Open Access Article
Open Access ArticleCurrent state of insect proteins: extraction technologies, bioactive peptides and allergenicity of edible insect proteins
Zidan
Ma
a,
Martin
Mondor
bc,
Francisco
Goycoolea Valencia
 a and
Alan Javier
Hernández-Álvarez
a and
Alan Javier
Hernández-Álvarez
 *a
*a
aSchool of Food Science and Nutrition, University of Leeds, Leeds, LS2 9JT, UK. E-mail: a.j.hernandezalvarez@leeds.ac.uk
bSaint-Hyacinthe Research and Development Centre, Agriculture and Agri-Food Canada, Saint-Hyacinthe, QC J2S 8E3, Canada
cInstitute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, QC G1V 0A6, Canada
First published on 1st September 2023
Abstract
This review aims to provide an updated overview of edible insect proteins and the bioactivity of insect-derived peptides. The essential amino acid content of edible insects is compared with well-known protein sources to demonstrate that edible insects have the potential to cover the protein quality requirements for different groups of the population. Then the current methodologies for insect protein extraction are summarized including a comparison of the protein extraction yield and the final protein content of the resulting products for each method. Furthermore, in order to improve our understanding of insect proteins, their functional properties (such as solubility, foaming capacity, emulsifying, gelation, water holding capacity and oil holding capacity) are discussed. Bioactive peptides can be released according to various enzymatic hydrolysis protocols. In this context, the bioactive properties of insect peptides (antihypertensive, antidiabetic, antioxidant and anti-inflammatory properties) have been discussed. However, the allergens present in insect proteins are still a major concern and an unsolved issue for insect-based product consumption; thus, an analysis of cross reactivity and the different methods available to reduce allergenicity are proposed. Diverse studies of insect protein hydrolysates/peptides have been ultimately promoting the utilization of insect proteins for future perspectives and the emerging processing technologies to enhance the wider utilization of insect proteins for different purposes.
Introduction
The global population requires sustainable and innovative protein sources as the demand for food protein is estimated to double by 2050 with the growing population. Insects are recognized, among all alternative sources, to have the potential to fulfill the huge demands for protein in the future. Insects are widely consumed; it is estimated that 1900 insect species are eaten by different groups of the population from around 80 countries in Asia, Africa and America. The most consumed and well-researched insects in recent years are A. domesticus, T. molitor and A. assamensi pupae. Insects are nutritious; the average protein content in insects is around 40% and for some species like A. domesticus and T. molitor larvae it is even higher.1–3 The essential amino acid requirement recommended by the FAO/WHO is also covered by insect proteins.4 Moreover, the vitamin, mineral (calcium, iron, and zinc) and monounsaturated and polyunsaturated fatty acid contents are high in edible insects.5 Insect protein was reported to be more digestible (usually between 76% to 98%) than plant proteins like peanuts and lentils (usually around 52%), but slightly less digestible than animal protein like beef (89%) and egg white (100%).6 Furthermore, the energy content of edible insects varies from 1821 to 1896 kJ per 100 g which is comparable to most meats except for pork (67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 kJ per 100 g).5 Other notable advantages compared to existing protein sources are related to positive environmental impacts such as less water use, less land occupied, and fewer gas emissions.7 Besides these benefits, sensory quality and acceptance are other important aspects that should be considered when evaluating alternative proteins. Although the willingness to consume insects is still an ongoing issue, earlier studies showed that a promising option was to use yellow mealworm flour as a supplement to fortify tortillas, muffins and beef/mealworm burgers, which were found to be acceptable by consumers.8–11 As a result of ongoing efforts, insects have received more attention in recent years.
750 kJ per 100 g).5 Other notable advantages compared to existing protein sources are related to positive environmental impacts such as less water use, less land occupied, and fewer gas emissions.7 Besides these benefits, sensory quality and acceptance are other important aspects that should be considered when evaluating alternative proteins. Although the willingness to consume insects is still an ongoing issue, earlier studies showed that a promising option was to use yellow mealworm flour as a supplement to fortify tortillas, muffins and beef/mealworm burgers, which were found to be acceptable by consumers.8–11 As a result of ongoing efforts, insects have received more attention in recent years.
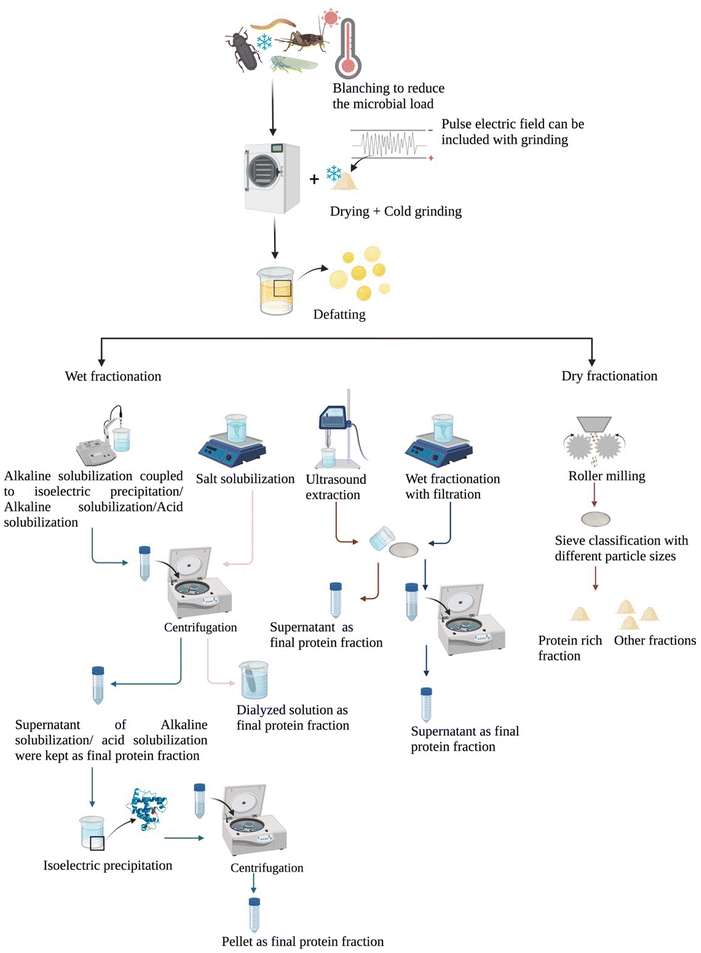
Compared to whole insects, insects as ingredients might be an easier alternative for people to consume if in an unrecognizable form. Del Valle et al. (1982) further indicated that insect protein extraction for future uses in the food industry and novel foods was extremely relevant for countries that did not consume insects, such as North American and European countries.12 As for insect protein extraction, the most commonly used protein extraction method is alkaline solubilization coupled to isoelectric precipitation, despite some publications also applying alkaline solubilization or acid solubilization separately. Other novel methods like dry fractionation and ultrasound-assisted extraction are gradually being researched.13,14
The bioactive properties of insect proteins can be improved through enzymatic hydrolysis either by in vivo or in vitro means, as these peptides within the sequence of the parent proteins are inactive.15,16 Currently, the widely demonstrated bioactivities of insect-derived peptides have been antihypertensive, antidiabetic and antioxidant properties. In recent years, numerous bioactive peptides were isolated from a wide variety of food proteins; significant ACE inhibitory activity has been observed in some common protein sources like egg white, rice, sweet potato, and cod.17–19 Similarly, ACE inhibitory activity has also been assessed in some insect species, such as B. mori, S. littoralis, S. gregaria, B. terrestris, A. assamensis pupae and G. sigillatus.20,21 As for diabetes, the WHO estimates that the number of people with diabetes will double by 2030; type 2 diabetes is the main contributor to cardiometabolic diseases.22 Insect proteins have presented antidiabetic properties, for example, whole G. sigillatus showed increased bioactivity by both simulated gastrointestinal digestion and enzymatic hydrolysis by food-grade enzymes such as alcalase.23 Other bioactivities such as anti-inflammatory activities are gradually studied in insects. But to date, no in vivo study has been performed on the antidiabetic and antioxidant properties of insect-derived peptides.
Furthermore, the new antigens and potential allergens that are released at the same time as bioactive peptides should also be taken into careful consideration. The allergenicity of insects can be caused in several ways including ‘sting, inhalation, direct contact and ingestion’. Cross reactivity with allergens in crustaceans and house dust mites can also be triggered after insect ingestion.24 Among the potential allergens found in insects, tropomyosin has been identified as a major one.25,26 Another allergen found in insects is arginine kinase. However, studies regarding the cross reactivity of arginine kinase are still limited. Some publications reported that hydrolysis under the assistance of microwave could reduce the immunoreactivity of insect protein. A possible explanation is that the compounds present may have some thermal and non-thermal interactions with microwave treatment, which is not the case for convection heating.21,27 Similar results were observed in other food products like dairy whey protein hydrolysates and fish frame protein hydrolysates.28
This review summarizes the current state of insect proteins including their nutritional value in terms of essential amino acid content and digestibility. Then, the identification and functional properties of insect proteins are discussed for various species. Protein extraction methods and their corresponding protein extraction yields and protein contents of the final products are also reported. Furthermore, the main methods and common enzymes used for protein hydrolysates production and release of bioactive peptides are outlined. Several bioactive properties including antihypertensive, antidiabetic, antioxidant and anti-inflammatory properties are also described along with potential mechanisms of action. Finally, the allergenic properties of insect proteins are reviewed and the potential use of some novel technologies to reduce their allergenic properties are discussed.
2. Proteins in edible insects
2.1. The nutritional properties of insect proteins
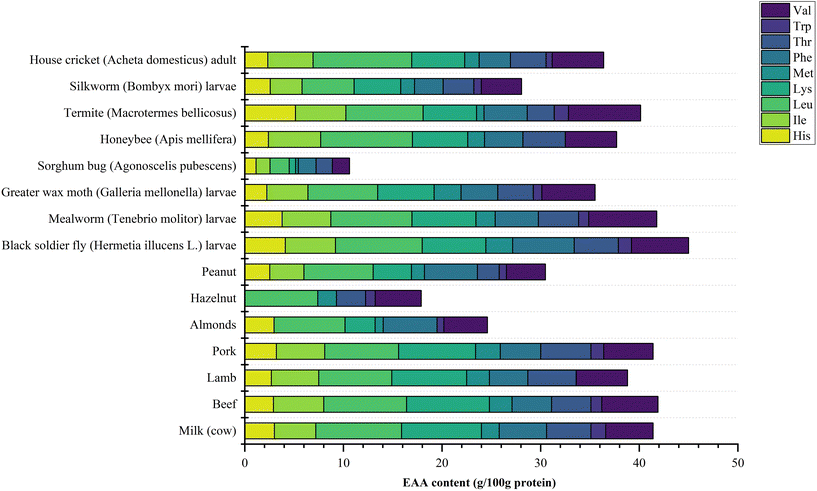
Legume proteins have been widely applied in many commercial food products, however insect protein is reported to have enhanced nutritional value, as it contains all the essential amino acids (EAAs) like Leu, Ile, Lys, Met, Thr, Trp, Phe, and Val, and the amount of EAAs prove to be higher than an adult's daily amino acid FAO requirement.7 A comparison of EAA contents of diverse insect sources and well-known protein sources is displayed in Fig. 1. The EAA levels of a diverse set of insects were comparable to soybean proteins, while being lower than casein.3H. illucens seems to be the insect containing the highest EAA amounts, reaching 45 g/100 g protein, comparable to beef (41.9 g/100 g protein). Widely researched T. molitor, A. domesticus and A. mellifera also contain a high content of EAAs: 33.84 g/100 g protein, 36.39 g/100 g protein and 37.7 g/100 g protein, respectively. Moreover, the protein content in common edible insects such as A. domesticus (72.45%), T. molitor (45%) and A. assamensis pupae (38.05%) are higher than that in common legumes such as lentils (26.7%), beans (23.5%) and soybean (41.1%).1,2,29,30Another advantage of insect proteins is their relatively high digestibility (as measured by in vitro assays). In vitro protein digestibility assays are rapid, easy to achieve, relatively cheap, give results similar to in vivo data and have been applied as a useful method to monitor protein digestibility.31 Several factors such as thermal unfolding, aggregation, carbonyl-amine reactions, cross-linking and interactions with other compounds such as carbohydrates or polyphenols have an impact on protein digestibility; furthermore, the polypeptide chain's accessibility and flexibility also affect protein digestibility. Digestibility values for insect proteins were first reported for edible insects from Mexico, for honeybees (89%), Liómetopum ants (82%), Sphenarium grasshoppers (75%), bees (71.7%), and ants (77.63%).32 Similar studies reported the digestibility values of S. ricinii pupae (86%) as well as T. molitor (54%), M. subhylanus (76.3%) and R. differens (90.5%).33–35 The digestibility of some insect proteins can be comparable to some common food proteins such as whole beef (89%), pork (90%), turkey (78%) and salmon (85%).
