerstrom, J. Dreiser, T. Greber, B. Buchner and A. A. Popov, Cluster-size dependent internal dynamics and magnetic anisotropy of Ho ions in HoM2N@C80 and Ho2MN@C80 families (M = Sc, Lu, Y), Nanoscale, 2014, 6, 11431–11438 RSC.
- M. Nie, J. Liang, C. Zhao, Y. Lu, J. Zhang, W. Li, C. Wang and T. Wang, Single-Molecule Magnet with Thermally Activated Delayed Fluorescence Based on a Metallofullerene Integrated by Dysprosium and Yttrium Ions, ACS Nano, 2021, 15(12), 19080–19088 CrossRef CAS PubMed.
- J. Dreiser, R. Westerström, Y. Zhang, A. A. Popov, L. Dunsch, K. Krämer, S.-X. Liu, S. Decurtins and T. Greber, The Metallofullerene Field-Induced Single-Ion Magnet HoSc2N@C80, Chem. – Eur. J., 2014, 20(42), 13536–13540 CrossRef CAS PubMed.
- N. Ishikawa, T. Iino and Y. Kaizu, Determination of Ligand-Field Parameters and f-Electronic Structures of Hetero-Dinuclear Phthalocyanine Complexes with a Diamagnetic Yttrium(III) and a Paramagnetic Trivalent Lanthanide Ion, J. Phys. Chem. A, 2002, 106(41), 9543–9550 CrossRef CAS.
- L. S. Natrajan, A. J. L. Villaraza, A. M. Kenwright and S. Faulkner, Controlled preparation of a heterometallic lanthanide complex containing different lanthanides in symmetrical binding pockets, Chem. Commun., 2009,(40), 6020–6022 RSC.
- D. Aguilà, L. A. Barrios, V. Velasco, O. Roubeau, A. Repollés, P. J. Alonso, J. Sesé, S. J. Teat, F. Luis and G. Aromí, Heterodimetallic [LnLn′] Lanthanide Complexes: Toward a Chemical Design of Two-Qubit Molecular Spin Quantum Gates, J. Am. Chem. Soc., 2014, 136(40), 14215–14222 CrossRef PubMed.
- C. D. Buch, S. H. Hansen, D. Mitcov, C. M. Tram, G. S. Nichol, E. K. Brechin and S. Piligkos, Design of pure heterodinuclear lanthanoid cryptate complexes, Chem. Sci., 2021, 12(20), 6983–6991 RSC.
- J. J. Le Roy, J. Cremers, I. A. Thomlinson, M. Slota, W. K. Myers, P. H. Horton, S. J. Coles, H. L. Anderson and L. Bogani, Tailored homo- and hetero- lanthanide porphyrin dimers: a synthetic strategy for integrating multiple spintronic functionalities into a single molecule, Chem. Sci., 2018, 9(45), 8474–8481 RSC.
- D. Aguilà, O. Roubeau and G. Aromí, Designed polynuclear lanthanide complexes for quantum information processing, Dalton Trans., 2021, 50(35), 12045–12057 RSC.
- D. Maniaki, D. Garay-Ruiz, L. A. Barrios, D. O. T. A. Martins, D. Aguilà, F. Tuna, D. Reta, O. Roubeau, C. Bo and G. Aromí, Unparalleled selectivity and electronic structure of heterometallic [LnLn′Ln] molecules as 3-qubit quantum gates, Chem. Sci., 2022, 13(19), 5574–5581 RSC.
- Z. Zhu, X.-L. Li, S. Liu and J. Tang, External stimuli modulate the magnetic relaxation of lanthanide single-molecule magnets, Inorg. Chem. Front., 2020, 7(18), 3315–3326 RSC.
- M. Feng, Z.-Y. Ruan, Y.-C. Chen and M.-L. Tong, Physical stimulus and chemical modulations of bistable molecular magnetic materials, Chem. Commun., 2020, 56(89), 13702–13718 RSC.
