Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in oral delivery systems of resveratrol: foreseeing their use in functional foods
Pedro M.
Silva
 *abc,
Catarina
Gonçalves
*abc,
Catarina
Gonçalves
 c,
Lorenzo M.
Pastrana
c,
Manuel A.
Coimbra
d,
Antonio A.
Vicente
c,
Lorenzo M.
Pastrana
c,
Manuel A.
Coimbra
d,
Antonio A.
Vicente
 ab and
Miguel A.
Cerqueira
*c
ab and
Miguel A.
Cerqueira
*c
aCentre of Biological Engineering (CEB), Campus de Gualtar, University of Minho, 4710-057 Braga, Portugal. E-mail: pedro.m.silva@inl.int
bAssociate Laboratory (LABBELS), Braga/Guimarães, Portugal
cInternational Iberian Nanotechnology Laboratory (INL), Av. Mestre José Veiga, 4715-330 Braga, Portugal. E-mail: miguel.cerqueira@inl.int
dLAQV/REQUIMTE, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal
First published on 10th November 2023
Abstract
Herein, we review the current state-of-the-art on the use of micro- and nano-delivery systems, a possible solution to some of the drawbacks associated with the incorporation of resveratrol in foods. Specifically, we present an overview of a wide range of micro-nanostructures, namely, lipidic and polymeric, used for the delivery of resveratrol. Also, the gastrointestinal fate of resveratrol-loaded micro-nanostructures, as a critical parameter for their use as functional food, is explored in terms of stability, bioaccessibility, and bioavailability. Different micro-nanostructures are of interest for the development of functional foods given that they can provide different advantages and properties to these foods and even be tailor-made to address specific issues (e.g., controlled or targeted release). Therefore, we discuss a wide range of micro-nanostructures, namely, lipidic and polymeric, used to deliver resveratrol and aimed at the development of functional foods. It has been reported that the use of some production methodologies can be of greater interest than others, for example, emulsification, solvent displacement and electrohydrodynamic processing (EHDP) enable a greater increase in bioaccessibility. Additionally, the use of coatings facilitates further improvements in bioaccessibility, which is likely due to the increased gastric stability of the coated micro-nanostructures. Other properties, such as mucoadhesion, can also help improve bioaccessibility due to the increase in gut retention time. Additionally, cytotoxicity (e.g., biocompatibility, antioxidant, and anti-inflammatory) and possible sensorial impact of resveratrol-loaded micro- and nano-systems in foods are highlighted.
1. Introduction
Over the last few years, the food industry has progressed from a low-tech industry, resulting in an increase in new and more innovative technologies and products.1,2 This modernization is attributed to the changes in consumer behaviors and choices. However, changes in dietary habits have been linked to an increasing rate of chronic diseases, causing consumers and the food industry to be more aware of the relationship between dietary habits and health. Consequently, the demand for healthier and more functional products has increased. Consumers desire products that are natural, less processed and have reduced amounts of fat, sugar, and salt. Furthermore, products that can provide health benefits by helping prevent nutrition-related diseases have led to a pivot in the food industry.1,3–6This increased interest is evident based on the growth of the global specialty food ingredient market. Specialty food ingredients can help maintain the typical properties of a food product (e.g., shelf life, texture, and color), while adding additional health benefits through an improved nutritional profile (e.g., micronutrients, bioactive compounds, and reduced fat). They are used to develop functional foods, which can be defined as foods that are demonstrated to improve health by adding health benefits or decreasing disease risk.1,7
Two major objectives can be achieved with functional foods, as follows: (i) improving physiological functions and (ii) reducing the risk of chronic problems. Furthermore, they can be categorized based on the mode and objective of fortification with bioactive compounds (Fig. 1).1,7
Several bioactive compounds and micronutrients can be used in food fortification, such as minerals, vitamins, prebiotics, flavonoids, polyphenols, and carotenoids, depending on the food matrix and the desired health benefit.7
Over the last few years, polyphenols have attracted increasing interest due to their numerous bioactive and health-promoting properties, such as antioxidant, antimicrobial, and anti-inflammatory,1 and thus are considered excellent candidates as bioactives in health-promoting and disease-preventing functional foods.
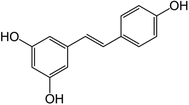
One of the polyphenols that has gained attention in the last few decades is resveratrol, (3,5,4′-trihydroxy-stilbene), a natural stilbene phytoalexin produced by plants as a defense mechanism against external stimuli. Resveratrol can be found naturally in two stereoisomeric forms, i.e., trans- and cis-, with the trans isoform (Fig. 2) reported to display higher bioactivities.1,8
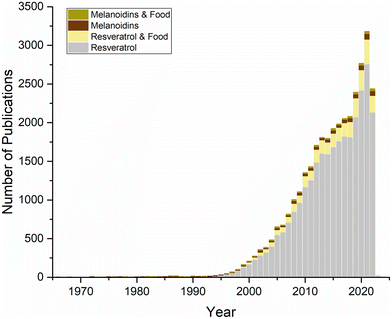
Interest in resveratrol dates back to the 1970s, but this interest has increased exponentially since it was reported to be one of the compounds that can explain the cardioprotective effects of red wines, also known as the ‘French paradox’, in 1992.9,10 This increase can be seen in Fig. 3, which shows the Scopus data regarding publications on resveratrol in food to date.
However, similar to most polyphenols, resveratrol possesses poor aqueous solubility and is chemically unstable and vulnerable to chemical degradation in food products, hindering its direct use as a functional food ingredient. Resveratrol degrades when exposed to light (namely at 360 and 254 nm), oxygen, low and high temperatures, and alkaline pH. This leads to its isomerization and auto-oxidation from its more bioactive trans- form to its cis-form.1,11,12 Thus, resveratrol is a prime candidate to be used in micro or nano delivery systems to overcome its limitations as a bioactive agent in functional food products.1,11
In the last decade, researchers and the food industry have increased their interest in micro- and nanotechnologies to produce delivery systems for bioactive compounds to respond to market demands and address some of the limitations of the most interesting bioactive compounds. In this case, the use of micro- and nano-delivery systems allows an increase in surface/volume ratio, facilitating an increase in the solubility and stability of bioactives, modifying their release, and most importantly, increasing their bioaccessibility.13 The selection of the most appropriate delivery system is dependent on the characteristics of the bioactive compound, the food matrix, and the objective, among other variables. An array of different delivery systems and techniques are available in the food industry, each with its own benefits and drawbacks.1,11,14 Currently, several materials (e.g., lipids, natural and synthetic polymers) and techniques (e.g., spray-drying, ionic gelation, electrohydrodynamic processing, and emulsification) are available for use in the food industry.15–21
Different from other recent reviews focusing on multiple bioactives of plant origin and specific techniques for their delivery22 or use in different areas (e.g., pharmaceutical,23 biomedical,24 and cosmetics25), this review highlights the diverse delivery systems and techniques that can be used as oral delivery systems in food applications.
Special consideration is given to research involving the assessment of the properties of bioactivities, such as their digestibility, cell-based assays (e.g., cytotoxicity and in vitro bioactivities), and incorporation in food products and their organoleptic influence, which is lacking in other reviews.26–28
The Scopus search engine was employed as a research tool to search for original reports regarding the development of resveratrol delivery systems, characterization of their physicochemical properties, in vitro digestion, cell-based assays (e.g., cytotoxicity and bioactivity), and food product incorporation over the past five years (with exception of up to ten years for less explored delivery systems). The search was performed within the article title, abstract, and keywords, and the following keywords were used: ‘resveratrol’ and ‘encapsulation’, and from the obtained list, publications associated with food applications were selected.
2. Benefits and challenges associated with resveratrol
Resveratrol is reported to have several impactful benefits in human health, which have been assessed in in vitro and in vivo studies.1,11,29 These benefits include anticarcinogenic, anti-inflammatory, antioxidant, antidiabetic, weight loss and wound healing properties.30–36 Several clinical trials have also been completed or are underway regarding the use and effect of resveratrol on several health conditions such as diabetes and pre-diabetes, systemic inflammation, and obesity.37–39Resveratrol can be found in a wide range of plants and edible sources in varying amounts ranging from a few ng g−1 (e.g., ∼32 ng g−1 in blueberries) to a few μg g−1 (e.g., ∼24 μg g−1 in grape skin) and a few mg L−1 in wines, namely in red wines (e.g., up to 2 mg L−1 in white wine and 14 mg L−1 in red wine).29 As seen in Fig. 2, resveratrol has the molecular formula of C14H12O3, corresponding to a molecular weight of 228.24 g mol−1 and melting point of around 254 °C.40 It presents a pKa of 8.99 and logP of 3.4, indicating that it is a highly hydrophobic compound, and thus has low water solubility (around 0.03 mg mL−1). However, it is soluble in other polar solvents such as ethanol (around 50 mg mL−1) and dimethyl sulfoxide (DMSO) (around 16 mg mL−1). Nevertheless, despite its low water solubility, resveratrol displays high membrane permeability due to its high lipophilicity.1,12
To date, despite its many potential health benefits, the use of resveratrol in the food industry has been limited due to some inherent challenges in using polyphenols and other bioactive compounds, namely, their low aqueous solubility, hampering their widespread use in the food industry.
Furthermore, despite the high oral absorption of resveratrol (around 70%), it displays low bioavailability (reported to be less than 1%) due to its fast and extensive metabolization in the liver and intestine through glucuronic acid conjugation and sulfation. After absorption, resveratrol is quickly metabolized in the liver, with a reported plasma half-life of 8 to 14 min, contributing to its low bioavailability.41,42
Currently, the daily intake of resveratrol is estimated to be around 4 mg day−1 and studies have shown that up to 5 g per day in healthy humans is considered a safe daily intake, whereas consumption of above 2.5 g per day can lead to some adverse gastrointestinal symptoms.1,11
Resveratrol can be consumed from natural sources and in crystalline form1,11,43,44 (e.g., powders, capsules, and tablets), either as a supplement† or incorporated in food products.‡
Natural sources of resveratrol are associated with certain challenges due to the variability in the content of resveratrol, and also it is typically impractical to consume the quantities needed to have a biological effect on human health, partially due to the fact that resveratrol is usually located in parts of fruits that may not be ordinarily consumed, such as the skins and seeds, and partially due to its low bioavailability.11
The powdered crystalline forms of resveratrol used in supplements can increase the daily intake of resveratrol but still display some of the known challenges, namely, low solubility and low bioavailability in the human gastrointestinal tract. Additionally, its incorporation in food products can lead to undesirable changes in organoleptic properties (e.g., appearance and mouthfeel).11
Given the ready availability of resveratrol supplements, in the next section, we present an overview of the different strategies to improve the aqueous dispersibility and/or solubility and stability of resveratrol, protecting it from chemical degradation during processing and storage in the food industry. Also, examples of the effect of micro- and nano-delivery systems on its oral bioavailability and bioactive properties are discussed.
3. Food-grade micro- and nano-delivery systems for resveratrol
Delivery systems can be produced using different techniques to entrap one or several ingredients or compounds in a structure (e.g., capsules, particles, and fibers). The compounds to be delivered are usually referred to as the active or bioactive materials, while the material that forms the surrounding matrix is referred to as the carrier or wall material.14,45 Additionally, particle diameter defines if the system is a micro- (1–1000 μm) or nano-delivery system (<1000 nm).At this scale, materials behave differently (physically, chemically, and biologically) than materials at the macro scale, which can improve the properties of materials and even enable novel functionalities and applications. The reduced particle size and high surface area-to-volume ratio allow for improvements in bioavailability, solubility, stability, sensory properties, aggregation rates, transport properties through biological barriers, reduced light scattering, etc. These properties can be extremely beneficial when planning to use delivery systems for bioactive compounds in foods.14,45–47
Over the last few years, research regarding delivery systems for resveratrol has been increasing, but the focus has been pharmaceutical applications. Alternatively, applications in the food industry need to address several other factors not considered in pharmaceutical applications, such as the need to use food-grade materials that are generally recognized as safe (GRAS) and both the encapsulation materials and technology need to be approved by the governing agencies, such as the European Food Safety Agency (EFSA) and the Food and Drug Administration (FDA).11,14,45 Additionally, the selected materials and methods should be economically feasible to be commercialized on a large scale. Also, the delivery system should not negatively impact the sensorial and physicochemical properties of the final product (e.g., texture, flavor, and appearance) and should maintain resveratrol in a metabolically active form, capable of exerting its health-promoting benefits when incorporated in a functional food.11,14,45
Several food-grade materials and techniques can be used to develop delivery systems, depending on the compatibility of the bioactive material with the selected food matrix, as well as the end goal of the delivery system (e.g., gastric protection, controlled release, and flavor masking).11,14,45 In this section, we review the recent advances in the different production techniques for food-grade micro- and nano-delivery systems suitable for the delivery of resveratrol (Table 1), including emulsification, spray-drying, anti-solvent precipitation, thin-film hydration, and electrohydrodynamic processing.48–53Table 1 presents a literature review of food-grade micro- and nano-delivery systems for resveratrol, focusing on works that assessed the properties of in vitro release, in vitro digestion, and/or cellular interactions (in vitro, ex vivo, and in vivo), which is organized by the production method.