2.2. Insect protein characterization
To date, SDS-PAGE has been widely applied to separate insect proteins with different molecular weights. Then bands are selected for in-gel extraction, trypsin digestion and LC-MS/MS analysis in order to identify proteins. Insect protein identification nowadays is based on multiple sequencing and alignments tools to find regions of local similarities between protein sequences.36 Although it is difficult to identify the proteins or peptides from most edible insects, as many of the expressed proteins have not been fully sequenced yet, there is a lack of complete insect proteomes, which are still needed. The proteins in insects such as D. melanogaster flies were fully identified, including 13 protein categories mainly classified as ‘gene expression transcription, protein metabolism, muscle structure, cytoskeleton organization and cell function’.37 More specifically, proteins in T. molitor have been gradually identified, but only a 10% match of mass data was obtained.35 For example, proteins with molecular weights less than 14 kDa in T. molitor are mainly proteins with anti-freezing properties, such as hemolymph proteins ranging from 8.5 to 13 kDa. Cuticle proteins range from 14 to 30 kDa and vitellogenin-like proteins are mainly over 95 kDa.3 Also, actin-like (42 kDa), α-actinin-4 (107 kDa), myosin heavy chain (262 kDa), myosin-2 essential light chain (16.8 kDa), tropomyosin 1 (75.2 kDa) and 2 (32.5 kDa), troponin I (23.8 kDa), troponin T (47.3 kDa), and putative troponin C (18.3 kDa) have been identified. Muscle proteins like α-actinin-4 (107 kDa), tropomyosin 1 and 2, and calponin (20.3 kDa) were present in significant amounts in the water-insoluble protein fraction.35Proteins in different parts of B. mori have also been gradually characterized. Zhou et al. identified five proteins in the midgut of B. mori, including myosin 1 light chain, tropomyosin 1, profilin, serpin-2 and glutathione peroxidase.38 Other studies reported that the most abundant proteins in B. mori heads were myosin and actin, and other identified proteins were related to the neurological system such as cuticle and chemosensory proteins.39 The proteins in the skeletal muscle of B. mori such as ‘contractile proteins, metabolic proteins, regulatory proteins and signal transducing proteins’ were identified during the change from larvae to pupae.40 More interestingly, insect protein expression was found to be linked to the insect's diet in B. mori. Comparing B. mori fed with fresh mulberry leaves and B. mori fed with an artificial diet, a decrease in proteins related to immunity and energy metabolism was found in those fed with an artificial diet.38
However, current proteomic studies mainly focus on specific parts of insects such as midgut proteins or insect secretions such as venom.41,42 Only limited studies revealed the intriguing traits behind whole protein groups in specific insects; among the proteomic studies available,37,38 the main focus has been on water soluble and water insoluble proteins, so potentially different protein groups such as those that are soluble in salts solutions, organic solvents or alkaline/acid solutions are of interest for further studies as these have been rarely characterized.
2.3. Functional properties of insect proteins
It is important to evaluate the functional properties of insect proteins such as solubility, foaming and emulsifying properties, gelation, water holding capacity and oil holding capacity.43,44Table 1 shows the functional properties of insect derived ingredients.| Insect | Type of ingredient and protein content | Functional properties | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein solubility | Water holding capacity | Oil holding capacity | Emulsifying properties | Foaming properties | Gelling properties | ||||
| Capacity | Stability | Capacity | Stability | ||||||
| a Data collected from ref. 47. b Data collected from ref. 127. c Data collected from ref. 128. d Data collected from ref. 129. e Data collected from ref. 130. ND: not determined. | |||||||||
| Mealworm (Tenebrio molitor)a | Protein ND | 97% (pH 11) | 3.95 ± 0.2 g g−1 | 2.74 ± 0.06 g g−1 | 66.6 ± 2.16% | 51.31 ± 0.46% | 32.67 ± 0.94% | 30.33 ± 0.47% | ND |
| Locusts (Schistocerca gregaria)a | Protein ND | 90% (pH 11) | 2.31 ± 0.19 g g−1 | 3.22 ± 0.16 g g−1 | 67.78 ± 1.6% | 50.41 ± 1.99% | 32.0 ± 1.88% | 6.17 ± 0.71% | ND |
| Crickets (Gryllodes sigillatus)a | Protein ND | 96% (pH 11) | 3.44 ± 0.13 g g−1 | 3.33 ± 0.11 g g−1 | 72.62 ± 1.9% | 38.3 ± 0.8% | 99.0 ± 1.41% | 92.0 ± 1.88% | ND |
| Mealworm (Tenebrio molitor)a | Insect flour 52.35% | ND | 1.29 ± 0.19 g g−1 | 1.71 ± 0.13 g g−1 | 65.96 ± 1.5% | 27.59 ± 1.18% | 31.0 ± 1.41% | 26.0 ± 0.94% | ND |
| Locusts (Schistocerca gregaria)a | Insect flour 76% | ND | 2.18 ± 0.07 g g−1 | 1.98 ± 0.16 g g−1 | 69.17 ± 0.59% | 48.11 ± 0.57% | 22.33 ± 1.41% | 19.33 ± 0.94% | ND |
| Crickets (Gryllodes sigillatus)a | Insect flour 70% | ND | 2.34 ± 0.28 g g−1 | 2.82 ± 0.08 g g−1 | 62.0 ± 1.25% | 31.65 ± 0.92% | 41.0 ± 1.41% | 34.67 ± 2.82% | ND |
| Cirina forda | Insect flour 20% | 90% (pH 5.5) | 248.3 ± 2.4% | 178.7 ± 0.0% | 135.7 ± 2.3% | ND | 0% | ND | 14 ± 0.1 w/v |
| Oryctes owariensis | Insect flour 50.64% | ND | 220.33 ± 1.5% | 265.9 ± 1.31% | 29.97 ± 0.12% | 104.84 ± 1.23% | 17.87 ± 0.16% | 12.36 ± 0.03% | ND |
| Cirina forda | Insect flour 55.5% | 55% (pH 11) | 300.0 ± 0.15% | 358.44 ± 0.21% | 36.67 ± 0.11% | 45.36 ± 0.21% | 7.10 ± 0.20% | 3.00 ± 0.00% | 6.0 ± 0.0% |
| House cricket (Acheta domesticus)e | Protein concentrate 71.7% (DW) | ND | 201.99 ± 10.01% | ND | 7.52 ± 0.24% | 97.36 ± 1.13% | 26.00 ± 2.00% | 86.79 ± 2.21% | ND |
| Field cricket (Gryllus bimaculatus)e | Protein concentrate 60.7% (DW) | ND | 201.22 ± 5.65% | ND | 7.19 ± 0.38% | 97.38 ± 0.44% | 16.67 ± 1.15% | 92.58 ± 0.91% | ND |
Among all the functional properties, solubility is certainly the most important one as many functional properties are influenced by the extent of protein solubilized in aqueous solution.45 The solubility of insect derived ingredients is influenced by protein structure, protein size, protein charge and pH. Less compact proteins are more likely to interact with water and have a higher solubility; also lower protein size improves solubility. In addition, enzymatic hydrolysis is believed to release more ionized groups, thus producing smaller proteins and peptides, and as a result, increasing solubility. As for the pH, the highest solubility tends to be observed at extreme pH values as more hydrophobic groups are exposed as a result of protein unfolding.46 The highest solubility among T. molitor, S. gregaria and G. sigillatus derived ingredients was observed for mealworm protein with 97% solubility at pH 11. S. gregaria protein and G. sigillatus protein also showed high solubility values of 90% and 96%, respectively, at pH 11.47 However, when the pH is close to the isoelectric point (pI) of insect proteins, these tend to aggregate and they become less soluble. For example, T. molitor protein showed approximately 15% solubility and H. illucens defatted flour also showed only 11% solubility at pH 4 (close to the pI; pI around 4).48
Foams can be defined as air bubbles imprisoned in liquid and stabilized by proteins at the interface of air and liquid. Foam formation is also affected by a variety of factors including protein structure. As the formation of foam requires protein unfolding, globular and compact proteins were demonstrated to be less effective for foam formation than other fibrous proteins.6 The main fibrous proteins found in insects are from muscle, keratin and connective tissues, and they are mainly responsible for the foaming properties of insects.49 So, except for the proteins commonly used due to their high foaming capacity like egg white protein, whey protein, caseins and soy protein, as well as insect protein sources extracted under the optimum conditions could be applied for foaming purposes although some insect species such as B. mori have a relatively low foaming capacity.6,50 The low foaming capacity of some insect proteins can be due to their conformational characteristics. For some insects, globular proteins are the main protein type. Since globular proteins are less unfolded at the interface between air and water, it limits their air bubble encapsulation ability.47 Also, the low foaming capacity of insect proteins is attributed to the large variation of protein molecular weights, which negatively impacts the formation of protein films.51 Besides the foaming capacity, the foaming stability of B. mori protein concentrates (93.46%) was reported to be higher than for other insect protein concentrates such as A. mellifera (44.4–55.5%), T. molitor (30.33%) and S. gregaria (6.17%) and even higher than milk protein concentrates (55.86%).6 This is due to the amino acid composition of B. mori protein concentrates; they contain a high ratio of non-polar/polar side amino acid chains, which seems to enhance the stability and cohesive nature of the membrane. On the other hand, the high sugar content in protein flour is another explanation for the low foaming stability for the aforementioned species, as it can decrease the protein–protein interactions and interrupt the formation of firm interfacial membranes, and as a result, reduce the ability to stabilize foams.47
Emulsions are homogenous mixtures of two immiscible liquids, either water droplets in an oil phase or oil droplets in an aqueous phase. Although whole Gryllidae sp. flour is found to have poor emulsifying properties, proteins are known to reduce the surface tension of the water–oil surface due to their amphiphilic character. Recent studies also showed that the emulsifying stability of T. molitor protein (51.3%) was higher than milk protein concentrates (33.50%). Furthermore, the hydrolysis of parent proteins is a valuable approach for improving the insect protein emulsifying properties, as it can facilitate the exposition of inaccessible amino acids and thus these residues can increase hydrophobic interactions and increase the formation of emulsions. For example, hydrolyzing G. sigillatus derived proteins with alcalase for 60 s resulted in an improvement in both emulsion capacity and emulsion stability after 60 min.52
Another key functional property is the oil holding capacity (oil binding capacity or oil holding capacity), which refers to the ability of the protein to absorb lipids. Small, low-density hydrophobic proteins were reported to be able to preserve more lipids, thus providing a tender structure and more palatable textures.53,54 Zielińska et al. (2018) reported that the lowest oil holding capacity was 2.74 g oil per g protein for T. molitor, due to its lower protein content and higher fat content than other insects.5,47 Zhao et al. (2016) also reported a similar oil holding capacity value for T. molitor protein with 2.33 g oil per g protein.55 In comparison, G. sigillatus protein and S. gregaria protein showed greater oil holding capacities, with values of 3.33 g oil per g protein and 3.22 g oil per g protein, respectively.47