- Y. Suzuki, K. Takeda and K. Awaga, Enhancement of Jahn-Teller isomerism in Mn12Ac under high quasi-hydrostatic pressure, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67(13), 132402 CrossRef.
- A. Sieber, R. Bircher, O. Waldmann, G. Carver, G. Chaboussant, H. Mutka and H.-U. Güdel, Effect of Pressure on the Magnetic Anisotropy in the Single-Molecule Magnet Mn12-Acetate: An Inelastic Neutron Scattering Study, Angew. Chem., Int. Ed., 2005, 44(27), 4239–4242 CrossRef CAS.
- R. Bircher, G. Chaboussant, C. Dobe, H. U. Güdel, S. T. Ochsenbein, A. Sieber and O. Waldmann, Single-Molecule Magnets Under Pressure, Adv. Funct. Mater., 2006, 16(2), 209–220 CrossRef CAS.
- A. Prescimone, C. J. Milios, S. Moggach, J. E. Warren, A. R. Lennie, J. Sanchez-Benitez, K. Kamenev, R. Bircher, M. Murrie, S. Parsons and E. K. Brechin, [Mn6] under Pressure: A Combined Crystallographic and Magnetic Study, Angew. Chem., Int. Ed., 2008, 47(15), 2828–2831 CrossRef CAS.
- A. Prescimone, C. J. Milios, J. Sanchez-Benitez, K. V. Kamenev, C. Loose, J. Kortus, S. Moggach, M. Murrie, J. E. Warren, A. R. Lennie, S. Parsons and E. K. Brechin, High pressure induced spin changes and magneto-structural correlations in hexametallic SMMs, Dalton Trans., 2009,(25), 4858–4867 RSC.
- P. Parois, S. A. Moggach, J. Sanchez-Benitez, K. V. Kamenev, A. R. Lennie, J. E. Warren, E. K. Brechin, S. Parsons and M. Murrie, Pressure-induced Jahn–Teller switching in a Mn12 nanomagnet, Chem. Commun., 2010, 46(11), 1881–1883 RSC.
- A. M. Thiel, E. Damgaard-Møller and J. Overgaard, High-Pressure Crystallography as a Guide in the Design of Single-Molecule Magnets, Inorg. Chem., 2020, 59(3), 1682–1691 CrossRef CAS.
- A. Etcheverry-Berrios, S. Parsons, K. V. Kamenev, M. R. Probert, S. A. Moggach, M. Murrie and E. K. Brechin, Putting the Squeeze on Molecule-Based Magnets: Exploiting Pressure to Develop Magneto-Structural Correlations in Paramagnetic Coordination Compounds, Magnetochemistry, 2020, 6(3), 32 CrossRef CAS.
- W.-B. Chen, Y.-C. Chen, J.-L. Liu, J.-H. Jia, L.-F. Wang, Q.-W. Li and M.-L. Tong, A Piezochromic Dysprosium(III) Single-Molecule Magnet Based on an Aggregation-Induced-Emission-Active Tetraphenylethene Derivative Ligand, Inorg. Chem., 2017, 56(15), 8730–8734 CrossRef CAS.
- M. S. Norre, C. Gao, S. Dey, S. K. Gupta, A. Borah, R. Murugavel, G. Rajaraman and J. Overgaard, High-Pressure Crystallographic and Magnetic Studies of Pseudo-D5h Symmetric Dy(III) and Ho(III) Single-Molecule Magnets, Inorg. Chem., 2020, 59(1), 717–729 CrossRef CAS PubMed.
- F. Pointillart, J. F. Gonzalez, H. Douib, V. Montigaud, C. McMonagle, B. L. Guennic, O. Cador, D. Pinkowicz and M. Probert, Reversible Pressure-Magnetic Modulation in a Tetrathiafulvalene-Based Dyad Piezochromic Dysprosium Single-Molecule Magnet, ChemRxiv, 2022. DOI:10.26434/chemrxiv-2022-sffw1.