| Material | Production Method | Particle/droplet size | Loading capacity | Encapsulation efficiency | In vitro release | In vitro digestion | Cellular interactions (in vitro, ex vivo, in vivo) | Ref. |
|---|---|---|---|---|---|---|---|---|
| FA – folic acid; F68 – Pluronic F68; GIT – gastrointestinal tract; GNP – galactosylated nanoparticles; INU – inulin; LG-g-PDLA – lignin-graft-poly(D-lactic acid); MCT – medium chain triglyceride; NF – nanofibers; NP – nanoparticles; O/W– oil-in-water; PLGA – poly(lactic-co-glycolic acid); PVA – polyvinyl alcohol; RSV – resveratrol; ROS – reactive oxygen species; SA – stearic acid; SGF – simulated gastric fluids; SIF – simulated intestinal fluids; and W/O/W – water-in-oil-in-water. | ||||||||
| Chitosan (coating); octenyl succinic anhydride-modified waxy maize starch nanocrystals | Emulsification (O/W) | 500–1500 nm | N/A | N/A | N/A | 14-Fold improved bioaccessibility of RSV from 7% (free RSV) to more than 90% (RSV emulsified in chitosan-coated or chitosan-uncoated Pickering emulsion) | Chitosan coating increased mucoadhesive properties of the emulsions (Nile Red used as a fluorescent dye) 7-fold mucoadhesiveness than uncoated Pickering emulsions and free RSV in porcine intestinal mucosa (ex vivo) | 58 |
| Casein sodium salt; gum Arabic; pectin | Emulsification (O/W) | 600–850 nm | N/A | N/A | N/A | RSV bioaccessibility increased to 83–90% when emulsified | N/A | 55 |
| Lecithin; Tween 80 | Emulsification (O/W) | 20–25 nm | N/A | N/A | Free RSV released easily in gastric conditions (90% in 15 min), while emulsified RSV had 55% release after 15 h; fast release followed by controlled and sustained release was verified | N/A | N/A | 137 |
| Sodium alginate; transglutaminase; zein | Emulsification (pickering emulsion followed by sequential crosslinking) | Emulsion size: 30–40 μm | N/A | N/A | N/A | Emulsions gels displayed an increased bioaccessibility of RSV (∼70%) compared to the loaded Pickering emulsions (∼40%) | N/A | 98 |
| Chitosan; PLGA; PVA | Emulsification (W/O/W double emulsion) | 11–20 μm | 0.4–0.5% | N/A | ∼50% at pH 1.2 (after 2 h); up to ∼100% at pH 7.4 (after 8 h) | N/A | N/A | 138 |
| Alginate | Emulsification/external gelation | 360 nm | 0.3% | N/A | Burst release (first 10 min) at both pH 1.2 and pH 7.2, followed by diffusion-governed release (max release up to 17%) | N/A | N/A | 139 |
| Propylene glycol alginate; rhamnolipids; zein | Emulsification–evaporation | 400–800 nm | 3–6% | N/A | N/A | 60–70% RSV released in the first hour of digestion, with > 95% RSV released after 3 h | N/A | 126 |
| Poly(lactic-co-glycolic acid); folic acid (active targeting) | Emulsification–evaporation | 120–130 nm | 42–78% | N/A | 10–13% of RSV was released in the first 120 min (pH 1.2), while within 50 h, 70% of the RSV content was released at pH 7.4 | % RSV transported through a Caco-2 monolayer after 180 min was 58% (PLGA-RSV) and 98% (PLGA-FA-RSV) compared to 3.8% for free RSV | 140 | |
| Propylene glycol alginate; tea saponin; zein | Emulsification–evaporation co-precipitation | 280–310 nm | 5.3–6.3% | N/A | N/A | The nanoparticles displayed a slight release of RSV in the gastric phase (∼23%), followed by a burst release when moved to the intestinal phase (∼63%) | N/A | 141 |
| Acrysol K-150; poly(D,L-lactide-co-glycolic acid); TGF-β1 | Emulsification-homogenization | 150–375 nm | N/A | N/A | The PLGA nanohybrid particles displayed a sustained release, with around 60% of RSV content released after 120 h, in contact with the PLGA nanoparticles, which released 60% of RSV content in less than 20 h | N/A | The PLGA nanohybrid particles were highly cytotoxic, revealing 60–74% growth inhibition in MFC-7 cells | 142 |
| Acrysol K-150; Labrafac lipophile WL 1349; poly(D,L-lactide-co-glycolic acid); Sefsol-218 | Emulsification-solvent evaporation | 20–250 nm | 1–30% | N/A | PLGA nanohybrid particles displayed controlled release; 10% released from the PLGA nanohybrid particles in the first 4 h, with 70% released after 96 h | N/A | The PLGA nanohybrid particles showed a lower % growth inhibition (SRB assay) at all concentration levels compared to free RSV; however, an RSV dose-dependent effect in Vero cells was observed. | 94 |
| Calcium chloride; propylene glycol alginate; tea saponin; zein | Emulsification–evaporation | 260–310 nm | 5–6% | N/A | N/A | Calcium improved the stability of the colloidal complexes and led to the sustained release of RSV. | N/A | 143 |
| Castor oil; olive oil; glycerol; glycol ether; Tween-80; Triton X-100; PEG-400 | Self-emulsification | 40–60 nm | N/A | N/A | N/A | N/A | Dose-dependent cytotoxic effect (CCK-8 assay) of free RSV (safe below 50 μM) in PC12 cells; emulsified RSV was less cytotoxic; dose-dependent intracellular antioxidant activity of RSV increased for emulsified RSV | 144 |
| N-Oleoyl-D-galactosamine; poly(lactic-co-glycolic acid); Tween 80 | Solvent diffusion | 100–350 nm | N/A | 85–95% | Free RSV was quickly released (95% in 4 h) in water, while encapsulated RSV displayed a controlled and prolonged release, 25% after 4 h, 45% at 8 h, 60% at 16 h, up to 80% at 36 h | N/A | Cellular uptake revealed that RES-GNPs can be more easily transported into the enterocytes (Caco-2 cells) than RSV solution after 4 h. The in vitro anti-inflammatory efficacy in RAW 264.7 cells of encapsulated RSV was higher than free RSV. | 145 |
| Ethyl acetate; Phospholipon 90G; stearic acid | Solvent diffusion–solvent evaporation | 90–200 nm | 2–7% | 80–96% | Initial burst release followed by sustained release at pH 7.4. After 120 h, between 80% and 90% of the solid lipid nanoparticle content had been released. Control sample released 94% of its content in under 8 h. | N/A | In vivo pharmacokinetic studies showed that the oral bioavailability of RSV was 8-fold improved when encapsulated | 146 |
| Inulin; Pluronic F68; stearic acid | Solvent evaporation | 65–175 nm | N/A | 52–56% | RSV-loaded INU-F68-SA nanomicelles released 35% of RSV at pH 1.2, compared to 90% for the RSV-loaded SA-F68 nanomicelles, and displayed sustained release at pH 6.8. | N/A | Free RSV and loaded nanomicelles displayed a dose- and time-dependent cytotoxic effect (MTT assay) against HCT 116 cancer cells. Pure RSV displayed an IC50 of 26 μg mL−1, while RSV-loaded nanomicelles had an IC50 of 17.5 μg mL−1; oral bioavailability (assayed in animals) improved for the RSV-loaded nanomicelles | 96 |
| Chitosan; ferulic acid; stearic acid | Solvent evaporation method and hot homogenization | 150–175 nm | N/A | N/A | Initial burst release followed by controlled release, up to 43% during 48 h at pH 7.4 | N/A | IC50 (MTT assay) between 10 and 25 μg mL−1 in HT-29 cells; chitosan ferulic acid solid lipid nanoparticles displayed better cytotoxicity results than uncoated or free RSV | 67 |
| Whey protein isolate | Cold gelation followed by crosslinking | Emulsion size: 20–280 nm; 190–960 nm | N/A | 60–63% | N/A | RSV release was slower with a higher the amount of oil in the emulsion gels; nevertheless, all the samples displayed a high release in the gastric phase (2 h, pH 1.2), followed by full release in the intestinal phase (4 h, pH 6.8). | N/A | 97 |
| Cholesterol; lactose; maltodextrin; Ppolysorbate 20; sorbitan monostearate | Thin film hydration followed by sonication | 180–280 nm | N/A | 75–84% | After two hours of gastric conditions, the proniosomes released between 25% and 50% of the RSV content after 2 h, while in intestinal conditions, between 50% and 70% of RSV was released in 8 h. | N/A | Biocompatibility assays (MTT assay) in Caco-2 cell lines demonstrated that all the tested formulations were safe to use, within normal oral dosage values (up to 73 μM RSV) | 93 |
| Cholesterol; maltodextrin; lactose monohydrate; pullulan; sorbitan monostearate | Thin-film hydration | 140–260 nm | N/A | 88–90% | RSV-loaded proniosomes released around 20% in gastro conditions, and between 70% and 80% in intestinal conditions within an additional 6 h | N/A | N/A | 115 |
| 2-Distearoyl-sn-glycero-3-phosphocholine 18:0 PC; cholesterol | Thin-film hydration | 280–330 nm | N/A | 85% | N/A | Liposomes increased in size (3682 nm) during gastric digestion and displayed a lower encapsulation efficiency (60%) due to the release of RES, decreased in size in intestinal conditions (682 nm), and further released RES (encapsulation efficiency of 34%) | The inhibition rate of cells increased with the concentration of RES, but all tested concentrations were safe to use. Encapsulation of RES improved its antioxidant activity, reducing the ROS levels of cells from 87% (unencapsulated) to 65% (encapsulated). | 129 |
| β-Sitosterol; L-α-phosphatidylcholine | Thin-lipid film hydration | 73–320 nm | N/A | 80% | N/A | Liposomal RSV displayed slower release compared to free RSV of almost 3-times less in the first 2 h | Cellular transport of RSV increased when loaded in liposomes (1.2- to 1.5-fold). Cellular antioxidant activity increased 1.5- to 2-fold for liposomal RSV. RSV in plasma also increased with the use of liposomes (1.5 to 3.5-fold increase). | 64 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; cholesterol; 2-hydroxypropyl β-cyclodextrins | Inclusion complexation by extrusion/thin film hydration | 115–130 nm | 3–11.5% | Encapsulated samples demonstrated burst release (∼60%) in the first hour, followed by prolonged release of up to 65–100% after 24 h, up from 30% and 40% release for free RSV, at 1 h and 24 h, respectively. | Nano formulations displayed a dose-dependent cytotoxic effect (MTS assay), with increased cytotoxicity compared to free RSV in HT-29 colon cancer cells | 62 | ||
| Arabic gum; coconut oil | Spray-drying | 8.2 μm | N/A | 87% | 100% in 20 min, in coconut oil | N/A | N/A | 147 |
| Carboxymethyl cellulose; sodium alginate | Spray-drying (3 fluid nozzle) | 5–9 μm | 1.5–2% | 76–99% | ∼35–58% in SGF (after 2 h); ∼70% in SIF (up to 4–32 h) | N/A | N/A | 148 |
| Protein microparticles (sodium caseinate or Whey protein concentrate) with or without anhydrous milk fat | Spray-drying | 1–20 μm | N/A | 68–83% | N/A | Digestive stability improved from 47% to 71–86%. Bioaccessibility increased from 23% to 68–81%. | N/A | 72 |
| Ammonium salts; Shellac resin | Spray-drying | ∼5–10 μm | 4–8% | 83–95% | <20% release at pH 1.0 (up to 18 h); up to 70% release at pH 6.8 (after 18 h); up to 50% release at pH 7.4 (after 18 h) | N/A | N/A | 149 |
| Kafirin; gallic acid (cross-linker) | Antisolvent precipitation | 80–250 nm | 1–21% | 75–96% | N/A | 10–20% of encapsulated RSV released in the oral phase, 35–60% in the gastric phase, and 45–75% in the intestinal phase versus 5% released in the oral, 15% in gastric and 15% in intestinal phases for free RSV | N/A | 150 |
| Bovine serum albumin; caffeic acid; zein | Antisolvent precipitation | 217 nm | 7.3% | 86% | N/A | Bioaccessibility increased from 44% to 63–73% after encapsulation | Cellular antioxidant activity (Caco-2 cells) of the encapsulated RSV was higher than the free RSV, with EC50 values of 19.3–20.8 and 41.6 μg mL−1, respectively. | 74 |
| Polycaprolactone | Antisolvent precipitation (GAS) | 100–135 μm | 50–65% | 98–99% | pH-independent burst release (first 9 h) up to 30 or 60%, followed by constant release | N/A | N/A | 151 |
| Pectin; zein | Antisolvent precipitation and electrostatic deposition | 230 nm | 10.2% (w/w) | 78% | N/A | N/A | Encapsulated RSV exhibited higher antiproliferative activity in human hepatocarcinoma cells (Bel-7402 cells); IC50 of encapsulated RSV was 17.6 mg mL−1versus 25.6 mg mL−1 for free RSV | 152 |
| Pectin; zein | Antisolvent precipitation and electrostatic deposition | 125–225 nm | N/A | N/A | N/A | Higher RSV release when encapsulated (37%) compared to free (13%) in the gastric phase and in the intestinal phase (80% versus 55%, respectively). Encapsulated RSV had much higher antioxidant activity than the non-encapsulated forms after simulated GIT. | Determination of the optimal dilution of digestion fluids for the intracellular antioxidant activity in HepG2 cells (dilution > 256 times), where the digested encapsulated RSV revealed superior activity. | 75 |
| Pectin; zein | Antisolvent precipitation and electrostatic deposition | 235 nm | N/A | 78% | N/A | N/A | High concentrations (>10 mg mL−1) inhibited the viability of Caco-2 or RAW 264.7 cells. Cellular uptake was higher for encapsulated RSV after 2 h (1.06 μg mL−1) only reaching 0.62 μg mL−1 for free RSV. Transmembrane transport was significantly higher for encapsulated RSV (4.7-fold) than free RSV after 4 h (∼1 μg mL−1vs. ∼0.2 μg m−1). | 153 |
| Zein-epigallocatechin gallate conjugate | Antisolvent precipitation method | 115–170 nm | 2.5–2.75% | 40–78% | N/A | Bioaccessibility increased from 43% (free RSV) to 90% (encapsulated RSV) | N/A | 154 |
| Gellan gum; chitosan | Electro-spinning | 180–300 nm | N/A | 67–86% | RSV release decreased at increasing pH. At pH 1.2, 53–65% of RSV was released, while at pH 6.8, around 50% was released, and at pH 7.5, around 45% of RSV was released | N/A | RSV-loaded nanofibers or free RSV displayed a dose-dependent cytotoxic effect (MTT assay) in HT-29 cell lines, with a higher effect for free RSV compared to loaded-NFs. | 84 |
| Zein | Electro-spinning | 375–500 nm | 1.94–7.8% | 78–97% | Up to 39% of RSV was released in PBS (pH 7.4) in 2 h, with controlled release up to 24 h | Bioaccessibility of RSV improved 1.4-fold when encapsulated in zein NFs (from 48% to 68%) | N/A | 83 |
| Polyethylene oxide; poly(lactide-glycolide) | Electro-spinning (coaxial) | 600–1000 nm | N/A | N/A | Constant release, in different polymer ratios, up to 55–72% in 350 h | N/A | RSV-loaded fibre mesh displayed anti-tumoral activity (MTT assay) in breast cancer MCF-7 cells. | 155 |
| Zein | Electro-spraying | 230–330 nm | N/A | 38–70% | All formulations followed a similar release profile, an initial burst in the first hour, followed by a controlled release up to 9 h; max. release rate for encapsulated RSV was between 15% and 55%, while free RSV was 10% | Only 35% RSV was released after 2 h in gastric digestion (pH 1.2), suffering a sudden increase (62%) after exposure to the intestinal digestion (pH 6.8) | Improved effective permeability in chicken intestinal segment (ex vivo) for nanoencapsulated RSV (1.4 × 10−4 cm s−1) compared to free RSV (1.2 × 10−4 cm s−1) | 156 |
| Egg shell membrane; silk fibroin | Electro-spraying | 20–250 nm | N/A | 35–96% | Encapsulated RSV had a sustained and controlled release, with 20% released in the first hour, with a maximum of around 80% released after 6 h for the two formulations assessed | N/A | N/A | 157 |
| Dextran; gliadin | Colloidal complexation | 15–22 nm | N/A | 70–75% | N/A | Bioaccessibility of free RSV was 55%, which increased to 70–76% when nanocomplexed with gliadin | N/A | 158 |
| Pea protein isolate; pectin; rhamnolipids | Colloidal complexation | 500–600 nm | 1% | 53–95% | N/A | Particle size increased during in vitro digestion; RSV release varied between 65% and 90% after 2 h of digestion, reaching ∼100% after 3 h | N/A | 159 |
| Lauroyl arginate; pea protein isolate; pectin; rhamnolipids; tea saponin | Colloidal complexation | 375–650 nm | 1.5–3% | 60–95% | N/A | The produced ternary complexes displayed a slow release of RSV in simulated gastrointestinal conditions | N/A | 160 |
| Spiral dextrin; RSV crystals | Crystallization and colloidal complexation | N/A | N/A | 7.5–60% | N/A | RSV crystals displayed low release in the small intestine phase, displaying promise for the colonic delivery of RSV | N/A | 161 |
| TEMPO-oxidized cellulose | Dissolution-freeze-drying | 18.6 m2 g−1 (pore size) | N/A | N/A | Burst release in the first 15 min, both in the gastric phase (pH 1.5) and intestinal phase (pH 7.4), followed by slow release up to 36% and 50%, respectively, which stabilized after 5 h. Free RSV released 90% in 5 h. | N/A | Both loaded (up to 40 μg mL−1 of RSV) and unloaded aerogels showed almost no cytotoxicity (MTT assay) in TC-28a2 chondrocytes (cell viability > 90%) | 162 |
| Lignin; D-lactide monomers | Film casting by solvent evaporation | N/A | N/A | N/A | Initial burst release followed by controlled release (pH 6.8 and 7.4, 37 °C). Samples with increased lignin content displayed a lower burst release. | N/A | All samples displayed good anti-tumoral properties (CCK-8 assay) in Lewis lung carcinoma cells; IC50 values of produced samples increased with the LG-g-PDLA content | 163 |
| Ethyl cellulose; hydroxypropyl cellulose | Film casting by solvent evaporation | Thickness: 0.10–0.12 mm | N/A | 93–99% | RSV release from the mucoadhesive buccal film was 33–55%. Drug release increased with the swelling degree of the films. | N/A | Ex vivo permeation was estimated to be 4.92 ± 0.22 μg cm−2 in goat buccal mucosa. No histopathological changes were observed (up to 8 h). | 85 |
| β-Cyclodextrin | Inclusion complexation | N/A | N/A | N/A | N/A | N/A | RSV free or encapsulated exhibited a dose-dependent cytotoxic effect (MTT assay) in Caco-2 cells. Non-cytotoxic against human erythrocytes. | 164 |
| Grape skin powder | Infusion by vacuum | N/A | 10% | N/A | N/A | The release of RSV was ∼45% during gastric digestion and the total release in the intestinal phase using low and high bile salts was ∼70% and ∼90%, respectively. | N/A | 100 |