Protein gels are able to retain a large amount of water as they are a structured protein network. Gelation depends on electrostatic interactions and temperature (the most important factors) as heat promotes the unfolding and denaturation of protein and causes their rearrangement and aggregation.56 However, studies focusing on the gelling properties of insect proteins are scarce. Yi et al. (2013) evaluated the relationship between the gelation properties and pH of five edible insects and found that no gel formation was observed at a concentration of 3% w/v at pH 3, 5, 7 and 10, except for A. domesticus protein, which formed a firm gel at pH 7. For 30% w/v systems, all the insect proteins led to firm gels at pH 7 and pH 10. The transparency was related to the pH applied, samples heated at pH 3 and 10 led to a greater transparency than those at pH 5 and 7. As for the heating time, after 10 minutes, the gels formed were stable, and no impact on the gelation properties was observed following additional heating.3,57 Another functional property highly related to gelation is the water-holding capacity (or water-binding capacity or water adsorption capacity), which reflects the ability of proteins to retain water. This functional property is generally increased by heat-induced denaturation. This functional property of insect proteins has been characterized mainly for T. molitor; the water holding capacity in T. molitor flour was 129%, while that in T. molitor protein concentrate was 395%, much higher than what was observed for soy protein concentrate (227%), cowpea flour (124.6%), soy flour (130%) and chickpea flour (131.6%).47,58
2.4. Current insect protein extraction methods
Protein extracted from insects as food ingredients is suggested to be a useful way to increase the acceptance of eating insects.59 Lipid removal before insect protein extraction was the most common step reported in the literature. Organic solvents like n-hexane have been applied to A. assamensis, T. molitor, Z. morio, A. diaperinus, A. domesticus, and B. dubia for lipid removal.3,30 Petroleum ether is another common solvent currently applied for defatting T. molitor powder and B. mori pupae.60,61 Other defatting protocols have used combinations of ethanol and isopropanol or hexane and isopropanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v)) to defat T. molitor.55 However, no comparison of the effectiveness of different defatting methods has been reported. The major drawback of applying organic solvents is protein loss, as proteins like prolamins may have affinity for the solvent and be washed away during defatting, but this has not yet been quantified. Another disadvantage of using organic solvents is safety issues. Another solvent of interest for defatting is ethanol, which is considered as GRAS (generally recognized as safe), but the efficiency of ethanol is lower than that of other organic solvents.62 Therefore, a more versatile method that extracted proteins from whole insect meals was carried out as follows, the whole insect meal was mixed with demineralized water and sieved through a 500 μm sieve, then the lipid layer was removed from the top of the supernatant after centrifugation (at 4 °C), thus this method could be employed as an alternative way to remove lipids from insect homogenates.3
2 (v/v)) to defat T. molitor.55 However, no comparison of the effectiveness of different defatting methods has been reported. The major drawback of applying organic solvents is protein loss, as proteins like prolamins may have affinity for the solvent and be washed away during defatting, but this has not yet been quantified. Another disadvantage of using organic solvents is safety issues. Another solvent of interest for defatting is ethanol, which is considered as GRAS (generally recognized as safe), but the efficiency of ethanol is lower than that of other organic solvents.62 Therefore, a more versatile method that extracted proteins from whole insect meals was carried out as follows, the whole insect meal was mixed with demineralized water and sieved through a 500 μm sieve, then the lipid layer was removed from the top of the supernatant after centrifugation (at 4 °C), thus this method could be employed as an alternative way to remove lipids from insect homogenates.3
To date, insect protein extraction protocols are mainly based on wet fractionation (Fig. 2). The most common/traditional wet fractionation method is alkaline solubilization coupled to isoelectric precipitation, although some research may apply alkaline solubilization or acid solubilization separately (Table 2). In brief, for alkaline solubilization coupled to isoelectric precipitation, the insect meal is solubilized at alkaline pH (8–11) to solubilize proteins, then centrifugation is applied to separate soluble proteins in solution, then the soluble protein solution is collected and the pellet discarded, afterwards the pH is adjusted to the isoelectric point (pI) to precipitate proteins. The protein content of final products with this type of extraction method was between 34.7% and 89.05% according to different insect species or individual differences observed for the same insect species (Table 2). The precipitation pH is vital to alkaline solubilization coupled to isoelectric precipitation, as it determines the protein content of the products and protein recovery. The pI distribution of different insect proteins has been reported for various insect species, and was first measured in Sarcophaga falculata larva; the outer layer proteins of Sarcophaga falculata had an isoelectric point of 5.1, and the chitin–protein complex (inner layer) had a different pI of 3.5.63 Later, Huang et al. (1984) categorized the insects in various groups according to the pI values of their proteins, “Coleoptera, Orthoptera, Odonata, Hemitera and Lepidoptera” were included in the first group where the pI ranged between 5.56 and 5.69, with no difference between thoracic and leg muscle actins. The pI of cicada's sound muscle (5.57) and thoracic (5.65) and leg muscle actins (5.68) were also in the same range without significant difference. Another group included Hymenoptera and Diptera (pl = 5.75–5.87). In this group, the thoracic muscles may contain two or three actin isoforms (α-actin, β-actin, γ-actin). So for instance, the pI values of honey bee thoracic muscle were 5.75 and 5.87, while the ones of fruitfly were 5.70, 5.77 and 5.84.64 More recently, the pI values of different insects such as A. mylitta, A. pernyi, A. yamamai, G. mellonella, B. mori and B. mandarina were reported to be quite similar with values of 5.6, 5.82, 5.35, 4.67, 4.59 and 4.64, respectively.57 Another widely used protocol is wet fractionation with mechanical separation, which is effective at obtaining different fractions such as fat and protein in separate layers. In brief, insect meal is mixed with ascorbic acid or sodium hydroxide, and the stainless filter sieve with a suitable pore size is applied to separate proteins, then the fat fraction is collected from the top of the supernatant after centrifugation (Table 2). The last novel protocol is ultrasound-assisted extraction, which results in extracts with protein contents varying between 35% and 94% (depending on the sample under study).65 Ultrasound-assisted extraction does not change the amino acid profile of the resulting extract,14 although its final protein content is higher than that from alkaline solubilization coupled to isoelectric precipitation. Mishyna et al. (2019) observed that only 39.6% of protein was found in the final product extracted by alkaline solubilization coupled to isoelectric precipitation, compared to 55.2% obtained by ultrasound-assisted extraction. But this result may have limitations, which may be due to the specific species studied and/or the insect life stage, as no further studies are available.66 Other novel technologies such as pulsed electric fields, which is a type of non-thermal technology, can enhance protein extraction from A. domesticus. Psarianos et al. demonstrated that the increase in protein extraction yield exceeded 18% when treated with pulsed electric fields, also, other components like fat also showed an increase (exceeded 40%) in extraction yield.1
| Source | Life stage | Protein content (starting material) | Method of extraction | Conditions | Protein content (final product) | Protein extraction yield/protein recovery | Ref. |
|---|---|---|---|---|---|---|---|
| ND: not determined. | |||||||
Muga silkworm (Antharaea assamensis)  |
Pupae | 38.05% (wet basis) | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 defatted powder 20 defatted powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water, at pH 9.0 for 2 h, centrifuged (8000g, 15 min at 20 °C). The pellets were freeze dried, supernatant pH 4.5, centrifuged (8000g, 15 min at 20 °C). Wash twice and freeze dry. Store at 4 °C. deionized water, at pH 9.0 for 2 h, centrifuged (8000g, 15 min at 20 °C). The pellets were freeze dried, supernatant pH 4.5, centrifuged (8000g, 15 min at 20 °C). Wash twice and freeze dry. Store at 4 °C. |
65.16 ± 0.53% | 28.07 ± 0.34% (protein recovery) | 30 |
Cricket (Acheta domesticus)  |
Adult | 72.45 ± 1.30 g/100 g (dry basis) | Alkaline solubilization | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 cricket powder 50 cricket powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 M NaOH, stir for 60 min; 2 mL of mixture are withdrawn at 15 min intervals and centrifuged (10 0.5 M NaOH, stir for 60 min; 2 mL of mixture are withdrawn at 15 min intervals and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min at 20 °C). The supernatant was isolated and stored at 4 °C. 000g, 10 min at 20 °C). The supernatant was isolated and stored at 4 °C. |
About 46 g/100 g | 63.49% (protein extraction yield) | 1 |
House cricket (Acheta domesticus)  |
ND | 53% | Alkaline solubilization coupled to isoelectric precipitation. | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 defatted powder 15 defatted powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 M NaOH, stir for 60 min at 40 °C, centrifuged (3500g, 20 min at 4 °C) twice. Adjust the pH of supernatants to 4.3–4.5 before centrifugation (2500g, 15 min at 4 °C). The residue was washed twice with distilled water at pH 4.5 and centrifuged (2500g, 10 min, at 4 °C). The residue and supernatant were freeze-dried. 0.25 M NaOH, stir for 60 min at 40 °C, centrifuged (3500g, 20 min at 4 °C) twice. Adjust the pH of supernatants to 4.3–4.5 before centrifugation (2500g, 15 min at 4 °C). The residue was washed twice with distilled water at pH 4.5 and centrifuged (2500g, 10 min, at 4 °C). The residue and supernatant were freeze-dried. |
For different defatting methods: Hexane: 74.7 ± 0.3%; Petroleum ether: 74.7 ± 0.3%; Ethyl acetate: 74.4 ± 1.2%; Ethanol: 78.5 ± 2.0% | For different defatting methods: Hexane: 32.4 ± 3.8%; Petroleum ether: 33.1 ± 1.0 %; Ethyl acetate: 31.6 ± 3.0%; Ethanol: 31.0 ± 4.0% | 2 |
Mealworm (Tenebrio molitor)  |