- M. Briganti and F. Totti, Magnetic anisotropy on demand exploiting high-pressure as remote control: an ab initio proof of concept, Dalton Trans., 2021, 50(30), 10621–10628 RSC.
- Q. Deng and A. A. Popov, Clusters encapsulated in Endohedral Metallofullerenes: How strained are they?, J. Am. Chem. Soc., 2014, 136(11), 4257–4264 CrossRef CAS PubMed.
- S. Stevenson, J. P. Phillips, J. E. Reid, M. M. Olmstead, S. P. Rath and A. L. Balch, Pyramidalization of Gd3N inside a C80 cage. The synthesis and structure of Gd3N@C80, Chem. Commun., 2004,(24), 2814–2815 RSC.
- T. M. Zuo, C. M. Beavers, J. C. Duchamp, A. Campbell, H. C. Dorn, M. M. Olmstead and A. L. Balch, Isolation and structural characterization of a family of endohedral fullerenes including the large, chiral cage fullerenes Tb3N@C88 and Tb3N@C86 as well as the Ih and D5h isomers of Tb3N@C80, J. Am. Chem. Soc., 2007, 129(7), 2035–2043 CrossRef CAS PubMed.
- R. Valencia, A. Rodriguez-Fortea, A. Clotet, C. de Graaf, M. N. Chaur, L. Echegoyen and J. M. Poblet, Electronic Structure and Redox Properties of Metal Nitride Endohedral Fullerenes M3N@C2n (M=Sc, Y, La, and Gd; 2n = 80, 84, 88, 92, 96), Chem. – Eur. J., 2009, 15(41), 10997–11009 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, J. Ashby, A. Kumbhar, A. M. Rao and L. Echegoyen, Lanthanum Nitride Endohedral Fullerenes La3N@C2n (43 < n < 55): Preferential Formation of La3N@C96, Chem. – Eur. J., 2008, 14(27), 8213–8219 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, B. Elliott, A. Kumbhar, A. J. Athans and L. Echegoyen, New M3N@C2n Endohedral Metallofullerene Families (M=Nd, Pr, Ce; n = 40–53): Expanding the Preferential Templating of the C88 Cage and Approaching the C96 Cage, Chem. – Eur. J., 2008, 14(15), 4594–4599 CrossRef CAS.
- A. A. Popov, M. Krause, S. F. Yang, J. Wong and L. Dunsch, C78 cage isomerism defined by trimetallic nitride cluster size: A computational and vibrational spectroscopic study, J. Phys. Chem. B, 2007, 111(13), 3363–3369 CrossRef CAS.
- A. A. Popov and L. Dunsch, Structure, Stability, and Cluster-Cage Interactions in Nitride Clusterfullerenes M3N@C2n (M = Sc, Y; 2n = 68–98): a Density Functional Theory Study, J. Am. Chem. Soc., 2007, 129(38), 11835–11849 CrossRef CAS.
- W. Shen, L. B. Bao, S. Hu, X. Gao, Y. Xie, X. Gao, W. Huang and X. Lu, Isolation and Crystallographic Characterization of Lu3N@C2n (2n = 80–88): Cage Selection by Cluster Size, Chem. – Eur. J., 2018, 24(62), 16692–16698 CrossRef CAS PubMed.
- S. F. Yang, S. I. Troyanov, A. A. Popov, M. Krause and L. Dunsch, Deviation from the planarity - a large Dy3N cluster encapsulated in an Ih-C80 cage: An X-ray crystallographic and vibrational spectroscopic study, J. Am. Chem. Soc., 2006, 128(51), 16733–16739 CrossRef CAS.
- Y. Hao, Y. Wang, L. Spree and F. Liu, Rotation of fullerene molecules in the crystal lattice of fullerene/porphyrin: C60 and Sc3N@C80, Inorg. Chem. Front., 2021, 8, 122–126 RSC.
- F. Liu and L. Spree, Molecular spinning top: visualizing the dynamics of M3N@C80 with variable temperature single crystal X-ray diffraction, Chem. Commun., 2019, 55(86), 13000–13003 RSC.