| Chitosan; γ-poly(glutamic acid) | Ionic gelation | 87–258 nm | 33–37% | N/A | N/A | N/A | Cellular antioxidant activity (HepG2 cell line) of the encapsulated RSV was significantly higher than the free RSV | 76 |
| γ-Cyclodextrins | Ionic gelation method | 170–350 nm | 17–22% | 65–92% | Free RSV exhibited a burst release, and was completely solubilized within 6 h, while the encapsulated RSV achieved the maximum release (84%) after 24 h | N/A | N/A | 165 |
| Alginate; pectin | Ionotropic gelation | 1450 μm | N/A | 42% | <10% release at pH 1.2 (2 h); up 70% release in pH 7.4 after 7 h | N/A | Reduction of triacylglycerol content in 3T3-L1 mature adipocytes (application in obesity treatment) | 166 |
| Chitosan; zein | Liquid–liquid dispersion | 220–300 nm | N/A | 51–53% | Initial burst release of 28% of RSV in the first 2 hours in SGF and up to 29% after 6 hours in SIF. In PBS, 25% of RSV was released in the first 4 h, and up to 40% in 120 h. | N/A | N/A | 167 |
| Zein | Nano-precipitation | 120–180 nm | 4–14% | 75–93% | Burst release in the first hour (20% and 50–60% for lyophilized particles), followed by slightly increased release up to 24 h at pH 6.8 | N/A | Zein NPs decreased the apparent cytotoxicity (MTT assay) of free RSV in Caco-2 or HT29-MTX cell lines. Fresh NPs led to reduced RSV transport across cell monolayers compared with free RSV, while freeze-dried counterparts showed similar permeability profiles to that of free RSV. | 132 |
| Chitosan (coating); zein | Phase separation | 100–150 nm | 3–15% | 50–91% | N/A | Bioaccessibility increased from 44% for free RSV to ∼90% for encapsulated RSV | N/A | 125 |
| Soy protein isolate | Rotary evaporation | 525 nm | N/A | N/A | ∼60% of encapsulated RSV was released at pH 1.2 (at 2 h), with an increase of up to ∼85% at pH 7.4, after 4 h vs. 16 and 30 5 for free RSV. | N/A | LPS-dependent NF-κB activation bioassay by measuring GFP expression using RAW 264.7 macrophages. After release from nanoparticles RSV is still bioactive. | 168 |
| Silica; tetraethyl orthosilicate | Sol–gel and freeze-drying | N/A | 19% | N/A | Initial burst release, followed by sustained release after 1 h; 90% and 80% release in gastrointestinal (pH 2.0) and intestinal conditions (pH 7.4), respectively | N/A | Cell viability (MTT assay) of TC-28a2 chondrocytes was higher than 90% for aerogels loaded with up to 20 μg mL−1 of RSV | 169 |
| Starch | Ultrasonication-freeze drying | 419–797 nm | 2.1–2.9% | 75–81% | N/A | 16–19% of RSV was released after 60 min in gastric conditions. In intestinal conditions, after 120 min 12–13% of RSV was released, while after 180 min, a burst release was observed with 55–65% of RSV released. Encapsulated RSV exhibited higher anti-obesity and anti-diabetic activities than free RSV after in vitro digestion. | N/A | 131 |
| Gelatin, Gellan gum | 3D printed gel | N/A | 3.82% | 86.41% | N/A | 11.2% release of RSV in the oral phase, followed by an additional 13.5% in the gastric phase, reaching a total of 89.2% bioaccessible RSV at the end of the intestinal phase, versus 74.7% for the control sample (RSV in MCT oil) | RSV intestinal absorption was analyzed via ex vivo everted sac model, with the 3D printed gel loaded with RSV displaying a 1.13-fold increase over the control MCT oil with RSV | 99 |
3.1 Lipid-based delivery systems
Emulsions can be produced with a wide range of droplet sizes, ranging from the micro to the nano scale, with smaller sizes typically presenting advantages, such as better stability to avoid aggregation and better transparency properties.3,47,54
Recently, emulsions and nanoemulsions have been used to develop delivery systems for resveratrol. Some examples are presented in Table 1. Cheng et al.55 developed nanoemulsions for the co-delivery of resveratrol and α-tocopherol through high-speed homogenization, followed by high-pressure homogenization. The sunflower oil nanoemulsions were stabilized with sodium caseinate in the absence or presence of gum Arabic and pectin. The sodium caseinate nanoemulsions displayed a bi-modal droplet size distribution (∼150 and 640 nm), which was not affected by the addition of gum Arabic, but increased with the addition of pectin (∼300 and 860 nm, respectively). In the study by Fang et al.,56 the addition of resveratrol to α-tocopherol improved its chemical stability, both in storage and during in vitro digestion. The authors also demonstrated that the addition of gum Arabic and low concentrations of pectin improved the bioaccessibility of both α-tocopherol and resveratrol (up to ∼40% and ∼90%, respectively), which is likely due to the increased protection conferred during digestion, leading to more stable resveratrol being available at the end of digestion.
Using a low-energy process, Mamadou et al.57 produced micro-emulsions through self-emulsification processes using liquid and semi-solid self-emulsification drug delivery systems (SEDDS) composed of Miglyol® 812 and Montanox® 80 for the liquid SEDDS. The nanoemulsions produced from the liquid SEDDS displayed a droplet size of ∼100 nm. The ex vivo permeability of these nanoemulsions was assayed using a Ussing chamber model with the jejunum of rats. The permeability of resveratrol tremendously improved using the nanoemulsions, where an 8.5-fold increase was obtained for the liquid SEDDS nanoemulsions compared to a resveratrol dispersion in an ethanolic medium. There were no significant changes in transepithelial conductance of the intestine (2 h of contact with the nanoemulsions), indicating that the intestinal functional viability was maintained.
To improve the gut-retention time and permeability of resveratrol, high-energy nanoemulsions produced using tricaprylin were coated with waxy maize starch nanocrystals and chitosan via the layer-by-layer methodology. The nanoemulsions displayed a Sauter mean diameter of ∼500 nm for the uncoated emulsions and ∼575 to 650 nm for the coated emulsions. The coated emulsions showed a 24-fold improvement in adhesion on porcine intestinal mucosa compared to resveratrol powder, while also displaying a 14-fold increase in bioavailability. The coated emulsions improved both the gut-retention time and absorption of resveratrol, while maintaining colloidal stability during storage for 60 days under acidic conditions.58
Huang et al.61 produced liposomes via thin-film hydration from egg yolk phosphatidylcholine and Tween 80 to co-encapsulate resveratrol and curcumin. The diameter of the liposomes was in the range of 75 to 90 nm, and increase in the bioactive loading increased the size of the liposomes. The co-encapsulated liposomes displayed better antioxidant and lipid peroxidation inhibition properties and improved stability compared to their individually loaded counterparts. An interesting strategy was presented by Soo et al.62 using liposomes to co-encapsulate pristine resveratrol and cyclodextrin-resveratrol inclusion complexes. They were co-encapsulated in the lipophilic and hydrophilic areas of the liposomes, respectively, using the thin film hydration method. The liposomes had a mean size of ∼130 nm, with a low polydispersity index (PDI = 0.089). The developed resveratrol-loaded liposomes exhibited improved drug release (100%) over a 24 h period compared to free resveratrol and conventional liposomal formulations (∼40–60%). Furthermore, the formulations were stable over 14-days storage (4 °C) and exhibited a dose-dependent cytotoxicity profile in HT-29 colon cancer cells.
In 2019, Peng et al.63 explored the use of pH-driven methods combined with high-pressure homogenization to produce polyphenol (curcumin, quercetin, and resveratrol)-loaded liposomes. The results showed different encapsulation efficiencies for each polyphenol, where the produced liposomes could load curcumin with an encapsulation efficiency of 100% and resveratrol at 93%, but quercetin at a much lower efficiency (54%). These differences were explained by the stability of the bioactives under alkaline conditions. Thus, the above-mentioned studies show that using pH-driven methods to encapsulate lipophilic polyphenols is dependent on the impact of pH on the stability and solubility of the bioactives.
Recently, Baek et al.64 developed nanoliposomes with resveratrol, which enabled a delayed in vitro release profile, resulting in an increase in the in vitro cellular transport, in vitro cellular antioxidant activity, and ex vivo intestinal permeability of resveratrol.
Qin et al.66 used the emulsification and low-temperature solidification method to produce SLNs from stearic acid, lecithin and Myrj52, and obtained a mean diameter of ∼150 nm (for resveratrol-loaded SLNs) and ∼80 nm for blank SLNs. The authors performed in vivo assays in mice to determine the effect of encapsulated resveratrol on physical fatigue. The results showed that the resveratrol-loaded SLNs prolonged the exhaustion time and running distance in the mice. Biochemical blood analysis showed that fatigue markers were also reduced (∼25% for aspartate aminotransferase and ∼40% for alanine aminotransferase) in the resveratrol-loaded SLNs, showing the great potential of using encapsulated resveratrol for this purpose. Another great application of resveratrol can be cancer treatment. Kumar et al.67 developed SLNs to target colon cancer cells. The SLNs were composed of stearic acid and Poloxamer-127, encapsulating resveratrol and ferulic acid. After preparation through the solvent evaporation method and hot homogenization method (homogenization via sonication), the SLNs were coated with chitosan and folic acid was conjugated. The uncoated SLNs displayed a lower mean size (∼150 nm) than the coated ones (∼170 nm). Over a 48 h period, the coated SLNs released ∼42% and ∼45% of their resveratrol and ferulic acid content, respectively. The authors reported that in vitro anti-cancer studies using HT-29 showed that the chitosan-coated SLNs effectively targeted and increased cytotoxicity in cancer cells.
Babazadeh et al.68 developed resveratrol-loaded NLCs to develop functional foods using high-speed homogenization and ultrasonication. Different NLC formulations composed of lauric acid, stearic acid or cocoa butter as the solid lipids, and glycerol, Miglyol 812, corn oil or oleic acid as the liquid lipids, and Poloxamer 407, Tween 80 or Tween 20 as surfactants, were prepared via the hot homogenization method. The mean size of the NLCs varied from ∼40 to ∼120 nm. The optimal formulation yielded an encapsulation efficiency of ∼99%, which was stable against resveratrol leakage during 45-day storage at 5 °C. Using similar surfactants, Shimojo et al.69 produced NLCs through high shear homogenization. The NLCs were composed of Compritol 888 ATO, Miglyol 812 in the lipid phase and Poloxamer 188 and Tween 80 in the aqueous phase. The mean size of the tested formulations ranged from ∼100 to ∼260 nm for the unloaded NLCs and ∼125 to ∼190 nm for the resveratrol-loaded NLCs. The optimum formulation was reported to have a mean diameter of ∼135 nm and PDI of ∼0.4. The NLCs were produced with high encapsulation efficiencies (∼93%) and production yield (∼65%).
Recently, Houacine at al.70 analyzed the effects of liquid lipids in the development of NLCs for the oral delivery of resveratrol. A hot melt homogenization process was used at high-speeds and several liquid lipids were assessed (Miglyol 808, Triolein, Capryol 90, Lauroglycol 90, decyl oleate and Labrasol). Trimyristin was used as the solid lipid, and the aqueous phase was composed of Tween 80 and sodium cholate. The more lipophilic liquid lipids (Triolein and decyl oleate) yielded NLCs with a smaller particle size, and increasing their concentration led to an increase in particle size. The drug loading capacity was dependent on the solubility of resveratrol in the liquid lipid. Tunable NLCs were produced with a mean particle diameter of less than 100 nm, low PDI (<0.3), high encapsulation efficiencies (91–99%) and a loading in the range of 2% to 7%. Additionally, it was reported that the selection of the liquid lipid influenced the storage stability (at 4 °C and 25 °C) of the resveratrol-loaded NLCs. Regarding resveratrol retention, all the NLCs stored at 4 °C remained stable after one month, while at 25 °C, only the Triolein NLCs remained stable.
Lipid-based systems seem to be a good strategy for the development of delivery systems for resveratrol with interesting results related to size, PDI, and stability. The most common applications of the proposed systems are liquid foods, while they can also be used in dried foods; however, in some cases, an additional drying step may be needed.
3.2 Polymeric-based delivery systems
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis resveratrol ratio (0.63) than the WPC microcapsules (0.43) and free resveratrol (0.49). The use of resveratrol in both the SC and WPC microparticles increased the digestive stability and bioaccessibility compared to free resveratrol (47% and 23%, respectively). Between the two, the SC microparticles displayed better digestive stability and bioaccessibility (86% and 81% compared to 71% and 68% for the WPC microparticles, respectively). Also using spray drying, Brotons-Canto et al.73 developed resveratrol-loaded zein-lysine nanoparticles through desolvation and spray drying and assessed their oral bioavailability in a human clinical trial. The developed nanoparticles had a mean size of 331 nm with an encapsulation efficiency of 87% and a resveratrol loading of 57 μg resveratrol per mg of formulation. The subjects of the clinical trial received 4.8 g of the developed formulation, equivalent to a dosage of 250 mg of resveratrol, in 100 mL of drinking water. The formulation was well tolerated by the participants in the clinical trial and quantifiable plasma levels of resveratrol and its metabolites were determined (maximum plasma concentration of 21.80 and 986.29 ng mL−1, respectively), indicating that resveratrol or its metabolites were absorbed in the blood stream. These values were higher than that reported in the literature for conventional resveratrol formulations (free compound), displaying the potential benefits of using delivery systems. The antisolvent precipitation method was used by Fan et al.74 to produce nanoparticles composed of zein and bovine serum albumin (BSA) and zein and BSA-caffeic acid (CA) conjugates. The particle size of the zein-BSA and zein-BSA-CA nanoparticles was ∼206 and ∼217 nm, with encapsulation efficiencies of ∼85% and ∼86%, respectively. The thermal- and photostability of resveratrol improved when it was encapsulated, namely, when encapsulated in the zein-BSA-CA nanoparticles. The bioaccessibility of free resveratrol after in vitro digestion was reported to be ∼44% and increased to ∼63% after its incorporation in zein-BSA nanoparticles and ∼73% in zein-BSA-CA nanoparticles. In addition, both the zein-BSA and zein-BSA-CA nanoparticles displayed higher cellular antioxidant activity values than that of free resveratrol.
cis resveratrol ratio (0.63) than the WPC microcapsules (0.43) and free resveratrol (0.49). The use of resveratrol in both the SC and WPC microparticles increased the digestive stability and bioaccessibility compared to free resveratrol (47% and 23%, respectively). Between the two, the SC microparticles displayed better digestive stability and bioaccessibility (86% and 81% compared to 71% and 68% for the WPC microparticles, respectively). Also using spray drying, Brotons-Canto et al.73 developed resveratrol-loaded zein-lysine nanoparticles through desolvation and spray drying and assessed their oral bioavailability in a human clinical trial. The developed nanoparticles had a mean size of 331 nm with an encapsulation efficiency of 87% and a resveratrol loading of 57 μg resveratrol per mg of formulation. The subjects of the clinical trial received 4.8 g of the developed formulation, equivalent to a dosage of 250 mg of resveratrol, in 100 mL of drinking water. The formulation was well tolerated by the participants in the clinical trial and quantifiable plasma levels of resveratrol and its metabolites were determined (maximum plasma concentration of 21.80 and 986.29 ng mL−1, respectively), indicating that resveratrol or its metabolites were absorbed in the blood stream. These values were higher than that reported in the literature for conventional resveratrol formulations (free compound), displaying the potential benefits of using delivery systems. The antisolvent precipitation method was used by Fan et al.74 to produce nanoparticles composed of zein and bovine serum albumin (BSA) and zein and BSA-caffeic acid (CA) conjugates. The particle size of the zein-BSA and zein-BSA-CA nanoparticles was ∼206 and ∼217 nm, with encapsulation efficiencies of ∼85% and ∼86%, respectively. The thermal- and photostability of resveratrol improved when it was encapsulated, namely, when encapsulated in the zein-BSA-CA nanoparticles. The bioaccessibility of free resveratrol after in vitro digestion was reported to be ∼44% and increased to ∼63% after its incorporation in zein-BSA nanoparticles and ∼73% in zein-BSA-CA nanoparticles. In addition, both the zein-BSA and zein-BSA-CA nanoparticles displayed higher cellular antioxidant activity values than that of free resveratrol.