ND | 45% | Alkaline solubilization coupled to isoelectric precipitation. | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 defatted powder 15 defatted powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 M NaOH, stir for 60 min at 40 °C, centrifuged (3500g, 20 min at 4 °C) twice. Adjust the pH of supernatants to 4.3–4.5 before centrifugation (2500g, 15 min at 4 °C). The residue was washed twice with distilled water at pH 4.5 and centrifuged (2500g, 10 min, at 4 °C). The residue and supernatant were freeze-dried. 0.25 M NaOH, stir for 60 min at 40 °C, centrifuged (3500g, 20 min at 4 °C) twice. Adjust the pH of supernatants to 4.3–4.5 before centrifugation (2500g, 15 min at 4 °C). The residue was washed twice with distilled water at pH 4.5 and centrifuged (2500g, 10 min, at 4 °C). The residue and supernatant were freeze-dried. |
For different defatting methods: Hexane: 74.0 ± 2.2%; Petroleum ether: 72.7 ± 1.5%; Ethyl acetate: 75.4 ± 0.5%; Ethanol: 75.3 ± 0.8% | For different defatting methods: Hexane: 33.7 ± 1.6%; Petroleum ether: 33.5 ± 1.2%; Ethyl acetate: 33.2 ± 0.6%; Ethanol: 33.9 ± 3.7% (Protein recovery) | 2 |
Mealworms (Tenebrio molitor)  |
Larvae | ND | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ground insects 10 ground insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2% NaOH, stir for 1 h at room temperature. Centrifugation (8000g, 20 min at 4 °C). Precipitation at isoelectric point. Then centrifuged (8000g, 20 min at 4 °C) and washed with distilled water. Freeze-dried at −18 °C. 0.2% NaOH, stir for 1 h at room temperature. Centrifugation (8000g, 20 min at 4 °C). Precipitation at isoelectric point. Then centrifuged (8000g, 20 min at 4 °C) and washed with distilled water. Freeze-dried at −18 °C. |
ND | ND | 97 |
Locusts (Schistocerca gregaria)  |
Adult | ND | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ground insects 10 ground insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2% NaOH, stir for 1 h at room temperature. Centrifugation (8000g, 20 min at 4 °C). Precipitation at isoelectric point. Then centrifuged (8000g, 20 min at 4 °C) and washed with distilled water. Freeze-dried at −18 °C. 0.2% NaOH, stir for 1 h at room temperature. Centrifugation (8000g, 20 min at 4 °C). Precipitation at isoelectric point. Then centrifuged (8000g, 20 min at 4 °C) and washed with distilled water. Freeze-dried at −18 °C. |
ND | ND | 97 |
Crickets (Gryllodes sigillatus)  |
Adult | ND | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ground insects 10 ground insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2% NaOH, stir for 1 h at room temperature. Centrifugation (8000g, 20 min at 4 °C). Precipitation at isoelectric point. Then centrifuged (8000g, 20 min at 4 °C) and washed with distilled water. Freeze-dried at −18 °C. 0.2% NaOH, stir for 1 h at room temperature. Centrifugation (8000g, 20 min at 4 °C). Precipitation at isoelectric point. Then centrifuged (8000g, 20 min at 4 °C) and washed with distilled water. Freeze-dried at −18 °C. |
ND | ND | 97 |
Crickets (Gryllidae)  |
ND | 63.43% | Alkaline solubilization or acid solubilization | Ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 defatted powder 1 defatted powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 M aqueous NaOH or ascorbic acid. Vortex and centrifuge (3500g, 20 min at 4 °C). Then the second extraction step was on the insoluble pellet. Vortex and centrifuge again. Keep the pellet and freeze dry. 0.5 M aqueous NaOH or ascorbic acid. Vortex and centrifuge (3500g, 20 min at 4 °C). Then the second extraction step was on the insoluble pellet. Vortex and centrifuge again. Keep the pellet and freeze dry. |
61.75% (alkaline); 69.69% (acid) | 82.95% (alkaline); 67.66% (acid) (protein extraction yield) | 131 |
Tenebrio molitor

|
Larvae | 19.1 ± 1.3% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 N2 frozen insects 0.005 N2 frozen insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) demineralized water demineralized water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15 ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. |
Supernatant (d.b.) : 50% to 61%; Pellet (d.b.): 65% to 75%; Residue (d.b.): 58% to 69% | 103% (protein extraction yield) | 3 |
Zophobas morio

|
Larvae | 20.7 ± 0.3% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 N2 frozen insects 0.005 N2 frozen insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) demineralized water demineralized water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15 ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. |
Supernatant (d.b.) : 50% to 61%; Pellet (d.b.): 65% to 75%; Residue (d.b.): 58% to 69% | 88.1%(protein extraction yield) | 3 |
Alphitobius diaperinus

|
Larvae | 20.6 ± 0.1% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 N2 frozen insects 0.005 N2 frozen insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) demineralized water demineralized water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15 ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. |
Supernatant (d.b.) : 50% to 61%; Pellet (d.b.): 65% to 75%; Residue (d.b.): 58% to 69% | 91.8%(protein extraction yield) | 3 |
Acheta domesticus

|
Adult | 21.5 ± 0.5% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 N2 frozen insects 0.005 N2 frozen insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) demineralized water demineralized water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15 ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. |
Supernatant (d.b.) : 50% to 61%; Pellet (d.b.): 65% to 75%; Residue (d.b.): 58% to 69% | 89.5%(protein extraction yield) | 3 |
Blaptica dubia

|
Adult | 19.3 ± 0.9% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 N2 frozen insects 0.005 N2 frozen insects![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) demineralized water demineralized water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15 ascorbic acid (w/v/w), blend for 1 min. The suspension was sieved through a stainless-steel filter sieve (pore size 500 μm). The filtrates and residues were collected. After centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. 000g, 30 min, at 4 °C) the supernatant, the pellet, and the fat fraction were obtained. The residue, the pellet and the supernatant fractions were freeze dried. |
Supernatant (d.b.) : 50% to 61%; Pellet (d.b.): 65% to 75%; Residue (d.b.): 58% to 69% | 86.5% (protein extraction yield) | 3 |
Crickets (Acheta domesticus)  |
ND | 59.84 ± 1.64% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 cricket meal 0.005 cricket meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water distilled water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid, blended. The suspension was sieved through a stainless steel filter sieve (pore size 500 μm). The residue was freeze dried, and stored at 4 °C. The filtrate was centrifuged (10 ascorbic acid, blended. The suspension was sieved through a stainless steel filter sieve (pore size 500 μm). The residue was freeze dried, and stored at 4 °C. The filtrate was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 30 min at 4 °C), then the precipitate was freeze dried. 000 rpm, 30 min at 4 °C), then the precipitate was freeze dried. |
Precipitate: 66.66 ± 0.82% (crude protein); Residue: 65.79 ± 1.3% (crude protein) | Precipitate: 32.72 ± 1.34%; Residue: 48.32 ± 0.92 (protein extraction yield) | 132 |
Migratory locust (Locusta migratoria)  |
ND | 65.87 ± 0.42% | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 locust meal 1 locust meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water at pH 9.0, stirred for 2 h. Chitin was separated by filtering the slurry through a fine-mesh cheese filtering cloth (pore size 0.2–0.5 mm, 100% cotton), then the filtrate was freeze-dried. deionized water at pH 9.0, stirred for 2 h. Chitin was separated by filtering the slurry through a fine-mesh cheese filtering cloth (pore size 0.2–0.5 mm, 100% cotton), then the filtrate was freeze-dried. |
82.26 ± 0.62% | ND | 133 |
Tenebrio molitor

|
Larvae | 58.44 ± 2.89 g/100 g | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 defatted insect powder 2 defatted insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02% ascorbic acid, homogenized and filtered by medical gauze. The filtered solution was centrifuged (15 0.02% ascorbic acid, homogenized and filtered by medical gauze. The filtered solution was centrifuged (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 30 min); the supernatant was collected. 000g 30 min); the supernatant was collected. |
ND | ND | 134 |
Allomyrina dichotoma

|
Larvae | 49.68 ± 0.54 g/100 g | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 defatted insect powder 2 defatted insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02% ascorbic acid, homogenized and filtered by medical gauze. The filtered solution was centrifuged (15 0.02% ascorbic acid, homogenized and filtered by medical gauze. The filtered solution was centrifuged (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 30 min); the supernatant was collected. 000g 30 min); the supernatant was collected. |
ND | ND | 134 |
Protaetia brevitarsis seulensis

|
Larvae | 51.08 ± 0.40 g/100 g | Wet fractionation with filtration | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 defatted insect powder 2 defatted insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02% ascorbic acid, homogenized and filtered by medical gauze. The filtered solution was centrifuged (15 0.02% ascorbic acid, homogenized and filtered by medical gauze. The filtered solution was centrifuged (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 30 min); the supernatant was collected. 000g 30 min); the supernatant was collected. |
ND | ND | 134 |
Crickets (G. Bimaculatus)  |
Adult | ND | Ultrasound extraction | Defatted sample was used for sonication: 20 kHz for 15 min, with 75% amplitude and pulse every 3 s with a 1 s interval. | ND | ND | 70 |
Mealworm (T. molitor)  |
Larvae | ND | Ultrasound extraction | Defatted sample was used for sonication: 20 kHz for 15 min, with 75% amplitude and pulse every 3 s with a 1 s interval. | ND | ND | 70 |
Silkworm (B. mori) pupae  |
ND | ND | Ultrasound extraction | Defatted sample was used for sonication: 20 kHz for 15 min, with 75% amplitude and pulse every 3 s with a 1 s interval. | ND | ND | 70 |
Yellow mealworm (Tenebrio molitor)  |
Larvae | ND | Ultrasound extraction | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 defatted insect coarse meal 16 defatted insect coarse meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water containing 9.46 mM ascorbic acid, sonication was carried out for 20 min on ice at 20 kHz with a 75% AMP and pulsed every 3 s. Then the aliquots were collected at 1, 2, 5, 10, 15, and 20 min. Samples were sieved through a stainless-steel filter (pore size of 1 mm) and filtrates were collected and freeze-dried. distilled water containing 9.46 mM ascorbic acid, sonication was carried out for 20 min on ice at 20 kHz with a 75% AMP and pulsed every 3 s. Then the aliquots were collected at 1, 2, 5, 10, 15, and 20 min. Samples were sieved through a stainless-steel filter (pore size of 1 mm) and filtrates were collected and freeze-dried. |