- N. Chen, E. Y. Zhang, K. Tan, C. R. Wang and X. Lu, Size effect of encaged clusters on the exohedral chemistry of endohedral fullerenes: A case study on the pyrrolidino reaction of ScxGd3−xN@C80 (x = 0–3), Org. Lett., 2007, 9(10), 2011–2013 CrossRef CAS PubMed.
- S. Aroua, M. Garcia-Borràs, S. Osuna and Y. Yamakoshi, Essential Factors for Control of the Equilibrium in the Reversible Rearrangement of M3N@Ih-C80 Fulleropyrrolidines: Exohedral Functional Groups versus Endohedral Metal Clusters, Chem. – Eur. J., 2014, 20(43), 14032–14039 CrossRef CAS.
- C. M. Cardona, A. Kitaygorodskiy and L. Echegoyen, Trimetallic nitride endohedral metallofullerenes: Reactivity dictated by the encapsulated metal cluster, J. Am. Chem. Soc., 2005, 127(29), 10448–10453 CrossRef CAS PubMed.
- N. Chen, L. Z. Fan, K. Tan, Y. Q. Wu, C. Y. Shu, X. Lu and C. R. Wang, Comparative Spectroscopic and Reactivity Studies of Sc3−xYxN@C80 (x = 0–3), J. Phys. Chem. C, 2007, 111(32), 11823–11828 CrossRef CAS.
- K. Junghans, K. B. Ghiassi, N. A. Samoylova, Q. Deng, M. Rosenkranz, M. M. Olmstead, A. L. Balch and A. A. Popov, Synthesis and Isolation of the Titanium-Scandium Endohedral Fullerenes – Sc2TiC@Ih-C80, Sc2TiC@D5h-C80, and Sc2TiC2@Ih-C80: Metal Size Tuning of the TiIV/TiIII Redox Potentials, Chem. – Eur. J., 2016, 22(37), 13098–13107 CrossRef CAS PubMed.
- K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerström, T. Greber, B. Büchner and A. A. Popov, Methane as a Selectivity Booster in the Arc-Discharge Synthesis of Endohedral Fullerenes: Selective Synthesis of the Single-Molecule Magnet Dy2TiC@C80 and Its Congener Dy2TiC2@C80, Angew. Chem., Int. Ed., 2015, 54(45), 13411–13415 CrossRef CAS.
- D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Büchner and A. A. Popov, Record-high thermal barrier of the relaxation of magnetization in the nitride clusterfullerene Dy2ScN@C80-Ih, Chem. Commun., 2017, 53, 7901–7904 RSC.
- D. Krylov, F. Liu, A. Brandenburg, L. Spree, V. Bon, S. Kaskel, A. Wolter, B. Buchner, S. Avdoshenko and A. A. Popov, Magnetization relaxation in the single-ion magnet DySc2N@C80: quantum tunneling, magnetic dilution, and unconventional temperature dependence, Phys. Chem. Chem. Phys., 2018, 20, 11656–11672 RSC.
- S. Yang, L. Zhang, W. Zhang and L. Dunsch, A Facile Route to Metal Nitride Clusterfullerenes by Using Guanidinium Salts: A Selective Organic Solid as the Nitrogen Source, Chem. – Eur. J., 2010, 16(41), 12398–12405 CrossRef CAS.
- Y. Zhang, K. B. Ghiassi, Q. Deng, N. A. Samoylova, M. M. Olmstead, A. L. Balch and A. A. Popov, Synthesis and Structure of LaSc2N@Cs(hept)-C80 with One Heptagon and Thirteen Pentagons, Angew. Chem., Int. Ed., 2015, 52(2), 495–499 Search PubMed.
- S. Stevenson, C. B. Rose, J. S. Maslenikova, J. R. Villarreal, M. A. Mackey, B. Q. Mercado, K. Chen, M. M. Olmstead and A. L. Balch, Selective Synthesis, Isolation, and Crystallographic Characterization of LaSc2N@Ih-C80, Inorg. Chem., 2012, 51(24), 13096–13102 CrossRef CAS PubMed.