In 2019, Huang et al.75 produced zein-pectin core–shell nanoparticles for the delivery of resveratrol through the combination of the antisolvent precipitation and electrostatic deposition methods. The nanoparticles were stable against aggregation over a wide pH range (2–7) and to thermal processing (80 °C for 1 h, pH 4), but degraded when exposed to high ionic strengths (>50 mmol L−1 NaCl, pH 4). The solubility of the nanoencapsulated resveratrol was higher in simulated gastric fluids than that of free resveratrol. The amount of resveratrol released at the end of the gastric phase was higher for the zein-pectin nanoparticle system (37%) than for the zein and free resveratrol (13.1%). The encapsulated resveratrol displayed higher levels of intracellular antioxidant activity compared to free resveratrol. Using a similar strategy, Chung et al.76 produced resveratrol-loaded chitosan/γ-poly(glutamic acid) (γ-PGA) nanoparticles via the ionic gelation method at different concentrations. Two optimum formulations (maximized UV stability and resveratrol solubility) were designed, i.e., one with a particle size of ∼90 nm and encapsulation efficiency of 33% and other with a particle size of ∼260 nm and encapsulation efficiency of 37%. The cellular antioxidant activity values of the resveratrol delivery system were higher than that of the free resveratrol, with the formulation with a smaller particle size (i.e., 90 nm) displaying higher cellular antioxidant activity.
The high interest in this technology has prompted researchers to explore its use for the delivery of resveratrol, given that it is possible to obtain dried and encapsulated materials without heating. In 2020, Seethu et al.82 developed whey protein isolate (WPI)-pullulan nanofibers (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio) at different concentrations (15%, 18% and 21% total solids (TS)). The produced fibers displayed a mean fiber diameter in the range of ∼60 to ∼200 nm and encapsulation efficiency of ∼75% to ∼95%. Uniform bead-free fibers were optimized under the electrospinning conditions of 18% TS, 18 kV applied voltage and 0.6 mL h−1 flow rate. The antioxidant properties of resveratrol were maintained. The release of resveratrol under simulated gastric (pH 1.2 for 2 h) and intestinal (pH 7.4 for 6 h) conditions was delayed when encapsulated in the nanofibers (∼30%, ∼40%, ∼73% and ∼80% for 2, 4, 6 and 8 h, respectively) versus free resveratrol (∼50%, ∼80%, ∼96% and ∼99%, for 2, 4, 6, and 8 h respectively). This enabled a more controlled and prolonged release, which is typically favorable for increasing the bioavailability of resveratrol in the gastrointestinal tract. Similar behavior was observed by Leena et al.83 in electrospun zein nanofibers produced at different concentrations (2%, 5% and 10%). The produced fibers had a ribbon-like morphology with a mean fiber diameter in the range of ∼400 to ∼500 nm and high encapsulation efficiency (∼80% to ∼95%). Furthermore, between ∼65% to ∼80% of the antioxidant capacity of resveratrol was maintained, indicating the effectiveness of the electrospinning process in maintaining the antioxidant properties of resveratrol. The in vitro release study indicated that the incorporation of resveratrol in the zein matrix resulted in its low release under gastric conditions (∼33% release) and quick release under intestinal conditions (up to ∼55% in less than 0.3 h), followed by its controlled and sustained release up to 8 h. The bioaccessibility of free resveratrol's was ∼48%, while that of the resveratrol-loaded fibers improved to ∼70%, a 1.4-fold increase.
50 ratio) at different concentrations (15%, 18% and 21% total solids (TS)). The produced fibers displayed a mean fiber diameter in the range of ∼60 to ∼200 nm and encapsulation efficiency of ∼75% to ∼95%. Uniform bead-free fibers were optimized under the electrospinning conditions of 18% TS, 18 kV applied voltage and 0.6 mL h−1 flow rate. The antioxidant properties of resveratrol were maintained. The release of resveratrol under simulated gastric (pH 1.2 for 2 h) and intestinal (pH 7.4 for 6 h) conditions was delayed when encapsulated in the nanofibers (∼30%, ∼40%, ∼73% and ∼80% for 2, 4, 6 and 8 h, respectively) versus free resveratrol (∼50%, ∼80%, ∼96% and ∼99%, for 2, 4, 6, and 8 h respectively). This enabled a more controlled and prolonged release, which is typically favorable for increasing the bioavailability of resveratrol in the gastrointestinal tract. Similar behavior was observed by Leena et al.83 in electrospun zein nanofibers produced at different concentrations (2%, 5% and 10%). The produced fibers had a ribbon-like morphology with a mean fiber diameter in the range of ∼400 to ∼500 nm and high encapsulation efficiency (∼80% to ∼95%). Furthermore, between ∼65% to ∼80% of the antioxidant capacity of resveratrol was maintained, indicating the effectiveness of the electrospinning process in maintaining the antioxidant properties of resveratrol. The in vitro release study indicated that the incorporation of resveratrol in the zein matrix resulted in its low release under gastric conditions (∼33% release) and quick release under intestinal conditions (up to ∼55% in less than 0.3 h), followed by its controlled and sustained release up to 8 h. The bioaccessibility of free resveratrol's was ∼48%, while that of the resveratrol-loaded fibers improved to ∼70%, a 1.4-fold increase.
Aiming to control the release of resveratrol in the gastrointestinal tract (GIT), Rostami et al.84 developed resveratrol-loaded chitosan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gellan nanofibers at two different ratios (95
gellan nanofibers at two different ratios (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 90
5 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). The fiber diameter varied in the range of ∼160 to ∼290 nm for 95
10). The fiber diameter varied in the range of ∼160 to ∼290 nm for 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 90
5 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratios. Resveratrol-loaded chitosan fibers were also prepared, with a diameter of ∼170 nm. The authors showed that the addition of gellan gum helped improve the controlled release of resveratrol from the nanofibers under gastro-simulated conditions, given that it reduced the amount of resveratrol released from ∼65% (for the chitosan fibers) to ∼50% (for the chitosan
10 ratios. Resveratrol-loaded chitosan fibers were also prepared, with a diameter of ∼170 nm. The authors showed that the addition of gellan gum helped improve the controlled release of resveratrol from the nanofibers under gastro-simulated conditions, given that it reduced the amount of resveratrol released from ∼65% (for the chitosan fibers) to ∼50% (for the chitosan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gellan nanofibers). At neutral pH (intestinal conditions), the release between the chitosan and the chitosan
gellan nanofibers). At neutral pH (intestinal conditions), the release between the chitosan and the chitosan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gellan fibers was similar. The in vitro cell cytotoxicity of the chitosan
gellan fibers was similar. The in vitro cell cytotoxicity of the chitosan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gellan nanofibers was assessed and the produced fibers displayed high biocompatibility.
gellan nanofibers was assessed and the produced fibers displayed high biocompatibility.
3.3 Other resveratrol delivery systems
In addition to the aforementioned systems, others can be used to encapsulate resveratrol. Recently, research has been conducted regarding the use of systems such as edible films, inclusion complexes, niosomes, proniosomes, micelles, gels, and ternary complexes.Edible films can be defined as a thin layer of edible material that can be formed into a film that can be used as food wrap, contact material, or in other applications such as buccal films for the delivery of nutraceutical compounds. They can be used to increase the shelf life of food products or promote a health effect if a bioactive compound is added.85–88
Ansari et al.85 developed mucoadhesive buccal films loaded with resveratrol through the solvent casting technique using hydroxypropyl cellulose (HPC) and ethyl cellulose (EC). The film thickness varied in the range of 0.10 to 0.12 mm and displayed an encapsulation efficiency of ∼93% to ∼99%. All the films displayed muco-adhesion and increasing the concentration of HPC led to the formation of films with better mucoadhesive properties. The release of resveratrol was found to be in the range of ∼33% to ∼55%. The films with higher amounts of HPC displayed a higher release of resveratrol, while higher amounts of EC led to the release of less resveratrol.
A popular material to produce inclusion complexes is cyclodextrins due to the simple and facile incorporation of bioactives in their cavities. Escobar-Avello et al.89 developed a hydroxypropyl β-cyclodextrin (HP-β-CD) inclusion complex via spray drying using a grape cane pilot-plant extract (GCPPE). The spray drying process had a yield of 83.8%, which was more than double that of the process yield for GCPPE alone (38.4%). The phenolic profile of the developed system was analyzed via LC-ESI-LTQ-Orbitrap-MS and displayed an encapsulation efficiency of 32.7% for resveratrol. The developed inclusion complex resulted in a size (d50, equivalent volume diameters at 50% cumulative volume) of 10.9 μm. The authors analyzed the antioxidant activity of the inclusion complex and determined it was 5300 μmol Trolox equivalents (TE) per g dry weight, which is comparable to that of a previously conducted study on non-encapsulated GCPFE (4612 μmol TE per g dry weight). The data showed that the inclusion complex maintained the antioxidant capacity of GCPFE, and it may improve its stability, solubility, and bioavailability.
Niosomes are delivery systems that possess a bilayer structure and are formed by the self-association of non-ionic surfactants and cholesterol in an aqueous phase. They are biodegradable, biocompatible, and nonimmunogenic. Proniosomes are enhanced versions of niosomes given that they are in the dried state, making them easier to store, transport and dose, and upon hydration they form the niosomes.90,91 Pando et al.92 developed niosomes for the delivery of resveratrol using a thin film hydration method followed by agitation and sonication. Niosomes were prepared from a mixture of surfactants (Span 60, Labrasol or Maisine 35-1) and dodecanoyls, and their mean diameter was in the range of ∼160 to ∼220 nm for Span 60 niosomes, ∼300 to ∼700 nm for Labrasol, and ∼160 to ∼400 nm for Maisine 35-1. The encapsulation efficiency was also dependent on the surfactant, with that of the Span 60 niosomes ranging from ∼16% to ∼72%, Labrasol niosomes from ∼16% to ∼64% and Maisine 35-1 niosomes from ∼25% to ∼58%. The niosomes displayed good stability, in particular the Span 60-based niosomes.
Recently, Schlich et al.93 also used the thin film hydration method, combined with sonication to develop niosomes. The solvent was removed under vacuum to produce proniosomes. Tween 20, Span 60, and cholesterol were used to produce the niosomes and lactose and maltodextrin were used to transform the niosomes into proniosomes through the slurry method. The produced proniosomes had a mean diameter of ∼190 to ∼280 nm, with a low PDI (<0.3), and a high EE (∼75% to ∼85%). The authors evaluated the release of resveratrol from the proniosomes under simulated gastrointestinal conditions and reported that after 2 h of gastric digestion, ∼25% to ∼50% of resveratrol was released from the different proniosome formulations. In simulated intestinal digestion, up to ∼70% of resveratrol was released for some of the proniosome formulations after 8 h, and a burst effect was reported during the first hour of intestinal digestion. The cytotoxicity effect of the developed proniosomes was assessed in Caco-2 cells and up to a concentration of 73 μmol L−1 of resveratrol for all the proniosome formulations was deemed safe to use, while only some of the formulations were safe to use at a loading of 146 μmol L−1 of resveratrol.
Currently, some of the researched nanocarriers have some limitations that can possibly be overcome through hybridization techniques, given that they enable the development of novel delivery systems by combining functionalities of different nano delivery systems into a single system.94
Nanomicelles are self-assembling colloidal structures made of amphiphilic monomers, which are composed of two parts, i.e., a small hydrophobic head and a long hydrophilic tail. The hydrophobic core enables the compatibility of hydrophobic nutraceuticals, while the hydrophilic tail helps prevent degradation and enhances the solubility of molecules.95 In 2020, Jangid et al.96 developed pH-responsive nanomicelles composed of stearic acid, Pluronic F68 and inulin. The developed resveratrol-loaded nanomicelles displayed a mean size of ∼170 nm, with a low PDI (∼0.24) and encapsulation efficiency of above 90% and 55% for resveratrol concentrations of 10% and 20% (w/w), respectively. Stability tests demonstrated the superior stability of resveratrol when loaded in the nanomicelles, given that the resveratrol content after 48 h storage at room and refrigerated temperatures was higher than ∼98% compared to ∼20% for a resveratrol solution. The resveratrol-loaded micelles displayed a release of ∼35% in an acidic environment (pH 1.2), which was much lower than the ∼95% obtained for a resveratrol solution or ∼65% for resveratrol-loaded nanomicelles developed without inulin. At a pH of 6.8, the resveratrol-loaded nanomicelles displayed controlled and sustained release. The blank nanomicelles displayed high biocompatibility in HCT 116 human colon cancer cell lines, while the resveratrol-loaded nanomicelles exhibited dose and time-dependent cytotoxicity. Resveratrol-loaded nanomicelles were also reported to have higher values of cellular uptake and improved oral bioavailability in in vivo studies when compared to a resveratrol solution.