ND | 35% (protein extraction yield) | 14 |
Field cricket (Gryllus bimaculatus)  |
Adult | ND | Ultrasound extraction | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 defatted insect coarse meal 16 defatted insect coarse meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water containing 9.46 mM ascorbic acid, sonication was carried out for 20 min on ice at 20 kHz with a 75% AMP and pulsed every 3 s. Then the aliquots were collected at 1, 2, 5, 10, 15, and 20 min. Samples were sieved through a stainless-steel filter (pore size of 1 mm) and filtrates were collected and freeze-dried. distilled water containing 9.46 mM ascorbic acid, sonication was carried out for 20 min on ice at 20 kHz with a 75% AMP and pulsed every 3 s. Then the aliquots were collected at 1, 2, 5, 10, 15, and 20 min. Samples were sieved through a stainless-steel filter (pore size of 1 mm) and filtrates were collected and freeze-dried. |
ND | 37% (protein extraction yield) | 14 |
Silkworm pupae (Bombyx mori)  |
ND | ND | Ultrasound extraction | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 defatted insect coarse meal 16 defatted insect coarse meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water containing 9.46 mM ascorbic acid, sonication was carried out for 20 min on ice at 20 kHz with a 75% AMP and pulsed every 3 s. Then the aliquots were collected at 1, 2, 5, 10, 15, and 20 min. Samples were sieved through a stainless-steel filter (pore size of 1 mm) and filtrates were collected and freeze-dried. distilled water containing 9.46 mM ascorbic acid, sonication was carried out for 20 min on ice at 20 kHz with a 75% AMP and pulsed every 3 s. Then the aliquots were collected at 1, 2, 5, 10, 15, and 20 min. Samples were sieved through a stainless-steel filter (pore size of 1 mm) and filtrates were collected and freeze-dried. |
ND | 94% (protein extraction yield) | 14 |
T. molitor

|
Larvae | 65.6% (larvae) 71.6% (larvae meal) blanched, dried and ground | Alkaline solubilization | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 larvae meal/distilled water, pH 10. The mixture was stirred for 1 h at 45 °C. After stirring, distilled water at pH 10 was added to reach 250 mL, then the mixture was centrifuged (10 40 larvae meal/distilled water, pH 10. The mixture was stirred for 1 h at 45 °C. After stirring, distilled water at pH 10 was added to reach 250 mL, then the mixture was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min) and the supernatants were freeze-dried. 000g for 30 min) and the supernatants were freeze-dried. |
83.7 ± 3.8% (larvae); 80.4 ± 0.9% (larvae meal) | 59.9% (protein extraction yield) (larvae); 26.4% (protein extraction yield) (larvae meal) | 135 |
Rhynchophorus phoenicis

|
Larvae | 8.2 g/100 g | Salt solubilization | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 larvae meal 10 larvae meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer solution with pH varied from 3.0–10.0 (a) 0.1 M citric acid/Na2HPO4 buffers (pH 3.0 to 7.0); (b) 0.1 M Na2HPO4/NaH2PO4 buffers (pH 8.0); (c) 0.1 M Na2CO3/NaHCO3 buffers (pH 9.0 and 10.0) Centrifugation and separation of fat fraction (upper), aqueous fraction (intermediate) and insoluble components (lower slurry). Then the protein content in the aqueous fraction (intermediate) was measured by BCA assay. buffer solution with pH varied from 3.0–10.0 (a) 0.1 M citric acid/Na2HPO4 buffers (pH 3.0 to 7.0); (b) 0.1 M Na2HPO4/NaH2PO4 buffers (pH 8.0); (c) 0.1 M Na2CO3/NaHCO3 buffers (pH 9.0 and 10.0) Centrifugation and separation of fat fraction (upper), aqueous fraction (intermediate) and insoluble components (lower slurry). Then the protein content in the aqueous fraction (intermediate) was measured by BCA assay. |
pH 3: 0.85 mg mL−1; pH 4: 0.9 mg mL−1; pH 5: 1.5 mg mL−1; pH 6: 1.7 mg mL−1; pH 7: 1.8 mg mL−1; pH 8: 2.2 mg mL−1; pH 9: 4.2 mg mL−1; pH 10: 4.6 mg mL−1 | (protein extraction yield) pH 3: 10.3%; pH 4: 10.5%; pH 5: 17%; pH 6: 18%; pH 7: 19%; pH 8: 23%; pH 9: 47%; pH 10: 53.6% | 136 |
Black soldier fly (Hermetica illucens)  |
Larvae | 30.0 ± 1.2 g per g DM | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 defatted meal 25 defatted meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water at pH 10, stirring (300 rpm, 30 min, at 60 °C). The extract was centrifuged (4000g, 20 min at 20 °C), then the pH of the supernatant was adjusted to 4. Re-extraction (pH 2, 60 °C, 30 min) and precipitation again. Both extracts were centrifuged (4000g, 20 min at 20 °C). Proteins and insoluble residues were freeze dried at −80 °C. distilled water at pH 10, stirring (300 rpm, 30 min, at 60 °C). The extract was centrifuged (4000g, 20 min at 20 °C), then the pH of the supernatant was adjusted to 4. Re-extraction (pH 2, 60 °C, 30 min) and precipitation again. Both extracts were centrifuged (4000g, 20 min at 20 °C). Proteins and insoluble residues were freeze dried at −80 °C. |
68.2 ± 0.3 g g−1 DM | ND | 48 |
Yellow mealworm (T. molitor)  |
31.7 ± 0.5 g per g DM | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 defatted meal 25 defatted meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water at pH 10, stirring (300 rpm, 30 min, at 60 °C). The extract was centrifuged (4000g, 20 min at 20 °C), then the pH of the supernatant was adjusted to 4. Re-extraction (pH 2, 60 °C, 30 min) and precipitation again. Both extracts were centrifuged (4000g, 20 min at 20 °C). Proteins and insoluble residues were freeze dried at −80 °C. distilled water at pH 10, stirring (300 rpm, 30 min, at 60 °C). The extract was centrifuged (4000g, 20 min at 20 °C), then the pH of the supernatant was adjusted to 4. Re-extraction (pH 2, 60 °C, 30 min) and precipitation again. Both extracts were centrifuged (4000g, 20 min at 20 °C). Proteins and insoluble residues were freeze dried at −80 °C. |
34.7 ± 0.2 g g−1 DM | ND | 48 | |
Tenebrio molitor

|
Larvae | 58.44 ± 2.89% | Acid solubilization and salt solubilization | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 defatted powder 2 defatted powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer, stepwise extraction was carried out.(a) Water-soluble protein buffer, stepwise extraction was carried out.(a) Water-soluble protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02% (w/v) ascorbic acid; (b) Salt-soluble protein 0.02% (w/v) ascorbic acid; (b) Salt-soluble protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.58 M saline solution (0.49 M NaCl, 17.8 mM Na5P3O10, and 1 mM NaN3; pH 8.3, 2 °C). Then homogenize at 10 0.58 M saline solution (0.49 M NaCl, 17.8 mM Na5P3O10, and 1 mM NaN3; pH 8.3, 2 °C). Then homogenize at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and filter through medical gauze. The filtrate was centrifuged (15 000 rpm and filter through medical gauze. The filtrate was centrifuged (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 30 min at 2 °C), the water-soluble and salt-soluble protein solutions were obtained. 000g, 30 min at 2 °C), the water-soluble and salt-soluble protein solutions were obtained. |
ND | ND | 137 |
Allomyrina dichotoma

|
Larvae | 49.68 ± 0.54% | Acid solubilization and salt solubilization | ND | ND | 137 | |
Protaetia brevitarsis seulensis

|
Larvae | 51.08 ± 0.40% | Acid solubilization and salt solubilization | ND | ND | 137 | |
Grasshopper (S. gregaria)  |
Adult | 38.1 ± 1.4% | 1. Alkaline solubilization coupled to isoelectric precipitation (ALK) 2. Alkaline and sonication extractions (SON) | ALK: ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 insect powder 15 insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water at pH 10, stirred (40 °C, 1 h). Then centrifuge (3000g, 20 min at 4 °C) and separate the insoluble residues. Adjust the supernatant pH to 4 (grasshopper) and 5 (honeybee) for 1 h (room temperature), then centrifuge solutions (15 distilled water at pH 10, stirred (40 °C, 1 h). Then centrifuge (3000g, 20 min at 4 °C) and separate the insoluble residues. Adjust the supernatant pH to 4 (grasshopper) and 5 (honeybee) for 1 h (room temperature), then centrifuge solutions (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 20 min at 4 °C). Precipitates were neutralized to pH 7. All fractions were freeze-dried; SON: ratio of 1 000g, 20 min at 4 °C). Precipitates were neutralized to pH 7. All fractions were freeze-dried; SON: ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 insect powder 15 insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water at pH 10 (using 0.1 M NaOH), sonication (amplitude – 70, pulse-on time – 30 s, pulse-off time – 30 s, process time – 6 min). distilled water at pH 10 (using 0.1 M NaOH), sonication (amplitude – 70, pulse-on time – 30 s, pulse-off time – 30 s, process time – 6 min). |
52.9 ± 1.3%(ALK); 57.5 ± 1.0%(SON) | 24.2 ± 1.2% (ALK); 30.6 ± 1.6% (SON) (ALK: Protein recovery; SON: Protein extraction yield) | 66 |
Honeybee (A. mellifera)  |
Larvae and pupae | 22.1 ± 0.1% | 1. Alkaline solubilization coupled to isoelectric precipitation (ALK) 2. Alkaline and sonication extractions (SON) | ALK: ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 insect powder 15 insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water at pH 10, stirred (40 °C, 1 h). Then centrifuge (3000g, 20 min at 4 °C) and separate the insoluble residues. Adjust the supernatant pH to 4 (grasshopper) and 5 (honeybee) for 1 h (room temperature), then centrifuge solutions (15 distilled water at pH 10, stirred (40 °C, 1 h). Then centrifuge (3000g, 20 min at 4 °C) and separate the insoluble residues. Adjust the supernatant pH to 4 (grasshopper) and 5 (honeybee) for 1 h (room temperature), then centrifuge solutions (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 20 min at 4 °C). Precipitates were neutralized to pH 7. All fractions were freeze-dried; SON: ratio of 1 000g, 20 min at 4 °C). Precipitates were neutralized to pH 7. All fractions were freeze-dried; SON: ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 insect powder 15 insect powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water at pH 10 (using 0.1 M NaOH), sonication (amplitude – 70, pulse-on time – 30 s, pulse-off time – 30 s, process time – 6 min). distilled water at pH 10 (using 0.1 M NaOH), sonication (amplitude – 70, pulse-on time – 30 s, pulse-off time – 30 s, process time – 6 min). |
39.6 ± 2.1%(ALK); 55.2 ± 1.3%(SON) | 27.5 ± 1.1% (ALK); 30.8 ± 0.8% (SON) (ALK: Protein recovery; SON: Protein extraction yield) | 66 |
Yellow mealworm (Tenebrio molito)  |
Larvae | 51.5 ± 0.5% | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 defatted powder 15 defatted powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 M NaOH solution at 40 °C for 60 min, and vortex every 15 min, centrifuge (3500g, 20 min at 4 °C). The supernatant and gel were adjusted to pH 4.3–4.5 (room temperature), centrifuged (2500g, 20 min at 4 °C). Washed twice and centrifuged twice (2500g, 20 min at 4 °C). and freeze dried. 0.25 M NaOH solution at 40 °C for 60 min, and vortex every 15 min, centrifuge (3500g, 20 min at 4 °C). The supernatant and gel were adjusted to pH 4.3–4.5 (room temperature), centrifuged (2500g, 20 min at 4 °C). Washed twice and centrifuged twice (2500g, 20 min at 4 °C). and freeze dried. |