- L. Dunsch, S. Yang, L. Zhang, A. Svitova, S. Oswald and A. A. Popov, Metal Sulfide in a C82 Fullerene Cage: A New Form of Endohedral Clusterfullerenes, J. Am. Chem. Soc., 2010, 132(15), 5413–5421 CrossRef CAS.
- U. Mueller, R. Förster, M. Hellmig, F. U. Huschmann, A. Kastner, P. Malecki, S. Pühringer, M. Röwer, K. Sparta, M. Steffien, M. Ühlein, P. Wilk and M. S. Weiss, The macromolecular crystallography beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current status and perspectives, Eur. Phys. J. Plus, 2015, 130(7), 141 CrossRef.
- W. Kabsch, XDS, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66(2), 125–132 CrossRef CAS PubMed.
- K. M. Sparta, M. Krug, U. Heinemann, U. Mueller and M. S. Weiss, XDSAPP2.0, J. Appl. Crystallogr., 2016, 49(3), 1085–1092 CrossRef.
- G. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3–8 Search PubMed.
- M. M. Olmstead, T. Zuo, H. C. Dorn, T. Li and A. L. Balch, Metal ion size and the pyramidalization of trimetallic nitride units inside a fullerene cage: Comparisons of the crystal structures of M3N@Ih-C80 (M = Gd, Tb, Dy, Ho, Er, Tm, Lu, and Sc) and some mixed metal counterparts, Inorg. Chim. Acta, 2017, 468, 321–326 CrossRef CAS.
- T. Zuo, M. M. Olmstead, C. M. Beavers, A. L. Balch, G. Wang, G. T. Yee, C. Shu, L. Xu, B. Elliott, L. Echegoyen, J. C. Duchamp and H. C. Dorn, Preparation and Structural Characterization of the Ih and the D5h Isomers of the Endohedral Fullerenes Tm3N@C80: Icosahedral C80 Cage Encapsulation of a Trimetallic Nitride Magnetic Cluster with Three Uncoupled Tm3+ Ions, Inorg. Chem., 2008, 47(12), 5234–5244 CrossRef CAS.
- L. Echegoyen, C. J. Chancellor, C. M. Cardona, B. Elliott, J. Rivera, M. M. Olmstead and A. L. Balch, X-Ray crystallographic and EPR spectroscopic characterization of a pyrrolidine adduct of Y3N@C80, Chem. Commun., 2006,(25), 2653–2655 RSC.
- C. Shu, W. Xu, C. Slebodnick, H. Champion, W. Fu, J. E. Reid, H. Azurmendi, C. Wang, K. Harich, H. C. Dorn and H. W. Gibson, Syntheses and Structures of Phenyl-C81-Butyric Acid Methyl Esters (PCBMs) from M3N@C80, Org. Lett., 2009, 11(8), 1753–1756 CrossRef CAS.
- S. Stevenson, H. R. Thompson, K. D. Arvola, K. B. Ghiassi, M. M. Olmstead and A. L. Balch, Isolation of CeLu2N@Ih-C80 through a Non-Chromatographic, Two-Step Chemical Process and Crystallographic Characterization of the Pyramidalized CeLu2N within the Icosahedral Cage, Chem. – Eur. J., 2015, 21(29), 10362–10368 CrossRef CAS.
- S. Stevenson, C. Chancellor, H. M. Lee, M. M. Olmstead and A. L. Balch, Internal and External Factors in the Structural Organization in Cocrystals of the Mixed-Metal Endohedrals (GdSc2N@Ih-C80, Gd2ScN@Ih-C80, and TbSc2N@Ih-C80) and Nickel(II) Octaethylporphyrin, Inorg. Chem., 2008, 47(5), 1420–1427 CrossRef CAS PubMed.