Some macrostructures, such as gels, have also been used as delivery systems for several bioactive compounds. Emulsion gels are produced from a stable liquid-like emulsion through the gelling of the aqueous phase. This process improves the emulsion properties such as stability against coalescence and phase separation. These emulsion gels can be used as fat substitutes in healthier functional foods or to develop products with novel textures.97,98 Yan et al.98 produced resveratrol and curcumin-loaded emulsion gels from Pickering emulsions via high-speed homogenization using zein and sodium alginate as gelling agents and transglutaminase and calcium ions as the crosslinking agents. The double-crosslinked gels presented the highest photostability, while the fastest resveratrol degradation occurred in the absence of crosslinking. The highest bioaccessibility was determined for the double-crosslinked gels (∼70%), while the lowest bioaccessibility was observed for the Pickering emulsions in the absence of crosslinking (∼40%). Alternatively, single crosslinking resulted in an intermediate bioaccessibility (∼55% for transglutaminase crosslinking and ∼60% for calcium crosslinking). This likely due to the increased protection provided by crosslinking and incorporation in the emulsion gel. The higher the protection during gastrointestinal digestion, the higher the amount of stable and active resveratrol will be bioaccessible at the end of the digestion. 3D printed gels can also be used, as shown recently by Kavimughil et al.,99 where they developed an MCT oleogel for the co-delivery of curcumin and resveratrol. Resveratrol loaded on the 3D printed gel was protected during oral and gastric digestion, and released in the intestinal phase, with a 1.2-fold increase in bioaccessibility compared to free resveratrol. Ex vivo assays showed that the liposome loaded-resveratrol could permeate 1.13-fold better than free resveratrol in oil.
A recent trend in the food industry is the use of food by-products to improve the stability and bioaccessibility of nutraceutical compounds. This strategy was explored by Rai et al.,100 where they developed a novel system in which they infused resveratrol in micron-scale grape skin powder (GSP) produced through the vacuum-assisted infusion of GSP. It improved photostability of infused resveratrol in GSP when exposed to UV-A light. Specifically, ∼45% of resveratrol was released from the infused GSP during gastric digestion and the total release in the intestinal phase reached ∼70% and ∼90% (using low and high bile salt concentration, respectively).
4. Food applications
Food fortification can be defined as the practice of increasing the content of essential micronutrients or other bioactive compounds in a food product. However, the incorporation of bioactives, such as resveratrol, can lead to undesirable consequences regarding changes in the sensory properties of functional food products, namely, their appearance and mouthfeel.Functional foods have only recently entered the shelves of supermarkets, which can lead to skepticism and reluctancy to adopt by consumers. It is also relevant that in many cases, functional foods do not have an immediate impact given that most are designed to mitigate specific health risks, which can result in the belief that products do not act as they are marketed, causing consumers to distrust the efficacy of functional foods, a phenomenon typically known as food neophobia.101,102 Food neophobia can be described as the reluctance of consumers to eat and/or their avoidance of novel foods.101,103 This can cause novel functional food products to be lower rated or have less interest simply due to consumer suspicion or unfamiliarity.
Familiarity has been previously linked with consumer preference, namely, regarding food preference, with more familiar foods or attributes rated higher in acceptance and less familiar products or attributes rated lower. Some of the parameters that can be affected by this are color, texture, aroma, flavor, and even the consumer willingness to buy products with differences to what they expect.104–112 Therefore, reducing organoleptic changes when adding functional compounds to food products is paramount.
The use of delivery systems can help reduce these alterations; nevertheless, careful analysis of the sensory changes in the developed functional food products needs to be conducted.3,47 Sensory analysis is one of the key elements in the development of a novel or modified food product and is essential for the development of functional foods to ensure that the incorporation of micro-nano delivery systems does not have a detrimental effect on sensory attributes such as aroma, flavor, mouthfeel, texture and overall consumer.3,113 There is a lack of information in the literature on how resveratrol can affect the development and acceptance of functional foods, namely, food products that involve encapsulation technology, and whether it can help reduce the effects on the sensory profile of a product compared to the direct addition of resveratrol to a food product.
Only a few studies explored the use of resveratrol delivery systems in food products. Recently, Silva et al.114 explored resveratrol-loaded γ-cyclodextrin for the development of functional lemon juices. A γ-cyclodextrin aqueous solution was homogenized with an ethanolic solution of resveratrol and snap-frozen in liquid nitrogen and freeze-dried. Then, the resulting product was rehydrated for 24 h in a water-saturated chamber. Lemon juices were supplemented with 25 mg of pure resveratrol or the equivalent quantity of resveratrol-loaded γ-cyclodextrin and their stability (resveratrol amount and antioxidant activity) was assessed over a 28-day period. Color differences were noted between the resveratrol-enriched juices and plain lemon juice. The quantity of resveratrol was revealed to be much higher, even immediately after formulation, in the inclusion complexes, indicating their ability to improve the dissolution of resveratrol. Over time, the amount of resveratrol in the inclusion complexes decreased from 43% to 20% at day 0 and day 28 of storage, respectively, which suggests some disintegration/degradation of the inclusion complex over time. Regarding antioxidant activity, no differences were observed between the fortified juices and the plain lemon juice. These results suggest that the antioxidant capacity of resveratrol was masked by the high antioxidant capacity of the plain juice due to the presence of other antioxidant compounds such as ascorbic acid and citric acid and other phenolic compounds.
Seethu et al.82 developed resveratrol-loaded whey protein isolate and pullulan nanofibers using EHDP and incorporated them into milk. The resveratrol-fortified milk exhibited no significant changes in its physiochemical and sensorial parameters. The physiochemical parameters such as pH, titratable acidity and milk viscosity were similar, given that no significant differences were found between the two samples. Regarding sensorial parameters, the color and appearance, consistency, flavor, and overall acceptability using a 9-point hedonic scale ratings were slightly higher for color and appearance and consistency and slightly lower for flavor and overall acceptability when comparing the resveratrol-enriched milk with the control formulation. Similarly, Pando et al.92 developed resveratrol-loaded niosomes and incorporated them in yoghurt. They studied their influence on the textural properties of the yoghurt and concluded that the addition of resveratrol-loaded niosomes did not cause changes in its textural properties. Shruthi et al.115 produced resveratrol-loaded proniosomes and tested them in milk and yoghurt. The incorporation of resveratrol-loaded niosomes in milk and yoghurt did not affect the organoleptic qualities of the products.
Koga et al.116 developed resveratrol-loaded sodium caseinate microcapsules through spray drying and evaluated their consumer acceptance when incorporated in snack bars and gummies. The use of 10 mg of resveratrol-loaded microcapsules in the snack bars did not significantly influence consumer acceptance compared to the control samples. However, when the resveratrol-loaded microcapsules were incorporated in the gummies, the consumer acceptance decreased. Also aimed at its use in snacks, Ahmad & Gani117 loaded resveratrol in different starches (horse-chestnut, water-chestnut, and lotus-stem) through ultrasonication and freeze-drying. The resveratrol-loaded starch nanoparticles were incorporated in wheat flour snacks, which were equally accepted by the sensory panel compared with the control, indicating that the addition of either free or loaded resveratrol at very low amounts did not affect the sensory properties of the snack.
In summary, sensory analysis conducted on foods fortified with resveratrol delivery systems indicated that their addition to food products slightly impacted their organoleptic properties. Nonetheless, only a few studies have added resveratrol delivery systems to food products, and thus more information on its use in the development of functional foods is still needed.
As the market for these products continues to grow, this is critical information for the development and consumption of functional food ingredients due to the potential negative impact of their addition to food products on the organoleptic properties.
5. In vitro models to assess safety and efficacy
Understanding the performance of colloidal systems in the human body is essential for the development of new formulations for oral delivery given that their behavior (changes and interactions under physiological conditions) determines their efficacy and safety.To date, different in vitro models are available in the literature to assess both bioaccessibility, which is the fraction of bioactive that reaches the intestine and is ready for absorption, and intestinal permeability, which is the fraction of bioactive that crosses the intestinal epithelium.118
Human digestion can be simulated in vitro following simple static digestion methods or using more complex dynamic models. However, static methods have limitations, given that they cannot mimic the complex dynamics of the digestion process, such as the constant pH in the gastric phase, the lack of gradual addition of gastric secretions, and the absence of gastric emptying. Moreover, the enzymatic activity is not adjusted to the type or amount of food. The intestinal phase is taken as a whole, not considering the sequential duodenal, jejunal, and ileal phases, which exhibit different conditions. More complex dynamic models are also available, such as the Dynamic Gastric Model (DGM) developed by the UK Institute of Food Research, TNO-Intestinal Models (TIMs) developed by the Netherlands Organization for Applied Scientific Research, DIDGI built at INRA and the Human Gastric Simulator (HGGS) conceived by the University of California-Davis.119 However, these models are expensive, labor intensive and time consuming. Recently, a semi-dynamic model built on a previously described standardized static protocol120,121 was published, including gradual acidification, enzyme addition and gastric emptying, in the gastric phase, while the intestinal phase remains static.122 The latter represents an intermediate approach regarding complexity and physiological relevance.
For the evaluation of intestinal permeability, in vitro mono- or co-cultures of intestinal human cell lines grown on semi-permeable membranes are widely used to mimic the gut epithelium and evaluate the intestinal absorption of test compounds.123 These models not only contribute to reducing the use of animal models, but also minimize interspecies variability given that human cell lines are used.124 However, they overlook important aspects of gut physiology, such as continuous flow in the lumen, peristaltic movements (mechanical stimulus), and co-cultures with microbiota. Microfluidic-based technology plays an important role in in vitro methodologies/models (organ-on-a-chip) for food and drug development. The possibility to use miniaturized tools to mimic the digestive system represents an advantage for scarce and expensive molecules, such as bioactives and nanoparticles, also allowing high throughput.
Several authors have studied the stability of resveratrol, both free and loaded in different delivery systems, under the digestive conditions, where static in vitro digestion methods are the most common approach (Table 1). However, despite their simplicity, different protocols are reported in the literature, where some have slight differences, but these differences are enough to hamper the comparison of results across laboratories. The most frequent differences among methods are the enzymes used, their origin and concentration, pH, digestion time in each phase, and how digestion is stopped and the micellar phase recovered. The enzymes used are often referred to in mass or molar concentration instead of enzymatic activity (U mg−1), which is critical given that the concentration does not reflect the activity of a specific enzyme, where it varies over the storage time or between lots.75,125,126 The quantification of bioaccessibility also differs, given that different authors use different conditions to recover the micellar phase, namely, regarding the centrifugation protocol (e.g., speed, time, and number of centrifugations).58,72 Small differences over the different stages of digestion protocols can add up and lead to bigger differences in the reported bioaccessibility of compounds, and thus the protocol for digestion and bioaccessibility should be carefully considered and standardized.121 Great effort has been devoted to the standardization of static digestion models, allowing the comparison of inter-laboratory results.127 Dynamic in vitro models have been identified as physiologically more relevant to evaluate the bioaccessibility and antioxidant activity of food polyphenols.128
Few studies have evaluated the behavior of formulations during the complete physiological process. Table 1 demonstrates that very few authors have reported in vitro digestion, followed by cellular interactions in the same study, where the studies reported by Huang et al.75 and Xu et al.129 are the only examples included in Table 1.
Reports are often limited to specific topics, such as physicochemical characterization and bioaccessibility or cytotoxicity and/or other cellular responses, such as oxidative stress, inflammation, cellular uptake, and transmembrane transport. Critically, intestinal permeability or cellular responses are often determined directly using freshly formulated systems. However, it will be more realistic to test them after their in vitro digestion for a better in vivo approximation. This evaluation is crucial to understand whether the tested nanomaterial or its cargo reaches the intestine. The main challenge to completely mimic the GIT is usually related to the cytotoxicity of the simulant fluids.130 Huang et al.75 tested digested samples after dilution (256 times) in hepatocarcinoma cells (HepG2), demonstrating the cell viability (MTT assay) and improved intracellular reactive oxygen species scavenging activity (H2DCF-DA assay) of resveratrol-loaded zein-pectin core/shell nanoparticles compared to free resveratrol. Fan et al.74 studied resveratrol loaded in zein-bovine serum albumin-caffeic acid nanoparticles, evaluating the bioaccessibility of resveratrol after in vitro digestion. The authors also evaluated their cellular antioxidant activity in human colon carcinoma cells (Caco-2 cells); however, the nanoparticles were tested in cells without previous digestion.
Xu et al.129 tested different concentrations of digested resveratrol-loaded liposomes (5 to 90 μM) in Caco-2 cells, demonstrating the dose-dependent effect of resveratrol on cell viability (CCK-8 assay) (all concentrations were biocompatible) and assessed the antioxidant effect of free resveratrol and resveratrol loaded in liposomes (CellROX Deep Red assay), with improved results for the delivery systems of resveratrol versus free resveratrol.
As summarized in Table 1, different approaches have been used following different production methodologies to develop resveratrol delivery systems, demonstrating high capacity to improve the bioaccessibility, reduce the cytotoxicity and increase the transport of resveratrol through a cell monolayer compared to that obtained for free resveratrol. The bioactivity of resveratrol has also been evaluated after in vitro digestion, such as antioxidant,75 anti-diabetic and anti-obesity.131 Cell responses have been assessed using different cell types, such as intestinal epithelium (Caco-2, HT29), chondrocytes (TC-28a2), macrophages (RAW 264.7), fibroblasts (NIH 3T3), erythrocytes, and adipocytes; however, few studies have used co-cultures of intestinal epithelium cells,132 which are definitely physiologically more relevant to test bioactives for oral administration.
Additionally, safety concerns also need to be addressed, given that materials behave differently at the nanoscale. These issues include questions regarding cytotoxicity and bioaccumulation of nanomaterials, which need to be considered when designing nanosized delivery systems. Moţ et al.133 evaluated the use of an emulsion delivery system and its impact on the gastrointestinal transit time and tissue distribution of resveratrol and reported an increase in its bioavailability in the blood and liver, which can lead to questions regarding the possibility of its bioaccumulation in these organs. Other studies have also shown that the use of resveratrol delivery systems can increase the permeability of resveratrol through the intestine, and thus increase its bioavailability.64,99
Currently, the use of nano-delivery systems in the European Union is covered by EC Regulation No 258/97,134 which that legislates “novel foods” and “novel food ingredients”. The guidelines for the risk assessment include the in vitro135 evaluation of the dissolution of these materials in lysosomal conditions, and genotoxicity and cytotoxicity studies, followed by the evaluation of parameters, such as in vivo genotoxicity and 90-day toxicity, in in vivo studies and organ histopathological studies.136
In conclusion, the standardization of methodologies to assess the bioaccessibility and cellular responses of a particular bioactive such as resveratrol will be very helpful. After proving the safety and efficacy of novel functional food ingredients, further steps need to be taken to ensure their proper use in food fortification, ensuring that the consumer is as unaffected as possible regarding organoleptic changes caused by the addition of these functional ingredients to foods.
6. Concluding remarks and future prospects
Interest in functional foods is projected to continue to increase over the next decade, reaching as high as 120 to 140 billion US dollars by 2030. Resveratrol has numerous health-promoting properties, making it a prime candidate for use in functional food ingredients, but it also has some drawbacks associated with its solubility, stability, bioavailability, and organoleptic impact, which has hindered its use as a widespread functional ingredient. In this case, the use of delivery systems has been shown to minimize some of these drawbacks, and recently explored for the delivery of resveratrol as a functional food ingredient.Recent studies have demonstrated that resveratrol can display in vitro health-promoting properties, although in vivo studies are still lacking, namely, regarding the use of resveratrol-loaded delivery systems. However, it can be concluded that their use is beneficial, namely, regarding the stability of resveratrol. Research into the gastrointestinal fate and cytotoxicity properties of delivery systems for resveratrol is also progressing, with in vitro studies indicating that resveratrol delivery systems are biocompatible and promote an increase in the bioavailability and cellular antioxidant activity of resveratrol in in vitro cell culture. Nevertheless, complete studies on the cytotoxicity properties of delivery systems for resveratrol after in vitro gastrointestinal digestion are lacking, especially when using complex food matrices.
Studies seem to show that applying coatings to the developed delivery systems can be helpful to improve some properties, namely, gastric stability, bioavailability, and mucoadhesion, given that they add additional properties to the system. For example, a chitosan coating can help the mucoadhesion and tailor the release of resveratrol during gastrointestinal digestion. Its bioavailability increased using delivery systems, and the increase was typically higher when using techniques such as emulsification, solvent displacement and EHDP.