79.0% (true protein) | 53% (protein recovery) | 55 |
Silkworm (Bombyx mori)  |
ND | 48.20 ± 0.84% | Alkaline extraction coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 sample 10 sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water, stirred for 1 h and vacuum filtered. The pH of the crude protein extract was adjusted to 3.5 or 4.5, stirred for 10 min and centrifuged (10 distilled water, stirred for 1 h and vacuum filtered. The pH of the crude protein extract was adjusted to 3.5 or 4.5, stirred for 10 min and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min at 4 °C). Collect the pellets and dry them with hot air (55–60 °C, 1 h). 000g, 10 min at 4 °C). Collect the pellets and dry them with hot air (55–60 °C, 1 h). |
0.462 ± 0.005 mg per mg defatted insect (Soxhlet method at pH 3.5) | ND | 138 |
Bamboo caterpillar (Omphisa fuscidentalis |
ND | 47.52 ± 0.55% | Alkaline extraction coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 sample 10 sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water, stirred for 1 h and vacuum filtered. The pH of the crude protein extract was adjusted to 3.5 or 4.5, stirred for 10 min and centrifuged (10 distilled water, stirred for 1 h and vacuum filtered. The pH of the crude protein extract was adjusted to 3.5 or 4.5, stirred for 10 min and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min at 4 °C). Collect the pellets and dry them with hot air (55–60 °C, 1 h). 000g, 10 min at 4 °C). Collect the pellets and dry them with hot air (55–60 °C, 1 h). |
0.18 ± 0.008 mg per mg defatted insect (Folch method at pH 4.5) | ND | 138 |
Field cricket (Gryllus bimaculatus |
ND | 52.08 ± 0.13% | Alkaline extraction coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 sample 10 sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water, stirred for 1 h and vacuum filtered. The pH of the crude protein extract was adjusted to 3.5 or 4.5, stirred for 10 min and centrifuged (10 distilled water, stirred for 1 h and vacuum filtered. The pH of the crude protein extract was adjusted to 3.5 or 4.5, stirred for 10 min and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min at 4 °C). Collect the pellets and dry them with hot air (55–60 °C, 1 h). 000g, 10 min at 4 °C). Collect the pellets and dry them with hot air (55–60 °C, 1 h). |
0.734 ± 0.019 mg per mg defatted insect (Folch method at pH 3.5) | ND | 138 |
Mealworm (Tenebrio molitor, L.)  |
Larvae | 38.64 ± 1.74 g/100 g | Alkaline solubilization | Deionized water was added to a concentration of 600 g L−1 and the slurry was mixed for 60 s, pH 10.5 and stirred for 2 h, then centrifuged (4000g, 10 min). The supernatant fraction was separated, frozen at −30 °C and freeze-dried at 0.2 mbar for 72 h. | 62.6 ± 1.3 g/100 g | 58.7 ± 1.2% (protein extraction yield) | 13 |
Black soldier fly (Hermetia illucens)  |
Larvae | 42.00 ± 0.33% | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.85 defatted meal 24.85 defatted meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 M NaOH, heated (52.23 °C, 59.43 min) and stirred (100 rpm). The slurry was centrifuged (4500g, 10 min, at 4 °C), and the supernatant pH was adjusted 4.4. The slurry was centrifuged (4500g, 10 min, at 4 °C), and the precipitate was washed and centrifuged twice (4500g, 10 min, at 4 °C). The pH was adjusted to 7, stored at −20 °C, and freeze-dried. 0.25 M NaOH, heated (52.23 °C, 59.43 min) and stirred (100 rpm). The slurry was centrifuged (4500g, 10 min, at 4 °C), and the supernatant pH was adjusted 4.4. The slurry was centrifuged (4500g, 10 min, at 4 °C), and the precipitate was washed and centrifuged twice (4500g, 10 min, at 4 °C). The pH was adjusted to 7, stored at −20 °C, and freeze-dried. |
80.42 ± 0.90% | 64.44% (protein recovery) | 139 |
Black soldier fly (Hermetia illucens)  |
Larvae | 37.3% | Alkaline solubilization coupled to isoelectric precipitation | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 defatted meal 15 defatted meal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 M NaOH, stirred (300 rpm, 1 h, at 40 °C) and centrifuged (2493g, 20 min, at 4 °C), same procedure was repeated as above. The solid fraction was collected, and the pH of supernatants was adjusted to 4.3–4.5, then the solution was centrifuged (1272g, 15 min, at 4 °C). The precipitate was washed twice with distilled water and freeze-dried. 0.25 M NaOH, stirred (300 rpm, 1 h, at 40 °C) and centrifuged (2493g, 20 min, at 4 °C), same procedure was repeated as above. The solid fraction was collected, and the pH of supernatants was adjusted to 4.3–4.5, then the solution was centrifuged (1272g, 15 min, at 4 °C). The precipitate was washed twice with distilled water and freeze-dried. |
61.1% | ND | 140 |
Another protein extraction protocol is dry fractionation, which avoids generating wastewater and energy-intensive processing. Similar to wet fractionation, defatting is also carried out before dry fractionation.13,67 So far, dry fractionation has not been widely applied to insect fraction separation. Dry fractionation produces different fractions with various nutrients; the protein content of T. molitor protein rich fraction (particle <500 μm) can reach 58%.13 Among dry fractionation approaches, fine milling coupled to air classification was reported as an efficient method for the generation of three fractions (lipid fraction, chitin-rich fraction and protein-rich fraction) from T. molitor and A. domesticus; the protein-rich fraction was reported to contain less chitin than other fractions. However, the water solubility of protein in the defatted meal can be decreased by air classification; the A. domesticus defatted meal showed 13–23% solubility and the T. molitor defatted meal showed 14–27% solubility according to different pH values. This may be attributable to the fact that the large particle size hinders protein extraction.67Table 2 summarizes the protein content of various insect species at different research stages and also a comparison of the final protein content and extraction yield when applying different insect protein extraction protocols.
3. Insect protein hydrolysis and bioactive properties of insect-derived peptides
Generally, bioactive peptides consist of 2 to 20 amino acids as a non-active sequence encoded in parent proteins.68 The amino acid sequence, length and other structural features such as secondary structure (β-sheets), cyclic structure (via disulfide bonds), relative ratio of a specific amino acid or group of amino acids, hydrophobic properties, molecular weight, type of residues at C- and N-terminals, among others, are linked to the structure–activity relationships of bioactive peptides and these are fundamental for the biological activities exerted (Fig. 3). Bioactive peptides can be released/produced through various processes, such as enzymatic hydrolysis, in vivo/in vitro digestion, microbial fermentation, chemical hydrolysis (not GRAS), peptide synthesis and novel processing technologies, such as high hydrostatic pressure, microwave and pulsed electric fields. Enzymatic hydrolysis has been identified as a good means to enhance or control bioactivity such as antihypertensive, antioxidant and antidiabetic properties.69–71 To date, farmed B. mori, which is the main by-product of the silk reel industry, appears to be identified as a potential medicinal source and has gradually become the most frequently studied insect to generate insect-derived bioactive peptides.69,72In general, enzymatic hydrolysis is applied to insect protein concentrates/isolates or directly to flours.73,74 Different studies outlined several available enzymes used in the generation of insect-derived peptides. For example, pepsin, as an aspartic acid endoprotease, has an active site with dual aspartic acids to hydrolyse peptide bonds. Trypsin is used mainly for cleaving arginine and lysine amino acid residues.75 Enzymes have different and specific excision sites that generate different peptides, thus displaying various bioactivities. Currently, mammalian digestive enzymes such as amylase, lipase and trypsin appear to be the most frequently employed ones and are used to simulate gastrointestinal digestion. S. littoralis, B. mori, S. gregaria, and B. terrestris were reported to be hydrolyzed by in vitro gastrointestinal digestion.20,76 Farmed insects such as G. sigillatus, S. gregaria and B. mori have also been successfully hydrolyzed through gastrointestinal digestion and new bioactive peptides have been isolated.5,77,78 Besides, microbial enzymes including alcalase, thermolysin, flavourzyme and acid protease were used for the hydrolysis of A. assamensis pupae and S. littoralis and T. molitor proteins.30,70,79 However, only limited research reported the use of plant-derived enzymes like papain for producing insect protein hydrolysates.80 One recent study reported that the utilization of papain mixed with alcalase at 3.0% enzyme–substrate ratio and incubation for 3 h achieved the highest ACE inhibition, and the highest inhibition of DPP-IV was achieved when mixing papain and flavourzyme at 2.3% enzyme–substrate ratio for 3 h incubation.30 Another innovative alternative is the combination of enzymatic hydrolysis with novel technologies: microwave-assisted enzymatic hydrolysis of whole crickets is demonstrated to be an advantageous method to hydrolyze whole insects as protein unfolding can be increased by microwave energy, and microwave energy can transport the peptide fragments that are not hydrolyzed by proteolytic enzymes.28,81,82
For different studies describing enzymatic hydrolysis of insect proteins, the degree of hydrolysis of insect protein has been assessed, varying from 3% to 100%. The large variation may be caused by the differences in hydrolysis conditions used such as the type of enzyme, E/S ratio, enzymatic activity, temperature, pH, the presence of protease inhibitors, and the insect species studied. For instance, when applying the same hydrolysis protocol (in vitro digestion with α-amylase, pepsin and pancreatin) to different insects, the degrees of hydrolysis of A. annulipes and L. migratoria were 15.8 and 36.3%, respectively.77 Results reported by Zielińska et al. (2015),5 for G. sigillatus, T. molitor and S. gregaria after in vitro digestion were approximately 32%, 14% and 30%, respectively. Also, the degree of hydrolysis was affected by different processing methods; boiling and baking increased the degree of hydrolysis of S. gregaria and T. molitor compared to raw samples. However, different results were observed for G. sigillatus: the degree of hydrolysis decreased from approximately 32% to 27% when applying boiling. But baked G. sigillatus still showed a higher degree of hydrolysis (37.5%) compared to the untreated sample. Table 3 summarizes the enzymatic conditions currently applied in some studies and the bioactivity of insect derived peptides from different insect species.