- V. Dubrovin, L.-H. Gan, B. Büchner, A. A. Popov and S. M. Avdoshenko, Endohedral metal-nitride cluster ordering in metallofullerene–NiII(OEP) complexes and crystals: a theoretical study, Phys. Chem. Chem. Phys., 2019, 21, 8197–8200 RSC.
- Y. Wang, G. Velkos, N. J. Israel, M. Rosenkranz, B. Büchner, F. Liu and A. A. Popov, Electrophilic Trifluoromethylation of Dimetallofullerene Anions en Route to Air-Stable Single-Molecule Magnets with High Blocking Temperature of Magnetization, J. Am. Chem. Soc., 2021, 143(43), 18139–18149 CrossRef CAS.
- A. A. Popov and L. Dunsch, The Bonding Situation in Endohedral Metallofullerenes as Studied by Quantum Theory of Atoms in Molecules (QTAIM), Chem. – Eur. J., 2009, 15(38), 9707–9729 CrossRef CAS.
- L. Ungur and L. F. Chibotaru, Ab Initio Crystal Field for Lanthanides, Chem. – Eur. J., 2017, 23(15), 3708–3718 CrossRef CAS PubMed.
- R. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32(5), 751–767 CrossRef.
- F. Aquilante, J. Autschbach, A. Baiardi, S. Battaglia, V. A. Borin, L. F. Chibotaru, I. Conti, L. D. Vico, M. Delcey, I. F. Galván, N. Ferré, L. Freitag, M. Garavelli, X. Gong, S. Knecht, E. D. Larsson, R. Lindh, M. Lundberg, P.Å Malmqvist, A. Nenov, J. Norell, M. Odelius, M. Olivucci, T. B. Pedersen, L. Pedraza-González, Q. M. Phung, K. Pierloot, M. Reiher, I. Schapiro, J. Segarra-Martí, F. Segatta, L. Seijo, S. Sen, D.-C. Sergentu, C. J. Stein, L. Ungur, M. Vacher, A. Valentini and V. Veryazov, Modern quantum chemistry with [Open]Molcas, J. Chem. Phys., 2020, 152(21), 214117 CrossRef CAS PubMed.
- L. F. Chibotaru and L. Ungur, Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation, J. Chem. Phys., 2012, 137(6), 064112 CrossRef CAS PubMed.
- J. Dreiser, Molecular lanthanide single-ion magnets: from bulk to submonolayers, J. Phys.: Condens. Matter, 2015, 27(18), 183203 CrossRef CAS PubMed.
- T. G. Ashebr, H. Li, X. Ying, X.-L. Li, C. Zhao, S. Liu and J. Tang, Emerging Trends on Designing High-Performance Dysprosium(III) Single-Molecule Magnets, ACS Mater. Lett., 2022, 4, 307–319 CrossRef CAS.
- W. Cai, J. D. Bocarsly, A. Gomez, R. J. Letona Lee, A. Metta-Magaña, R. Seshadri and L. Echegoyen, High blocking temperatures for DyScS endohedral fullerene single-molecule magnets, Chem. Sci., 2020, 11, 13129–13136 RSC.
- C. Schlesier, L. Spree, A. Kostanyan, R. Westerström, A. Brandenburg, A. U. B. Wolter, S. Yang, T. Greber and A. A. Popov, Strong carbon cage influence on the single molecule magnetism in Dy–Sc nitride clusterfullerenes, Chem. Commun., 2018, 54(70), 9730–9733 RSC.
- A. Brandenburg, D. S. Krylov, A. Beger, A. U. B. Wolter, B. Büchner and A. A. Popov, Carbide clusterfullerene DyYTiC@C80 featuring three different metals in the endohedral cluster and its single-ion magnetism, Chem. Commun., 2018, 54(76), 10683–10686 RSC.
- G. Velkos, W. Yang, Y.-R. Yao, S. M. Sudarkova, F. Liu, S. Avdoshenko, N. Chen and A. A. Popov, Metallofullerene single-molecule magnet Dy2O@C2v(5)-C80 with a strong antiferromagnetic Dy⋯Dy coupling, Chem. Commun., 2022, 58, 7164–7167 RSC.