Although there are some studies on the organoleptic impact of resveratrol in functional food products, this is another key area in which information is still lacking. This is particularly relevant when assessing the potential benefits of resveratrol delivery systems and comparing the sensory properties of foods with resveratrol as an additive.
Some issues still exist regarding the use of delivery systems in food products, namely, those with sizes in the nano range. Most delivery systems present a low loading capacity, indicating that the addition of dried delivery systems to food products must consider the fact that the replacement level of the product will be higher compared to free resveratrol, thus possibly leading to unintended changes in some of the organoleptic properties of the functional food product.
Safety issues are also a concern, and hence additional knowledge regarding the behaviour of nano-sized structures when orally ingested is required. Thus, it is necessary to examine current food regulations and guidelines to ensure the proper design and safe use of nano-sized delivery systems.
An ideal delivery system for resveratrol should provide a high loading capacity and encapsulation efficiency, while providing chemical and physical stability to resveratrol. This should allow its controlled and/or targeted release to the target site, thus increasing its bioavailability, and consequently its bioactivity in vivo. Additionally, flavor masking should also be considered to ensure that the consumer does not experience any organoleptic changes when consuming functional food products.
Although some of the current technologies can adequately improve the bioaccessibility and bioavailability of resveratrol, such as EHDP, they still present some drawbacks, namely, regarding the complexity of the delivery system, cost, and production yield of the technology, as well as scaling up issues.
Addressing the above-mentioned issues can help further the use and development of functional food products loaded with resveratrol, which are currently lacking in the market, allowing for the better exploitation of the current interest in functional food products.
Author contributions
Conceptualization: P. M. S., C. G., L. M. P., M. A. C., A. A. V., M. C.; writing – original draft: P. M. S, C. G., and M. C. Data curation: P. M. S., C. G., M. C.; writing – review & editing: P. M. S., C. G., L. M. P., M. A. C., A. A. V., M. C.; funding acquisition: C. G., L. M. P., M. A. C., A. A. V., M. C.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit, and by LABBELS – Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020. This work was funded by the SbDtoolBox – Nanotechnology-based tools and tests for Safer-by-Design nanomaterials, with the reference NORTE-01-0145-FEDER-000047, funded by Norte 2020 – North-Regional Operational Programme under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). The research also received funding from the European Union's H2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement N 778388 (H2020 MSCA-RISE-2017) project Food for Diabetes and Cognition (FODIAC), and grant agreement N 713640 (MSCA-2015-COFUND-FP). Pedro Silva is the recipient of a fellowship (SFRD/BD/130247/2017) supported by Fundação para a Ciência e a Tecnologia, (FCT, Portugal). Graphical abstract was created with BioRender.com.References
- M. Chávarri and M. C. Villarán, Encapsulation technologies for resveratrol in functional food, in New Polymers for Encapsulation of Nutraceutical Compounds, ed. J. Ruiz and M.Campos, 2017, DOI:10.1002/9781119227625.ch8.
- V. Orlien, Innovative Food Processing Technologies: A Comprehensive Review, Ref. Modul. Food Sci, 2019, 1 Search PubMed.
- S. F. Hosseini, L. Ramezanzade and D. J. McClements, Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods, Trends Food Sci. Technol., 2021, 109, 322 CrossRef CAS.
- H. Koc, C. J. Vinyard, G. K. Essick and E. A. Foegeding, Food oral processing: Conversion of food structure to textural perception, Annu. Rev. Food Sci. Technol., 2013, 4, 237 CrossRef CAS PubMed.
- F. Temelli, Perspectives on the use of supercritical particle formation technologies for food ingredients, J. Supercrit. Fluids, 2018, 134, 244 CrossRef CAS.
- M. A. Sani, M. Tavassoli, M. Azizi-Lalabadi, K. Mohammadi and D. J. McClements, Nano-enabled plant-based colloidal delivery systems for bioactive agents in foods: Design, formulation, and application, Adv. Colloid Interface Sci., 2022, 305, 102709 CrossRef CAS PubMed.
- S. Pund, A. Joshi and R. Banerjee, Engineered Nanomaterials in Functional Foods, in Innovative Food Processing Technologies, ed. K. Knoerzer and K. Muthukumarappan, 2021, DOI:10.1016/b978-0-08-100596-5.22981-8.
- R. W. Sabnis, Patents on Natural Products for Diagnosing/Preventing/Treating Alzheimer's Disease, in Studies in Natural Products Chemistry, 2019 Search PubMed.
- E. H. Siemann and L. L. Creasy, Concentration of the phytoalexin resveratrol in wine, Am. J. Enol. Vitic., 1992, 43, 49 CrossRef CAS.
- P. Kopp, Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the “French paradox”?, Eur. J. Endocrinol., 1998, 138, 619 CAS.
- G. Davidov-Pardo and D. J. McClements, Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues, Trends Food Sci. Technol., 2014, 38, 88 CrossRef CAS.
- B. V. Fiod Riccio, B. Fonseca-Santos, P. Colerato Ferrari and M. Chorilli, Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review, Crit. Rev. Anal. Chem., 2020, 50, 339 CrossRef CAS PubMed.
- M. A. Cerqueira, A. C. Pinheiro, H. D. Silva, P. E. Ramos, M. A. Azevedo, M. L. Flores-López, M. C. Rivera, A. I. Bourbon, Ó. L. Ramos and A. A. Vicente, Design of Bio-nanosystems for Oral Delivery of Functional Compounds, Food Eng. Rev., 2014, 6, 1 CrossRef CAS.
- L. S. Simões, D. Madalena, A. Pinheiro, J. Teixeira, A. Vicente and O. Ramos, Micro- and nano bio-based delivery systems for food applications: In vitro behavior, Adv. Colloid Interface Sci., 2017, 243, 23–45 CrossRef PubMed.
- P. E. Ramos, P. Silva, M. M. Alario, L. M. Pastrana, J. A. Teixeira, M. A. Cerqueira and A. A. Vicente, Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics, Food Hydrocolloids, 2018, 77, 8 CrossRef CAS.
- R. M. Rodrigues, P. E. Ramos, M. F. Cerqueira, J. A. Teixeira, A. A. Vicente, L. M. Pastrana, R. N. Pereira and M. A. Cerqueira, Electrosprayed whey protein-based nanocapsules for β-carotene encapsulation, Food Chem., 2020, 314, 126157 CrossRef CAS PubMed.
- M. A. Azevedo, M. A. Cerqueira, P. Fuciños, B. F. B. Silva, J. A. Teixeira and L. Pastrana, Rhamnolipids-based nanostructured lipid carriers: Effect of lipid phase on physicochemical properties and stability, Food Chem., 2021, 344, 128670 CrossRef CAS PubMed.
- R. Nunes, B. D. D. Pereira, M. A. Cerqueira, P. M. Silva, L. M. Pastrana, A. A. Vicente, J. T. Martins and A. I. Bourbon, Lactoferrin-based nanoemulsions to improve the physical and chemical stability of omega-3 fatty acids, Food Funct., 2020, 11, 1966 RSC.
- A. M. Marques, A. I. Bourbon, J. A. Teixeira, L. M. Pastrana and M. A. Cerqueira, Nano spray drying for the encapsulation of bioactive ingredients, in Spray Drying Encapsulation of, Bioactive Materials, 2021 Search PubMed.
- A. M. Marques, A. I. Bourbon, R. M. Rodrigues, J. A. Teixeira, L. M. Pastrana and M. A. Cerqueira, Lactoferrin as a carrier of iron: Development and physicochemical characterization, Food Hydrocolloids, 2023, 142, 108772 CrossRef CAS.
- A. R. Machado, P. M. P. Silva, A. A. Vicente, L. A. Souza-Soares, A. C. Pinheiro and M. A. Cerqueira, Alginate Particles for Encapsulation of Phenolic Extract from Spirulina sp. LEB-18: Physicochemical Characterization and Assessment of In Vitro Gastrointestinal Behavior, Polymers, 2022, 14, 4759 CrossRef CAS PubMed.
- R. Castro-Muñoz, M. S. Kharazmi and S. M. Jafari, Chitosan-based electrospun nanofibers for encapsulating food bioactive ingredients: A review, Int. J. Biol. Macromol., 2023, 245, 125424 CrossRef.
- I. Robertson, W. Hau, F. Sami, S. Ali, V. Badgujar, S. Hasnain, A. Khan, S. Majeed and M. Tahir Ansari, The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential, Int. J. Pharm., 2022, 618, 121605 CrossRef CAS.
- I. M. Chung, U. Subramanian, P. Thirupathi, B. Venkidasamy, R. Samynathan, B. H. Gangadhar, G. Rajakumar and M. Thiruvengadam, Resveratrol nanoparticles: A promising therapeutic advancement over native resveratrol, Processes, 2020, 8, 1 Search PubMed.
- M. L. Castro, J. P. Ferreira, M. Pintado, O. L. Ramos, S. Borges and S. Baptista-Silva, Grape By-Products in Sustainable Cosmetics: Nanoencapsulation and Market Trends, Appl. Sci., 2023, 13, 9168 CrossRef CAS.
- Z. Xie and X. Chen, Healthy benefits and edible delivery systems of resveratrol: a review, Food Rev. Int., 2021, 39(7), 3879–3905 CrossRef.
- A. C. Santos, I. Pereira, M. Pereira-Silva, L. Ferreira, M. Caldas, M. Magalhães, A. Figueiras, A. J. Ribeiro and F. Veiga, Nanocarriers for resveratrol delivery: Impact on stability and solubility concerns, Trends Food Sci. Technol., 2019, 91, 483 CrossRef CAS.
- N. D. Machado, M. A. Fernández and D. D. Díaz, Recent Strategies in Resveratrol Delivery Systems, ChemPlusChem, 2019, 84, 951 CrossRef CAS.
- J. A. Baur and D. A. Sinclair, Therapeutic potential of resveratrol: The in vivo evidence, Nat. Rev. Drug Discovery, 2006, 5, 493 CrossRef CAS PubMed.
- K. Wang, Z. Chen, J. Shi, Y. Feng, M. Yu, Y. Sun, Q. Zhuang, B. Liang, G. Luo, X. Xu and M. Fan, Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells, Prostate, 2020, 80, 977 CrossRef CAS PubMed.
- A. Davoodvandi, M. Darvish, S. Borran, M. Nejati, S. Mazaheri, O. R. Tamtaji, M. R. Hamblin, N. Masoudian and H. Mirzaei, The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis, Int. Immunopharmacol., 2020, 88, 106905 CrossRef CAS.
- M. Shabani, A. Sadeghi, H. Hosseini, M. Teimouri, R. B. Khorzoughi, P. Pasalar and R. Meshkani, Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population, Sci. Rep., 2020, 10, 1 CrossRef PubMed.
- R. Torregrosa-Muñumer, E. Vara, J. Á. Fernández-Tresguerres and R. Gredilla, Resveratrol supplementation at old age reverts changes associated with aging in inflammatory, oxidative and apoptotic markers in rat heart, Eur. J. Nutr., 2021, 60, 2683–2693 CrossRef PubMed.
- T. T. Cai, X. L. Ye, R. R. Li, H. Chen, Y. Y. Wang, H. J. Yong, M. L. Pan, W. Lu, Y. Tang, H. Miao, A. M. Snijders, J. H. Mao, X. Y. Liu, Y. B. Lu and D. F. Ding, Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice, Front. Pharmacol., 2020, 11, 1249 CrossRef CAS PubMed.
- X. Zhou, Q. Ruan, Z. Ye, Z. Chu, M. Xi, M. Li, W. Hu, X. Guo, P. Yao and W. Xie, Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration, Burns, 2021, 47, 133 CrossRef PubMed.
- P. Wang, J. Gao, W. Ke, J. Wang, D. Li, R. Liu, Y. Jia, X. Wang, X. Chen, F. Chen and X. Hu, Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota, Free Radicals Biol. Med., 2020, 156, 83 CrossRef CAS PubMed.
- ClinicalTrials.gov, Short Interval Resveratrol Trial in Cardiovascular Surgery.
- ClinicalTrials.gov, The Effects of Resveratrol Supplementation on Inflammation and Cognitive Performance in Healthy Adults.
- ClinicalTrials.gov, Effects of High-Dose Resveratrol in Non-Diabetic Obese Males.
- M. Williams, The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th Edition Edited by M.J.O'Neil, Royal Society of Chemistry, Cambridge, UK ISBN 9781849736701; 2708 pages. April 2013, $150 with 1-year free access to The Merck Index Online, Drug Dev. Res., 2013, 74, 339 CrossRef CAS.
- T. Walle, F. Hsieh, M. H. DeLegge, J. E. Oatis and U. K. Walle, High absorption but very low bioavailability of oral resveratrol in humans, Drug Metab. Dispos., 2004, 32, 1377 CrossRef CAS PubMed.
- T. Walle, Bioavailability of resveratrol, Ann. N. Y. Acad. Sci., 2011, 1215, 9 CrossRef CAS PubMed.
- Buckwheat Healthcare Products, Buckwheat Healthcare Products Pte Ltd.
- M. Mesías and C. Delgado-Andrade, Melanoidins as a potential functional food ingredient, Curr. Opin. Food Sci., 2017, 14, 37 CrossRef.
- G. Davidov-Pardo, I. J. Joye and D. J. Mcclements, Food-Grade Protein-Based Nanoparticles and Microparticles for Bioactive Delivery: Fabrication, Characterization, and Utilization, Adv. Protein Chem. Struct. Biol., 2015, 98, 293 CAS.
- M. J. Costa, P. E. Ramos, P. Fuciños, J. A. Teixeira, L. M. Pastrana and M. Â. Cerqueira, Development of Bio-Based Nanostructured Systems by Electrohydrodynamic Processes, in Nanotechnology Applications in the Food Industry, 2018, DOI:10.1201/9780429488870-1.
- A. M. Marques, M. A. Azevedo, J. A. Teixeira, L. M. Pastrana, C. Gonçalves and M. A. Cerqueira, Engineered Nanostructures for Enrichment and Fortification of Foods, Food Appl. Nanotechnol., 2019 DOI:10.1201/9780429297038-4.
- S. Pimentel-Moral, M. C. Teixeira, A. R. Fernandes, D. Arráez-Román, A. Martínez-Férez, A. Segura-Carretero and E. B. Souto, Lipid nanocarriers for the loading of polyphenols – A comprehensive review, Adv. Colloid Interface Sci., 2018, 260, 85 CrossRef CAS PubMed.
- A. Maqsoudlou, E. Assadpour, H. Mohebodini and S. M. Jafari, Improving the efficiency of natural antioxidant compounds via different nanocarriers, Adv. Colloid Interface Sci., 2020, 278, 102122 CrossRef CAS PubMed.
- H. Rostamabadi, S. R. Falsafi, M. M. Rostamabadi, E. Assadpour and S. M. Jafari, Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules, Adv. Colloid Interface Sci., 2021, 290, 102384 CrossRef CAS PubMed.
- D. J. McClements, Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems, Adv. Colloid Interface Sci., 2015, 219, 27 CrossRef CAS PubMed.
- Y. Ozogul, G. T. Karsli, M. Durmuş, H. Yazgan, H. M. Oztop, D. J. McClements and F. Ozogul, Recent developments in industrial applications of nanoemulsions, Adv. Colloid Interface Sci., 2022, 304, 102685 CrossRef CAS PubMed.