| Source | Life stage | Protein content (starting material) | Enzymatic conditions | Main highlights | Ref. |
|---|---|---|---|---|---|
| ND: not determined. | |||||
| Silkworm (Bombyx mori); Cotton leafworm (Spodoptera littoralis); Locust (Schistocerca gregaria); Buff-tailed bumblebee (Bombus terrestris) | Larvae; Larvae; Adults; Adults | ND | 1. Simulated gastric phase: pepsin (enzyme/substrate: 1/250 w/w for 2 h at 37 °C, pH 2). And simulated intestinal phase: trypsin and α-chymotrypsin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (enzyme/substrate: 1/250 w/w for 2.5 h at 37 °C, pH 6.5); 2. Alcalase (enzyme/substrate: 48 U kg−1 for 3 h at 55 °C, pH 8) and thermolysin (enzyme/substrate: 1/1600 w/w for 5 h at 37 °C, pH 8) 1) (enzyme/substrate: 1/250 w/w for 2.5 h at 37 °C, pH 6.5); 2. Alcalase (enzyme/substrate: 48 U kg−1 for 3 h at 55 °C, pH 8) and thermolysin (enzyme/substrate: 1/1600 w/w for 5 h at 37 °C, pH 8) |
1. ACE inhibitory activity increased when hydrolyzed by enzymes such as gastrointestinal proteases, alcalase, and thermolysin; 2. Insect protein contains antihypertensive components and can be applied to functional foods and nutraceuticals. | 20 |
| Cotton leafworm (Spodoptera littoralis) | Larvae | ND | 1. Simulated gastric phase: pepsin (enzyme/substrate: 1/250 w/w for 2 h at 37 °C, pH 2). And simulated intestinal phase: trypsin and α-chymotrypsin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (enzyme/substrate: 1/250 w/w for 2.5 h at 37 °C, pH 6.5); 2. Alcalase (48 U kg−1 for 3 h at 55 °C and at pH 8) and thermolysin (enzyme/substrate: 1/1600 w/w for 5 h at 37 °C, pH 8) 1) (enzyme/substrate: 1/250 w/w for 2.5 h at 37 °C, pH 6.5); 2. Alcalase (48 U kg−1 for 3 h at 55 °C and at pH 8) and thermolysin (enzyme/substrate: 1/1600 w/w for 5 h at 37 °C, pH 8) |
1. A new tripeptide Ala-Val-Phe was identified to have ACE inhibitory ability (IC50 = 2123 μM); 2. The cotton leafworm (S. littoralis) hydrolysates (hydrolyzed with gastrointestinal enzymes) showed ACE inhibition activity; 3. New applications as functional foods, dietary supplements and antihypertensive agents. | 79 |
| Silkworm (Bombyx mori) | Larvae | ND | 1. Pepsin (1750 U mg−1 for 90 min at 37 °C, pH 2.0), followed by hydrolysis with trypsin (2500 U mg−1 for 2.5 h at 37 °C, pH 6.5) and α-chymotrypsin (1000 U mg−1 for 2.5 h at 37 °C, pH 6.5) | 1. The EAA composition of silkworm larvae protein isolate is balanced, and it is a high-quality protein source; 2. Silkworm larvae protein isolates displayed ACE inhibitory activity (IC50 = 8.3 μg mL1), DPPH scavenging activity (IC50 = 57.91 μg mL−1) and ferrous ion chelating capacity (IC50 = 2.03 mg mL−1). | 141 |
| Silkworm (Bombyx mori) | Pupae | ND | 1. Pepsin (1000 U mg−1 for 1.5 h at 37 °C, pH 2.0). Trypsin and α-chymotrypsin (1000 U mg−1 for each enzyme for 2.5 h at 37 °C, pH 6.5). | 1. A novel ACE-inhibitory tripeptide (Ala-Ser-Leu) (IC50 = 102.15 μM) was isolated, showing competitive inhibition behavior; 2. The docking complex was stabilized by amino acids (Lys453, Asp415, His383, Val380, Val397, Ala354, His353, Gln281) in the ACE active site. 3. Strong hydrogen bonds formed by Ala354 and Gln281 and His353 are the main contributors to the ACE inhibition. | 112 |
| Cricket (Gryllodes sigillatus); Mealworm (Tenebrio molitor); Locust (Schistocerca gregaria) | Adult; Larvae; Adult | 70.0 ± 1.7 (dry basis); 52.35 ± 1.1 (dry basis); 76.0 ± 0.9 (dry basis) | 1. Stimulated saliva: α-amylase (50 U mg−1, enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate 1 substrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/w for 10 min at 37 °C, pH 6.75); 2. Simulated gastric phase: pepsin (250 U mg−1, enzyme 10 w/w for 10 min at 37 °C, pH 6.75); 2. Simulated gastric phase: pepsin (250 U mg−1, enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate 1 substrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 w/w for 2 h at 37 °C, pH = 2.5); 3. Simulated intestinal phase: 0.7% pancreatin (for 1 h at 37 °C, pH 7.0) and 2.5% solution of bile extract (1 100 w/w for 2 h at 37 °C, pH = 2.5); 3. Simulated intestinal phase: 0.7% pancreatin (for 1 h at 37 °C, pH 7.0) and 2.5% solution of bile extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 v/v) 2.5 v/v) |
1. Baked cricket (Gryllodes sigillatus) showed the highest degree of hydrolysis (37.76%); 2. The hydrolysates of raw, cooked and baked crickets, mealworm, and locust showed inhibition of human skin fibroblasts CRL-2522 (cytotoxicity). | 5 |
| Silkworm (Bombyx mori) | Pupae | ND | 1. Stimulated saliva: α-amylase (50 U mg−1, enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate 1 substrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/w for 10 min at 37 °C, pH 6.75); 2. Simulated gastric phase: pepsin (250 U mg−1, enzyme:substrate 1 10 w/w for 10 min at 37 °C, pH 6.75); 2. Simulated gastric phase: pepsin (250 U mg−1, enzyme:substrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 w/w for 2 h at 37 °C, pH = 2.5); 3. Simulated intestinal phase: 0.7% pancreatin (for 1 h at 37 °C, pH 7.0) and 2.5% solution of bile extract (1 100 w/w for 2 h at 37 °C, pH = 2.5); 3. Simulated intestinal phase: 0.7% pancreatin (for 1 h at 37 °C, pH 7.0) and 2.5% solution of bile extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 v/v) 2.5 v/v) |
1. The peptides from silkworm pupa showed antiradical activity (via ion chelation); 2. The highest peptide concentration before digestion was 3.13 mg mL−1 for locust, but after digestion locust peptide concentration was still highest at 5.88 mg mL−1. | 77 |
| Mealworms (Tenebrio molitor); Locusts (Schistocerca gregaria); Crickets (Gryllodes sigillatus) | Larvae; Adult; Adult | ND | 1. Stimulated saliva solution: 7 mM NaHCO3 and 0.35 mM NaCl, pH 6.75, for 10 min; 2. Stimulated gastric phase: pepsin (250 U mg−1 for 2 h at 37 °C, pH 2.5); 3. Simulated intestinal phase: 0.7% pancreatin and 2.5% bile extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, v/v) and incubated at 37 °C for 1 h. 2.5, v/v) and incubated at 37 °C for 1 h. |
1. Heat treatment increased LOX and COX-2 (referred to as anti-inflammatory) inhibition in mealworm, locusts and crickets; 2. Anti-inflammatory properties were shown in both whole insect hydrolysates and insect protein hydrolysates. The highest anti-inflammatory activity was shown in cricket (LOX: IC50 = 0.13 μg mL−1; COX-2: IC50 = 0.26 μg mL−1); 3. The highest antioxidant activity: highest Fe2+ chelating (EC50 = 2.21 μg mL−1) shown in mealworm protein hydrolysates. Highest ABTS and DPPH scavenging shown in cricket protein hydrolysates (EC50 = 2.75 μg mL−1 and 6.91 μg mL−1, respectively). | 104 |
| Mealworms (Tenebrio molitor); Locusts (Schistocerca gregaria); Crickets (Gryllodes sigillatus) | Larvae; Adult; Adult | ND | 1. Stimulated saliva solution: 7 mM NaHCO3 and 0.35 mM NaCl, pH 6.75 for 10 min; 2. Stimulated gastric phase: pepsin (250 U mg−1 for 2 h at 37 °C, pH 2.5); 3. Simulated intestinal phase: 0.7% pancreatin and 2.5% bile extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, v/v) and incubated at 37 °C for 1 h; 4. Simulated absorption process: a membrane (molecular weight cut-off 3.5 kDa) was used for dialyzing hydrolysates, for 1 h at 37 °C without light. 2.5, v/v) and incubated at 37 °C for 1 h; 4. Simulated absorption process: a membrane (molecular weight cut-off 3.5 kDa) was used for dialyzing hydrolysates, for 1 h at 37 °C without light. |
1. S. gregaria peptide fractions showed the highest enzyme inhibitory activities: ACE (IC50 = 3.95 μg mL−1) for boiled samples, lipase (IC50 = 9.84 μg mL−1) for baked, and α-glucosidase (IC50 = 1.89 μg mL−1) for raw S. gregaria, respectively; 2. The sequences of synthesized peptides with highest inhibitory activity were KVEGDLK, YETGNGIK, AIGVGAIR, IIAPPER, and FDPFPK; 3. When applying heat treatment to edible insects, it led to positive effects on the enzyme's inhibitory activity of peptides produced. | 97 |
| Muga silkworm (Antheraea assamensis) | Pupae | ND | 1. Alcalase (enzyme/substrate: 0.5% v/w at 50 °C, pH 8.0), flavourzyme (enzyme/substrate: 1.5% v/w at 50 °C, pH 7.0), papain (enzyme/substrate: 2.3% v/w at 60 °C, pH 6.0) and thermolysin (enzyme/substrate: 3.0% v/w at 70 °C, pH 8.0). Aliquots were taken at 0, 1, 2, 3, 5, 8, 12, 24 h to measure bioactive properties. | 1. The highest ACE inhibition activity was obtained when alcalase and papain were used at 3% enzyme–substrate ratio and incubated for 3 h; 2. 3% enzyme–substrate ratio and incubated for 5 h resulted in the highest DPPH scavenging activities; 3. Flavourzyme and papain enzymatic system at 2.3% enzyme–substrate ratio and incubated for 5 and 3 h, respectively, resulted in the highest DPP-IV inhibition activity. | 30 |
| Mealworm (Tenebrio molitor) | Larvae | ND | 1. Alcalase (enzyme/substrate: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 w/w at 50 °C, pH 8.5) 100 w/w at 50 °C, pH 8.5) |
1. Tenebrio molitor (L.) larvae can be used as a source of ACE inhibitory peptides after hydrolysis with alcalase; 2. Single oral administration of ACE inhibitory peptide (Tyr-Ala-Asn) showed a significant reduction of systolic blood pressure in rats; 3. RP-HPLC was applied to purify Tyr-Ala-Asn (novel ACE inhibitory peptide); 4. The in vivo antihypertensive activity of Tyr-Ala-Asn needs to be further confirmed. | 60 |
| Cricket (Gryllodes sigillatus) | ND | ND | 1. Establish nine conditions to test different enzyme–substrate concentrations and hydrolysis time on protein functionality. Alcalase (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 0.5, 1.5, and 3% w/w for 30, 60, and 90 min at 50 °C, respectively) substrate: 0.5, 1.5, and 3% w/w for 30, 60, and 90 min at 50 °C, respectively) |
1. Whole cricket can produce protein hydrolysates with improved functionality; 2. The protein solubility can be improved over a wide range of pH, so the cricket protein hydrolysates have potential to be applied in acidic foods; 3. Higher foamability was observed for protein hydrolysates, but the foam stability was not as good as the non-hydrolyzed protein. | 52 |
| Cotton leafworm (Spodoptera littoralis) | Larvae | ND | 1. Alcalase (48 U kg−1 for 3 h at 55 °C, pH 8); thermolysin (enzyme/substrate: 1/1600 w/w for 5 h at 37 °C, pH 8); 2. To simulate the human gastrointestinal digestion process as follows: pepsin (enzyme/substrate: 1/250 w/w for 2 h at 37 °C, pH 2) and small intestine: trypsin and α-chymotrypsin (enzyme/substrate: 1/250 w/w for 2.5 h at 37 °C, pH 6.5); 3. Hydrolysis with mucosal peptidases (enzyme/substrate: 1/500 w/w for 2 h at 37 °C); 4. Different combinations of hydrolysis were used: a. Gastrointestinal digestion; b. Gastrointestinal digestion + mucosal peptidases; c. Digestion with thermolysin; d. Digestion with thermolysin + gastrointestinal digestion; e. Digestion with thermolysin + gastrointestinal digestion + mucosal peptidases; f. Digestion with alcalase; g. Digestion with alcalase + gastrointestinal digestion; h. Digestion with alcalase + gastrointestinal digestion + mucosal peptidases. | 1. The S. littoralis hydrolysates showed both ACE inhibitory activity and antioxidant activity (in vitro), but these showed no correlation; 2. The S. littoralis hydrolysates showed a relatively low antioxidant activity; 3. Gastrointestinal digestion (IC50 = 320 μg mL−1) and digestion with mucosal enzyme (IC50 = 211 μg mL−1) showed higher ACE inhibitory activity compared to alcalase hydrolysis (IC50 = 827 μg mL−1), thermolysin hydrolysis (IC50 = 1392 μg mL−1). | 76 |