- W. Yang, G. Velkos, F. Liu, S. M. Sudarkova, Y. Wang, J. Zhuang, H. Zhang, X. Li, X. Zhang, B. Büchner, S. M. Avdoshenko, A. A. Popov and N. Chen, Single Molecule Magnetism with Strong Magnetic Anisotropy and Enhanced Dy⋯Dy Coupling in Three Isomers of Dy-Oxide Clusterfullerene Dy2O@C82, Adv. Sci., 2019, 6(20), 1901352 CrossRef CAS.
- C.-H. Chen, D. S. Krylov, S. M. Avdoshenko, F. Liu, L. Spree, R. Yadav, A. Alvertis, L. Hozoi, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter and A. A. Popov, Selective arc-discharge synthesis of Dy2S-clusterfullerenes and their isomer-dependent single molecule magnetism, Chem. Sci., 2017, 8(9), 6451–6465 RSC.
- D. Krylov, G. Velkos, C.-H. Chen, B. Büchner, A. Kostanyan, T. Greber, S. Avdoshenko and A. A. Popov, Magnetic hysteresis and strong ferromagnetic coupling of sulfur-bridged Dy ions in clusterfullerene Dy2S@C82, Inorg. Chem. Front., 2020, 7, 3521–3532 RSC.
- M. E. Lines, Orbital Angular Momentum in the Theory of Paramagnetic Clusters, J. Chem. Phys., 1971, 55(6), 2977–2984 CrossRef CAS.
- L. Ungur, W. Van den Heuvel and L. F. Chibotaru, Ab initio investigation of the non-collinear magnetic structure and the lowest magnetic excitations in dysprosium triangles, New J. Chem., 2009, 33(6), 1224–1230 RSC.
- L. F. Chibotaru, L. Ungur and A. Soncini, The Origin of Nonmagnetic Kramers Doublets in the Ground State of Dysprosium Triangles: Evidence for a Toroidal Magnetic Moment, Angew. Chem., Int. Ed., 2008, 47(22), 4126–4129 CrossRef CAS PubMed.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes, J. Comput. Chem., 2013, 34(13), 1164–1175 CrossRef CAS.
- Y.-S. Ding, K.-X. Yu, D. Reta, F. Ortu, R. E. P. Winpenny, Y.-Z. Zheng and N. F. Chilton, Field- and temperature-dependent quantum tunnelling of the magnetisation in a large barrier single-molecule magnet, Nat. Commun., 2018, 9(1), 3134 CrossRef PubMed.
- P. G. Klemens, Localized Modes and Spin-Lattice Interactions, Phys. Rev., 1962, 125(6), 1795–1798 CrossRef.
- C.-Y. Huang, Optical Phonons in Electron Spin Relaxation, Phys. Rev., 1967, 154(2), 215–219 CrossRef CAS.
- A. Lunghi, F. Totti, R. Sessoli and S. Sanvito, The role of anharmonic phonons in under-barrier spin relaxation of single molecule magnets, Nat. Commun., 2017, 8, 14620 CrossRef PubMed.
- M. Zalibera, F. Ziegs, S. Schiemenz, V. Dubrovin, W. Lubitz, A. Savitsky, S. Deng, X.-B. Wang, S. Avdoshenko and A. A. Popov, Metallofullerene photoswitches driven by photoinduced fullerene-to-metal electron transfer, Chem. Sci., 2021, 12, 7818–7838 RSC.
- M. Zalibera, D. S. Krylov, D. Karagiannis, P.-A. Will, F. Ziegs, S. Schiemenz, W. Lubitz, S. Reineke, A. Savitsky and A. A. Popov, Thermally Activated Delayed Fluorescence in a Y3N@C80 Endohedral Fullerene: Time-Resolved Luminescence and EPR Studies, Angew. Chem., Int. Ed., 2018, 57(1), 277–281 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2184669–2184673. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi02224a |
| This journal is © the Partner Organisations 2023 |