- J. L. Guía-García, A. V. Charles-Rodríguez, P. Silva, A. Robledo-Olivo, M. A. Cerqueira and M. L. Flores-López, Electrosprayed hydroxypropyl methylcellulose microcapsules containing Rhus microphylla fruit extracts and their application in strawberry (Fragaria × ananassa) preservation, LWT–Food Sci. Technol., 2023, 184, 115048 CrossRef.
- J. S. Komaiko and D. J. McClements, Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods, Compr. Rev. Food Sci. Food Saf., 2016, 15, 331 CrossRef CAS PubMed.
- H. Cheng, Q. Fan, T. Liu, Wusigale and L. Liang, Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility, J. Food Eng., 2020, 264, 109685 CrossRef CAS.
- Z. Fang, Wusigale, H. Bao, Y. Ni, N. Choijilsuren and L. Liang, Partition and digestive stability of α-tocopherol and resveratrol/naringenin in whey protein isolate emulsions, Int. Dairy J., 2019, 93, 116 CrossRef CAS.
- G. Mamadou, C. Charrueau, J. Dairou, N. Limas-Nzouzi, B. Eto and G. Ponchel, Increased intestinal permeation and modulation of presystemic metabolism of resveratrol formulated into self-emulsifying drug delivery systems, Int. J. Pharm., 2017, 521, 150 CrossRef CAS PubMed.
- M. Jo, C. Ban, K. K. T. Goh and Y. J. Choi, Enhancement of the gut-retention time of resveratrol using waxy maize starch nanocrystal-stabilized and chitosan-coated Pickering emulsions, Food Hydrocolloids, 2021, 112, 106291 CrossRef CAS.
- M. R. Mohammadabadi and M. R. Mozafari, Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form, J. Drug Delivery Sci. Technol., 2018, 47, 445 CrossRef CAS.
- L. Ramezanzade, S. F. Hosseini and M. Nikkhah, Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides, Food Chem., 2017, 234, 220 CrossRef CAS PubMed.
- M. Huang, C. Liang, C. Tan, S. Huang, R. Ying, Y. Wang, Z. Wang and Y. Zhang, Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol, Food Funct., 2019, 10, 6447 RSC.
- E. Soo, S. Thakur, Z. Qu, S. Jambhrunkar, H. S. Parekh and A. Popat, Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach, J. Colloid Interface Sci., 2016, 462, 368 CrossRef CAS PubMed.
- S. Peng, L. Zou, W. Zhou, W. Liu, C. Liu and D. J. McClements, Encapsulation of Lipophilic Polyphenols into Nanoliposomes Using pH-Driven Method: Advantages and Disadvantages, J. Agric. Food Chem., 2019, 67, 7506 CrossRef CAS PubMed.
- Y. Baek, E. W. Jeong and H. G. Lee, Encapsulation of resveratrol within size-controlled nanoliposomes: Impact on solubility, stability, cellular permeability, and oral bioavailability, Colloids Surf., B, 2023, 224, 113205 CrossRef CAS PubMed.
- R. Mohseni, Z. Arab Sadeghabadi, N. Ziamajidi, R. Abbasalipourkabir and A. Rezaei, Oral Administration of Resveratrol-Loaded Solid Lipid Nanoparticle Improves Insulin Resistance Through Targeting Expression of SNARE Proteins in Adipose and Muscle Tissue in Rats with Type 2 Diabetes, Nanoscale Res. Lett., 2019, 14, 227 CrossRef PubMed.
- L. Qin, T. Lu, Y. Qin, Y. He, N. Cui, A. Du and J. Sun, In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements, Molecules, 2020, 25(22), 5302 CrossRef CAS PubMed.
- C. S. Kumar, R. Thangam, S. A. Mary, P. R. Kannan, G. Arun and B. Madhan, Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells, Carbohydr. Polym., 2020, 231, 115682 CrossRef PubMed.
- A. Babazadeh, B. Ghanbarzadeh and H. Hamishehkar, Formulation of food grade nanostructured lipid carrier (NLC) for potential applications in medicinal-functional foods, J. Drug Delivery Sci. Technol., 2017, 39, 50 CrossRef CAS.
- A. A. M. Shimojo, A. R. V. Fernandes, N. R. E. Ferreira, E. Sanchez-Lopez, M. H. A. Santana and E. B. Souto, Evaluation of the influence of process parameters on the properties of resveratrol-loaded NLC using 22 full factorial design, Antioxidants, 2019, 8(8), 272 CrossRef CAS PubMed.
- C. Houacine, D. Adams and K. K. Singh, Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol, J. Mol. Liq., 2020, 316, 113734 CrossRef CAS.
- A. Karim, A. Rehman, J. Feng, A. Noreen, E. Assadpour, M. S. Kharazmi, Z. Lianfu and S. M. Jafari, Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds, Adv. Colloid Interface Sci., 2022, 307, 102744 CrossRef CAS PubMed.
- C. C. Koga, J. E. Andrade, M. G. Ferruzzi and Y. Lee, Stability of Trans-Resveratrol Encapsulated in a Protein Matrix Produced Using Spray Drying to UV Light Stress and Simulated Gastro-Intestinal Digestion, J. Food Sci., 2016, 81(2), C292–C300 CrossRef CAS PubMed.
- A. Brotons-Canto, C. J. Gonzalez-Navarro, J. Gurrea, C. González-Ferrero and J. M. Irache, Zein nanoparticles improve the oral bioavailability of resveratrol in humans, J. Drug Delivery Sci. Technol., 2020, 57, 101704 CrossRef CAS.
- Y. Fan, Y. Liu, L. Gao, Y. Zhang and J. Yi, Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate, Food Chem., 2018, 261, 283 CrossRef CAS PubMed.
- X. Huang, Y. Liu, Y. Zou, X. Liang, Y. Peng, D. J. McClements and K. Hu, Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion, Food Hydrocolloids, 2019, 93, 261 CrossRef CAS.
- J. H. Chung, J. S. Lee and H. G. Lee, Resveratrol-loaded chitosan–γ-poly(glutamic acid) nanoparticles: Optimization, solubility, UV stability, and cellular antioxidant activity, Colloids Surf., B, 2020, 186, 110702 CrossRef CAS PubMed.
- T. Senthil Muthu Kumar, K. Senthil Kumar, N. Rajini, S. Siengchin, N. Ayrilmis and A. Varada Rajulu, A comprehensive review of electrospun nanofibers: Food and packaging perspective, Composites, Part B, 2019, 175, 107074 CrossRef.
- P. M. Silva, C. Prieto, C. C. P. Andrade, J. M. Lagarón, L. M. Pastrana, M. A. Coimbra, A. A. Vicente and M. A. Cerqueira, Hydroxypropyl methylcellulose-based micro- and nanostructures for encapsulation of melanoidins: Effect of electrohydrodynamic processing variables on morphological and physicochemical properties, Int. J. Biol. Macromol., 2022, 202, 453 CrossRef CAS PubMed.
- P. M. Silva, S. Torres-Giner, A. A. Vicente and M. A. Cerqueira, Management of Operational Parameters and Novel Spinneret Configurations for the Electrohydrodynamic Processing of Functional Polymers, Macromol. Mater. Eng., 2022, 307(5), 2100858 CrossRef CAS.
- P. M. Silva, C. Prieto, J. M. Lagarón, L. M. Pastrana, M. A. Coimbra, A. A. Vicente and M. A. Cerqueira, Food-grade hydroxypropyl methylcellulose-based formulations for electrohydrodynamic processing: Part I – role of solution parameters on fibre and particle production, Food Hydrocolloids, 2021, 118, 106761 CrossRef CAS.
- P. M. Silva, S. Torres-Giner, A. A. Vicente and M. A. Cerqueira, Electrohydrodynamic processing for the production of zein-based micro- and nanostructures, Curr. Opin. Colloid Interface Sci., 2021, 56, 101504 CrossRef CAS.
- B. G. Seethu, H. A. Pushpadass, F. M. E. Emerald, B. S. Nath, N. L. Naik and K. S. Subramanian, Electrohydrodynamic Encapsulation of Resveratrol Using Food-Grade Nanofibres: Process Optimization, Characterization and Fortification, Food Bioprocess Technol., 2020, 13, 341 CrossRef CAS.
- M. M. Leena, K. S. Yoha, J. A. Moses and C. Anandharamakrishnan, Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility, Food Biosci., 2020, 36, 100669 CrossRef.
- M. Rostami, M. Ghorbani, M. Aman mohammadi, M. Delavar, M. Tabibiazar and S. Ramezani, Development of resveratrol loaded chitosan-gellan nanofiber as a novel gastrointestinal delivery system, Int. J. Biol. Macromol., 2019, 135, 698 CrossRef CAS PubMed.
- M. Ansari, B. Sadarani and A. Majumdar, Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol, J. Drug Delivery Sci. Technol., 2018, 44, 278 CrossRef CAS.
- M. J. Costa, A. M. Marques, L. M. Pastrana, J. A. Teixeira, S. M. Sillankorva and M. A. Cerqueira, Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio, Food Hydrocolloids, 2018, 81, 442 CrossRef CAS.
- E. de J. Salas-Méndez, A. Vicente, A. C. Pinheiro, L. F. Ballesteros, P. Silva, R. Rodríguez-García, F. D. Hernández-Castillo, M. de L. V. Díaz-Jiménez, M. L. Flores-López, J. Á. Villarreal-Quintanilla, F. M. Peña-Ramos, D. A. Carrillo-Lomelí and D. Jasso de Rodríguez, Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit, Postharvest Biol. Technol., 2019, 150, 19 CrossRef.
- N. Soares, P. Silva, C. Barbosa, R. Pinheiro and A. A. A. Vicente, Comparing the effects of glazing and chitosan-based coating applied on frozen salmon on its organoleptic and physicochemical characteristics over six-months storage, J. Food Eng., 2017, 194, 79 CrossRef CAS.
- D. Escobar-Avello, J. Avendaño-Godoy, J. Santos, J. Lozano-Castellón, C. Mardones, D. von Baer, J. Luengo, R. M. Lamuela-Raventós, A. Vallverdú-Queralt and C. Gómez-Gaete, Encapsulation of phenolic compounds from a grape cane pilot-plant extract in hydroxypropyl beta-cyclodextrin and maltodextrin by spray drying, Antioxidants, 2021, 10, 1130 CrossRef CAS PubMed.
- M. Khatoon, K. U. Shah, F. U. Din, S. U. Shah, A. U. Rehman, N. Dilawar and A. N. Khan, Proniosomes derived niosomes: recent advancements in drug delivery and targeting, Drug Delivery, 2017, 24, 56 CrossRef CAS PubMed.
- S. Khoee and M. Yaghoobian, Niosomes: a novel approach in modern drug delivery systems, Nanostruct. Drug Delivery, 2017 DOI:10.1016/b978-0-323-46143-6.00006-3.
- D. Pando, M. Beltrán, I. Gerone, M. Matos and C. Pazos, Resveratrol entrapped niosomes as yoghurt additive, Food Chem., 2015, 170, 281 CrossRef CAS PubMed.
- M. Schlich, F. Lai, R. Pireddu, E. Pini, G. Ailuno, A. M. Fadda, D. Valenti and C. Sinico, Resveratrol proniosomes as a convenient nanoingredient for functional food, Food Chem., 2020, 310, 125950 CrossRef CAS PubMed.
- S. Kumar, P. Sangwan, V. Lather and D. Pandita, Biocompatible PLGA-oil hybrid nanoparticles for high loading and controlled delivery of resveratrol, J. Drug Delivery Sci. Technol., 2015, 30, 54 CrossRef CAS.
- H. M. Trinh, M. Joseph, K. Cholkar, R. Mitra and A. K. Mitra, Nanomicelles in Diagnosis and Drug Delivery, in Emerging Nanotechnologies for Diagnostics, Drug Delivery Med. Devices, 2017 DOI:10.1016/B978-0-323-42978-8.00003-6.
- A. K. Jangid, K. Patel, P. Jain, S. Patel, N. Gupta, D. Pooja and H. Kulhari, Inulin-pluronic-stearic acid based double folded nanomicelles for pH-responsive delivery of resveratrol, Carbohydr. Polym., 2020, 247, 116730 CrossRef CAS PubMed.
- H. Bao, Y. Ni, Wusigale, H. Dong and L. Liang, α-Tocopherol and resveratrol in emulsion-filled whey protein gels: Co-encapsulation and in vitro digestion, Int. Dairy J., 2020, 104, 104649 CrossRef CAS.
- J. Yan, X. Liang, C. Ma, D. J. McClements, X. Liu and F. Liu, Design and characterization of double-cross-linked emulsion gels using mixed biopolymers: Zein and sodium alginate, Food Hydrocolloids, 2021, 113, 106473 CrossRef CAS.
- M. Kavimughil, M. M. Leena, J. A. Moses and C. Anandharamakrishnan, 3D printed MCT oleogel as a co-delivery carrier for curcumin and resveratrol, Biomaterials, 2022, 287, 121616 CrossRef CAS PubMed.
- R. Rai, C. Merrell, W. Yokoyama and N. Nitin, Infusion of trans-resveratrol in micron-scale grape skin powder for enhanced stability and bioaccessibility, Food Chem., 2021, 340, 127894 CrossRef CAS PubMed.
- M. T. Baker, P. Lu, J. A. Parrella and H. R. Leggette, Consumer Acceptance toward Functional Foods: A Scoping Review, Int. J. Environ. Res. Public Health, 2022, 19, 1217 CrossRef PubMed.
- L. Frewer, J. Scholderer and N. Lambert, Consumer acceptance of functional foods: Issues for the future, Br. Food J., 2003, 105, 714 CrossRef.
- P. Pliner and K. Hobden, Development of a scale to measure the trait of food neophobia in humans, Appetite, 1992, 19, 105 CrossRef CAS PubMed.
- F. M. Clydesdale, Color as a factor in food choice, Crit. Rev. Food Sci. Nutr., 1993, 33, 83 CrossRef CAS PubMed.
- C. Spence, On the Relationship(s) between Color and Taste/Flavor, Exp. Psychol., 2019, 66, 99 CrossRef PubMed.
- C. Spence, C. A. Levitan, M. U. Shankar and M. Zampini, Does food color influence taste and flavor perception in humans?, Chemosens. Percept., 2010, 3, 68 CrossRef.
- M. Lyly, K. Roininen, K. Honkapää, K. Poutanen and L. Lähteenmäki, Factors influencing consumers’ willingness to use beverages and ready-to-eat frozen soups containing oat β-glucan in Finland, France and Sweden, Food Qual. Prefer., 2007, 18, 242 CrossRef.
- A. M. Muñoz and G. V. Civille, Factors affecting perception and acceptance of food texture by american consumers, Food Rev. Int., 1987, 3, 285 CrossRef.
- H. S. G. Tan, E. van den Berg and M. Stieger, The influence of product preparation, familiarity and individual traits on the consumer acceptance of insects as food, Food Qual. Prefer., 2016, 52, 222 CrossRef.
- D. Labbe, L. Damevin, C. Vaccher, C. Morgenegg and N. Martin, Modulation of perceived taste by olfaction in familiar and unfamiliar beverages, Food Qual. Prefer., 2006, 17, 582 CrossRef.
- M. Borgogno, S. Favotto, M. Corazzin, A. V. Cardello and E. Piasentier, The role of product familiarity and consumer involvement on liking and perceptions of fresh meat, Food Qual. Prefer., 2015, 44, 139 CrossRef.
- D. D. Torrico, S. Fuentes, C. Gonzalez Viejo, H. Ashman and F. R. Dunshea, Cross-cultural effects of food product familiarity on sensory acceptability and non-invasive physiological responses of consumers, Food Res. Int., 2019, 115, 439 CrossRef PubMed.
- R. Sun, J. Lu and A. Nolden, Nanostructured foods for improved sensory attributes, Trends Food Sci. Technol., 2021, 108, 281 CrossRef CAS.
- A. F. R. Silva, M. Monteiro, D. Resende, S. S. Braga, M. A. Coimbra, A. M. S. Silva and S. M. Cardoso, Inclusion complex of resveratrol with γ-cyclodextrin as a functional ingredient for lemon juices, Foods, 2020, 10, 16 CrossRef PubMed.
- P. A. Shruthi, H. A. Pushpadass, M. E. E. Franklin, S. N. Battula and N. Laxmana Naik, Resveratrol-loaded proniosomes: Formulation, characterization and fortification, LWT–Food Sci. Technol., 2020, 134, 110127 CrossRef CAS.
- C. C. Koga, S. Y. Lee and Y. Lee, Consumer Acceptance of Bars and Gummies with Unencapsulated and Encapsulated Resveratrol, J. Food Sci., 2016, 81, S1222 CAS.
- M. Ahmad and A. Gani, Development of novel functional snacks containing nano-encapsulated resveratrol with anti-diabetic, anti-obesity and antioxidant properties, Food Chem., 2021, 352, 129323 CrossRef CAS PubMed.
- D. E. Lefebvre, K. Venema, L. Gombau, L. G. Valerio, J. Raju, G. S. Bondy, H. Bouwmeester, R. P. Singh, A. J. Clippinger, E. M. Collnot, R. Mehta and V. Stone, Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices, Nanotoxicology, 2015, 9, 523 CrossRef CAS PubMed.
- D. Dupont, M. Alric, S. Blanquet-Diot, G. Bornhorst, C. Cueva, A. Deglaire, S. Denis, M. Ferrua, R. Havenaar, J. Lelieveld, A. R. Mackie, M. Marzorati, O. Menard, M. Minekus, B. Miralles, I. Recio and P. Van den Abbeele, Can dynamic in vitro digestion systems mimic the physiological reality?, Crit. Rev. Food Sci. Nutr., 2019, 59, 1546 CrossRef CAS PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. MacIerzanka, A. MacKie, S. Marze, D. J. McClements, O. Ménard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food-an international consensus, Food Funct., 2014, 5, 1113 RSC.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991 CrossRef CAS PubMed.
- A. I. Mulet-Cabero, L. Egger, R. Portmann, O. Ménard, S. Marze, M. Minekus, S. Le Feunteun, A. Sarkar, M. M. L. Grundy, F. Carrière, M. Golding, D. Dupont, I. Recio, A. Brodkorb and A. Mackie, A standardised semi-dynamic: in vitro digestion method suitable for food-an international consensus, Food Funct., 2020, 11, 1702 RSC.
- F. Antunes, F. Andrade, F. Araújo, D. Ferreira and B. Sarmento, Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs, Eur. J. Pharm. Biopharm., 2013, 83, 427 CrossRef CAS PubMed.
- B. Sarmento, F. Andrade, S. B. Da Silva, F. Rodrigues, J. Das Neves and D. Ferreira, Cell-based in vitro models for predicting drug permeability, Expert Opin. Drug Metab. Toxicol., 2012, 8, 607 CrossRef CAS PubMed.
- M. A. Khan, L. Chen and L. Liang, Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles, Food Hydrocolloids, 2021, 113, 106477 CrossRef CAS.
- Y. Wei, Z. Yu, K. Lin, C. Sun, L. Dai, S. Yang, L. Mao, F. Yuan and Y. Gao, Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion, Food Hydrocolloids, 2019, 95, 336 CrossRef CAS.
- R. Colombo, L. Ferron, I. Frosi and A. Papetti, Advances in static: In vitro digestion models after the COST action Infogest consensus protocol, Food Funct., 2021, 12, 7619 RSC.
- K. Wojtunik-Kulesza, A. Oniszczuk, T. Oniszczuk, M. Combrzyński, D. Nowakowska and A. Matwijczuk, Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review, Nutrients, 2020, 12, 1401 CrossRef CAS PubMed.
- X. Xu, M. Tian, L. Deng, H. Jiang, J. Han, C. Zhen, L. Huang and W. Liu, Structural degradation and uptake of resveratrol-encapsulated liposomes using an in vitro digestion combined with Caco-2 cell absorption model, Food Chem., 2023, 403, 133943 CrossRef CAS PubMed.
- M. Xavier, P. M. Rodrigues, M. D. Neto, M. I. Guedes, V. Calero, L. Pastrana and C. Gonçalves, From mouth to gut: microfluidic in vitro simulation of human gastro-intestinal digestion and intestinal permeability, Analyst, 2023, 148(14), 3193–3203 RSC.
- M. Ahmad and A. Gani, Ultrasonicated resveratrol loaded starch nanocapsules: Characterization, bioactivity and release behaviour under in-vitro digestion, Carbohydr. Polym., 2021, 251, 117111 CrossRef CAS PubMed.
- R. Nunes, A. Baião, D. Monteiro, J. das Neves and B. Sarmento, Zein nanoparticles as low-cost, safe, and effective carriers to improve the oral bioavailability of resveratrol, Drug Delivery Transl. Res., 2020, 10, 826 CrossRef CAS PubMed.
- A. C. Moţ, R. Silaghi-Dumitrescu and C. Sârbu, Rapid and effective evaluation of the antioxidant capacity of propolis extracts using DPPH bleaching kinetic profiles, FT-IR and UV-vis spectroscopic data, J. Food Compos. Anal., 2011, 24, 516 CrossRef.
- Official Journal of the European Union, Regulation (EC) No 2015/2283 of the European Parliament, 2015.
- M. Xavier, I. A. Parente, P. M. Rodrigues, M. A. Cerqueira, L. Pastrana and C. Gonçalves, Safety and fate of nanomaterials in food: The role of in vitro tests, Trends Food Sci. Technol., 2021, 109, 593 CrossRef CAS.
- S. More, V. Bampidis, D. Benford, C. Bragard, T. Halldorsson, A. Hernández-Jerez, S. Hougaard-Bennekou, K. Koutsoumanis, C. Lambré, K. Machera, H. Naegeli, S. Nielsen, J. Schlatter, D. Schrenk, V. Silano, D. Turck, M. Younes, J. Castenmiller, Q. Chaudhry, F. Cubadda, R. Franz, D. Gott, J. Mast, A. Mortensen, A. G. Oomen, S. Weigel, E. Barthelemy, A. Rincon, J. Tarazona and R. Schoonjans, Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health, EFSA J., 2021, 19(8), e06768 CAS.
- R. Kumar, K. Kaur, S. Uppal and S. K. Mehta, Ultrasound processed nanoemulsion: A comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stability, Ultrason. Sonochem., 2017, 37, 478 CrossRef CAS PubMed.
- V. Sanna, A. M. Roggio, N. Pala, S. Marceddu, G. Lubinu, A. Mariani and M. Sechi, Effect of chitosan concentration on PLGA microcapsules for controlled release and stability of resveratrol, Int. J. Biol. Macromol., 2015, 72, 531 CrossRef CAS PubMed.
- K. Istenič, B. D. Balanč, V. B. Djordjević, M. Bele, V. A. Nedović, B. M. Bugarski and N. P. Ulrih, Encapsulation of resveratrol into Ca-alginate submicron particles, J. Food Eng., 2015, 167, 196 CrossRef.
- M. Naserifar, H. Hosseinzadeh, K. Abnous, M. Mohammadi, S. M. Taghdisi, M. Ramezani and M. Alibolandi, Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats, Life Sci., 2020, 262, 118555 CrossRef CAS PubMed.
- Y. Wei, C. Li, L. Dai, L. Zhang, J. Liu, L. Mao, F. Yuan and Y. Gao, The construction of resveratrol-loaded protein-polysaccharide-tea saponin complex nanoparticles for controlling physicochemical stability and: In vitro digestion, Food Funct., 2020, 11, 9973 RSC.
- S. Kumar, V. Lather and D. Pandita, A facile green approach to prepare core-shell hybrid PLGA nanoparticles for resveratrol delivery, Int. J. Biol. Macromol., 2016, 84, 380 CrossRef CAS PubMed.
- Y. Wei, C. Li, L. Zhang, L. Dai, S. Yang, J. Liu, L. Mao, F. Yuan and Y. Gao, Influence of calcium ions on the stability, microstructure and in vitro digestion fate of zein-propylene glycol alginate-tea saponin ternary complex particles for the delivery of resveratrol, Food Hydrocolloids, 2020, 106, 105886 CrossRef CAS.
- Y. Chen, H. Zhang, J. Yang and H. Sun, Improved antioxidant capacity of optimization of a self-microemulsifying drug delivery system for resveratrol, Molecules, 2015, 20, 21167 CrossRef CAS PubMed.
- F. Y. K. Siu, S. Ye, H. Lin and S. Li, Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity, Int. J. Nanomed., 2018, 13, 4133 CrossRef CAS PubMed.
- D. Pandita, S. Kumar, N. Poonia and V. Lather, Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol, Food Res. Int., 2014, 62, 1165 CrossRef CAS.
- T. Cardoso, A. Gonçalves, B. N. Estevinho and F. Rocha, Potential food application of resveratrol microparticles: Characterization and controlled release studies, Powder Technol., 2019, 355, 593 CrossRef CAS.
- M. Maria Leena, M. Gover Antoniraj, J. A. Moses and C. Anandharamakrishnan, Three fluid nozzle spray drying for co-encapsulation and controlled release of curcumin and resveratrol, J. Drug Delivery Sci. Technol., 2020, 57, 101678 CrossRef CAS.
- J. Zhou, W. Cheng, T. Liu, J. Li and X. Li, Preparation, characterization, and in vitro antioxidant activity of pH-sensitive resveratrol microcapsule in simulated intestinal fluids, Int. J. Food Prop., 2019, 22, 804 CrossRef CAS.
- C. Pu, W. Tang, M. Liu, Y. Zhu and Q. Sun, Resveratrol-loaded hollow kafirin nanoparticles via gallic acid crosslinking: An evaluation compared with their solid and non-crosslinked counterparts, Food Res. Int., 2020, 135, 109308 CrossRef CAS PubMed.
- G. S. B. Sakata, M. M. Ribas, C. Dal Magro, A. E. Santos, G. P. S. Aguiar, J. V. Oliveira and M. Lanza, Encapsulation of trans-resveratrol in poly(ε-caprolactone) by GAS antisolvent, J. Supercrit. Fluids, 2021, 171, 2 CrossRef.
- X. Huang, Y. Dai, J. Cai, N. Zhong, H. Xiao, D. J. McClements and K. Hu, Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities, Food Hydrocolloids, 2017, 64, 157 CrossRef CAS.
- Y. Liu, X. Liang, Y. Zou, Y. Peng, D. J. McClements and K. Hu, Resveratrol-loaded biopolymer core-shell nanoparticles: Bioavailability and anti-inflammatory effects, Food Funct., 2020, 11, 4014 RSC.
- F. Liu, D. Ma, X. Luo, Z. Zhang, L. He, Y. Gao and D. J. McClements, Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol, Food Hydrocolloids, 2018, 79, 450 CrossRef CAS.
- X. Zhang, L. Han, Q. Sun, W. Xia, Q. Zhou, Z. Z. Zhang and X. Song, Controlled release of resveratrol and xanthohumol via coaxial electrospinning fibers, J. Biomater. Sci., Polym. Ed., 2020, 31, 456 CrossRef CAS PubMed.
- H. Jayan, M. Maria Leena, S. K. Sivakama Sundari, J. A. Moses and C. Anandharamakrishnan, Improvement of bioavailability for resveratrol through encapsulation in zein using electrospraying technique, J. Funct. Foods, 2019, 57, 417 CrossRef CAS.
- O. Bayraktar, Y. Yahsi and M. D. Köse, Electroencapsulation of Trans-resveratrol in Nanoparticles Composed of Silk Fibroin and Soluble Eggshell Membrane Protein, Food Bioprocess Technol., 2021, 14, 334 CrossRef CAS.
- C. Qiu, B. Wang, Y. Wang and Y. Teng, Effects of colloidal complexes formation between resveratrol and deamidated gliadin on the bioaccessibility and lipid oxidative stability, Food Hydrocolloids, 2017, 69, 466 CrossRef CAS.
- Q. Guo, X. Shu, Y. Hu, J. Su, S. Chen, E. A. Decker and Y. Gao, Formulated protein-polysaccharide-surfactant ternary complexes for co-encapsulation of curcumin and resveratrol: Characterization, stability and in vitro digestibility, Food Hydrocolloids, 2021, 111, 106265 CrossRef CAS.
- Q. Guo, J. Su, X. Shu, F. Yuan, L. Mao and Y. Gao, Development of high methoxyl pectin-surfactant-pea protein isolate ternary complexes: Fabrication, characterization and delivery of resveratrol, Food Chem., 2020, 321, 126706 CrossRef CAS PubMed.
- P. P. Wang, Z. G. Luo and T. M. Tamer, Spiral-Dextrin Complex Crystals: Efficient Approach for Colon-Targeted Resveratrol Delivery, J. Agric. Food Chem., 2021, 69, 474 CrossRef CAS PubMed.
- L. Qin, X. Zhao, Y. He, H. Wang, H. Wei, Q. Zhu, T. Zhang, Y. Qin and A. Du, Preparation, characterization, and in vitro evaluation of resveratrol-loaded cellulose aerogel, Materials, 2020, 13, 1 Search PubMed.
- R. Liu, L. Dai, Z. Zou and C. Si, Drug-loaded poly(L-lactide)/lignin stereocomplex film for enhancing stability and sustained release of trans-resveratrol, Int. J. Biol. Macromol., 2018, 119, 1129 CrossRef CAS PubMed.
- A. Duarte, A. Martinho, Â. Luís, A. Figueiras, M. Oleastro, F. C. Domingues and F. Silva, Resveratrol encapsulation with methyl-β-cyclodextrin for antibacterial and antioxidant delivery applications, LWT–Food Sci. Technol., 2015, 63, 1254 CrossRef CAS.
- C. Qiu, D. J. McClements, Z. Jin, Y. Qin, Y. Hu, X. Xu and J. Wang, Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation, Food Chem., 2020, 317, 126328 CrossRef CAS PubMed.
- O. Gartziandia, A. Lasa, J. L. Pedraz, J. Miranda, M. P. Portillo, M. Igartua and R. M. Hernández, Preparation and characterization of resveratrol loaded pectin/alginate blend gastro-resistant Microparticles, Molecules, 2018, 23, 1 CrossRef PubMed.
- D. Pauluk, A. K. Padilha, N. M. Khalil and R. M. Mainardes, Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity, Food Hydrocolloids, 2019, 94, 411 CrossRef CAS.
- N. Pujara, S. Jambhrunkar, K. Y. Wong, M. McGuckin and A. Popat, Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate, J. Colloid Interface Sci., 2017, 488, 303 CrossRef CAS PubMed.
- L. Qin, Y. He, X. Zhao, T. Zhang, Y. Qin and A. Du, Preparation, Characterization, and in Vitro Sustained Release Profile of Resveratrol-Loaded Silica Aerogel, Molecules, 2020, 25(12), 2752 CrossRef CAS PubMed.
Footnotes |
| † https://www.bulk.com/eu/resveratrol-powder-99-trans-resveratrol.html |
| ‡ https://buckwheat.com.sg/products/bhp-buckwheat-lycopene-resveratrol-cookies-100g. |
| This journal is © The Royal Society of Chemistry 2023 |