| Lesser mealworm (A. diaperinus) | ND | ND | 1. Alcalase and the enzyme corolase PP (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 0.5, 1.5 and 3.0% v/w, 50 °C, aliquots at different time points were taken 0, 30 min, 1, 2, 4, 6, 8 and 24 h, pH 8.0). substrate: 0.5, 1.5 and 3.0% v/w, 50 °C, aliquots at different time points were taken 0, 30 min, 1, 2, 4, 6, 8 and 24 h, pH 8.0). |
1. The enzymatic hydrolysis process increased the nutritional value of insect powder such as improving the free amino acid content and small peptides; 2. The hydrolysates have antioxidant (TEAC value: 95 ± 0.8 μmol TE per g) and antihypertensive properties (IC50 = 55.5 ± 6.2 μg mL−1); 3. No antimicrobial activity or α-glucosidase inhibition activity were found. | 15 |
| Cricket (Gryllodes sigillatus) | Adult (6 weeks old) | ND | 1. Establish nine hydrolysis conditions: alcalase (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 0.5, 1.5, and 3% w/w for 30, 60, and 90 min, respectively at 50 °C, pH 8.0); 2. Pepsin (enzyme substrate: 0.5, 1.5, and 3% w/w for 30, 60, and 90 min, respectively at 50 °C, pH 8.0); 2. Pepsin (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 2 h at 37 °C, pH 2), then bile salts and pancreatin (enzyme substrate: 4% w/w for 2 h at 37 °C, pH 2), then bile salts and pancreatin (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 2 h at 37 °C, pH 6.8) substrate: 4% w/w for 2 h at 37 °C, pH 6.8) |
1. Hydrolysates displayed good ACE (IC50 = 0.062 mg mL−1), DPP-IV inhibition (69%), and antioxidant activity (ABTS assay: 799.4 ± 8.2 μmol TE per mg sample; DPPH assay: 1926.3 ± 4.6 μmol TE per mg sample); 2. Bioactivity increased after simulated gastrointestinal digestion; 3. A degree of hydrolysis between 60 and 85% can completely remove allergenicity. | 23 |
| Mealworm (Tenebrio molitor); Cricket (Gryllus bimaculatus); Silkworm (Bombyx mori) | Larvae; Adult; Pupae | ND | 1.- Two doses: a) Flavourzyme with 30 and 60 U per g protein; b) Alcalase with 12 and 72 mU per g protein; c) Neutrase with 4 and 24 mU per g protein; d) Protamex with 7.5 and 45 mU per g protein 2.- A mixture group a) Flavourzyme 30 U per g protein with Alcalase 12 mU per g protein; b) Flavourzyme 60 U per g protein with Alcalase 72 mU per g protein All hydrolysis were performed at 55 °C for 8 h. | 1. All the protein hydrolysates showed higher solubility than the unhydrolyzed proteins. The highest solubility was presented for the sample cotreated with two enzymes (flavourzyme 60 U g−1 + alcalase 72 mU per g protein). The solubility of mealworm larvae showed a significant increase (30%–50%); 2. Alcalase hydrolysates were the best emulsifiers because of the high emulsifying stability. But the combination of flavourzyme and alcalase led to a decreased emulsifying property; 3. The greatest inhibition of ACE was shown for the alcalase-treated group; 4. The highest α-glucosidase inhibitory activity was shown for silkworm pupae treated with alcalase and mealworms treated with the flavourzyme and alcalase; 5. Anti-inflammatory activity was only observed for silkworm pupae, but this decreased with hydrolysis time. | 70 |
| Silkworm (Bombyx mori) | Pupae | 66.83% | 1. Acid protease (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 1% w/w for 4.92 h at 35 °C, pH 2.18). substrate: 1% w/w for 4.92 h at 35 °C, pH 2.18). |
1. Albumin was the easiest protein fraction to hydrolyze showing a higher degree of hydrolysis (17.32%) and ACE inhibitory activity (81%) than the other three protein fractions (prolamin, globulin and glutelin) obtained by the Osborne method; 2. A peptide sequence APPPKK, which inhibits the angiotensin I-converting enzyme activity, was identified in the albumin fraction; 3. The highest ACE inhibitory activity was in silkworm albumin (IC50 = 0.047 mg mL−1). | 142 |
| Silkworm (Bombyx mori) | Pupae | ND | 1. Acid protease (protein/water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/v), 3% acid protease (E/S = 3000 U g−1) for 5.0 h at 35 °C, pH 2.0 7 w/v), 3% acid protease (E/S = 3000 U g−1) for 5.0 h at 35 °C, pH 2.0 |
1. Silkworm pupae protein hydrolysates showed in vivo ACE inhibition (blood pressure of rats was 139.0 ± 10.4 mmHg after consumption and after 6 h, blood pressure was recovered); 2. Acute toxicity research showed that protein hydrolysates were safe for human consumption; 3. The systolic blood pressure of non-hypertensive rats is not affected by hydrolysates in a long-term test. | 143 |
| Silkworm (Bombyx mori) | Pupae | ND | 1. Alcalase (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 5 h at 55 °C, pH 8.0); 2. Flavourzyme (enzyme:substrate: 4% w/w for 5 h at 55 °C, pH 7.0); 3. Protamex (enzyme substrate: 4% w/w for 5 h at 55 °C, pH 8.0); 2. Flavourzyme (enzyme:substrate: 4% w/w for 5 h at 55 °C, pH 7.0); 3. Protamex (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 5 h at 40 °C, pH 7.0); 4. Trypsin (enzyme substrate: 4% w/w for 5 h at 40 °C, pH 7.0); 4. Trypsin (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 5 h at 40 °C, pH 8.0); 5. Papain (enzyme substrate: 4% w/w for 5 h at 40 °C, pH 8.0); 5. Papain (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 5 h at 50 °C, pH 7.0); 6. Pepsin (enzyme substrate: 4% w/w for 5 h at 50 °C, pH 7.0); 6. Pepsin (enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 4% w/w for 5 h at 40 °C, pH 2.0). substrate: 4% w/w for 5 h at 40 °C, pH 2.0). |
1. A high degree of hydrolysis was necessary to reach a high antioxidant activity of the protein hydrolysates; 2. The optimum conditions for silkworm protein alcalase hydrolysis were E/S 7.38%, pH 7.97 at 60 °C, and the maximum antioxidant activity was 66.1%. | 69 |
| Silkworm (Bombyx mori) | Pupae | ND | 1. 7% alkaline protease (for 4 h at 50 °C, pH 9.5). | 1. The α-P3 fraction showed the highest ACE inhibition before purification; after purification with RP-HPLC and HPLC, α-P3-6-b showed the highest ACE inhibition; 2. Good solubility, heat and acid resistance was shown in purified pupa angiotensin I-converting enzyme inhibitory peptides. | 144 |
| Silkworm (Bombyx mori) | Pupae | ND | 1. Ultrasound-assisted enzymatic hydrolysis by alcalase. Alcalase (3500 U per g protein) at 50 °C for 50 min, pH 9.0. The ultrasound-pretreated (20 kHz, 250 W to 600 W, 24 min) protein solution was adjusted with distilled water to 1% (w/v) at 50 °C for 10 min before hydrolysis. | 1. The ACE inhibitory activity of silkworm pupae can be improved by ultrasound pretreatment; it showed a 39.9%–67.3% increase (ultrasonic power 250–600 W) compared to non-ultrasonic treatment; 2. A novel peptide (Lys-His-Val) resistant to gastrointestinal proteases exhibited ACE inhibitory activity (IC 50 = 12.82 μM); 3. The active site of ACE could effectively interact with Lys-His-Val peptide as result of molecular docking analysis. | 61 |
| Crickets (Gryllodes sigillatus) | Adults (6 weeks old) | ND | 1. Microwave-assisted enzymatic hydrolysis by alcalase. Alcalase (7.54 U): enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate: 3% for 10 min or 20 min at 55 °C, pH 8.0. Microwave unit with varying power (600 W; 80% power maximum). substrate: 3% for 10 min or 20 min at 55 °C, pH 8.0. Microwave unit with varying power (600 W; 80% power maximum). |
1. Microwave-assisted hydrolysis led to highest inhibition of ACE (IC50 = 0.096 mg mL−1) and DPP-IV (IC50 = 0.27 mg mL−1); 2. Low immunoreactivity peptides can be generated through microwave-assisted enzymatic hydrolysis. | 21 |
3.1. Antihypertensive properties
ACE inhibition is associated with antihypertensive properties; peptides with ACE inhibiting activity have already been extracted from various food proteins like soy, milk, fish and egg white.83–86 Studies have demonstrated that insect protein hydrolysates have bioactive properties including antihypertensive.87–89 Enzyme selection is a key factor for the generation of ACE inhibitory peptides.76,90 To achieve the highest ACE inhibition, recent studies reported different enzymes and optimum hydrolysis conditions for different insect proteins. Enzymes such as pepsin, trypsin and α-chymotrypsin were commonly used to simulate stomach and gastrointestinal digestion; α-amylase and pancreatin were applied to simulate saliva and intestinal digestion; and alcalase, thermolysin and flavourzyme were frequently applied to generate ACE-inhibiting hydrolysates. Other enzymes like neutrase, protamex, acid protease and alkaline protease were also applied. Some studies combined different enzymes to achieve increased bioactivity. Vercruysse et al. (2009) combined gastrointestinal digestion and thermolysin, alcalase or mucosal peptidases and found that the combination of these enzymes increased ACE inhibition, for example, gastrointestinal digestion (with mucosal peptidases) significantly lowered the IC50 value to 211 μg mL−1 and showed the best ACE inhibitory ability, compared to gastrointestinal digestion (IC50 = 320 μg mL−1).76 Similarly, insect protein hydrolyzed by gastrointestinal proteases, alcalase, and thermolysin led to an increase of ACE inhibitory activity with IC50 values of 1.26 mg mL−1 for B. terrestris and 0.77 mg mL−1 for S. littoralis, compared to 43.22 mg mL−1 and 2.58 mg mL−1 before hydrolysis.20Combining conventional hydrolysis with techniques such as microwave and ultrasound treatment can achieve a lower IC50.76 Hall and Liceaga (2020) reported that the microwave-hydrolyzed G. sigillatus protein had the highest ACE inhibition (IC50 = 0.096 mg mL−1) compared to enzymatically hydrolyzed protein (IC50 = 0.20 mg mL−1).21 Similar to microwave treatment, ultrasound treatment is another good alternative for hydrolyzing proteins and increasing ACE inhibition, as the treatment induces the molecular unfolding of proteins and increases the surface hydrophobicity, which promotes the release of peptides with an ACE inhibitory function. However, parameters for ultrasound hydrolysis must be optimized as sonication with high power and long duration leads to the formation of a stable structure and hinders the release of ACE-inhibiting peptides.61,91Table 3 summarizes the common enzymes and suitable conditions applied to insect protein hydrolysis.
More specifically for ACE inhibitory peptides (ACEIPs), their inhibitory activity is related to peptide properties such as hydrophobicity, charge, and structural conformation. ACE inhibition activity is improved by the presence of hydrophobic rings in structural amino acids.92 Similarly, another study also demonstrated the importance of aromatic amino acids in ACE inhibitory peptides.93 Peptides such as IIe-Pro-Pro and Val-Pro-Pro with a proline ring at the C-terminus were recognized as potent ACEIPs. Zhang et al. (2020) also observed that a C-terminal hydrophobic amino acid was more effective than an N-terminal one in ACE inhibition.92,94 Known antihypertensive peptides AF, GW, GY and PH in M. domestica and GKDAVIV and VAPEEHPV in S. gregaria also agreed with the fundamental features of ACEIPs, and thus displayed great antihypertensive activities.95,96
However, only limited in vivo studies confirmed the antihypertensive activity of insect-derived peptides. Dai et al. (2013) reported a significant decrease in blood pressure when applying multiple dose oral administration of protein hydrolysates of T. molitor to spontaneously hypertensive rats; the systolic blood pressure decreased to 27 mm Hg at 400 mg per kg weight after 4 h post-administration.60
3.2. Antidiabetic properties
High postprandial blood glucose is caused by DPP-IV, but to date, the application of DPP-IV inhibitors is expensive and clinicians lack experience.97 Some publications reported the DPP-IV inhibition activity of insect protein hydrolysates obtained with various enzymes. For enzymatic hydrolysis by simulated gastrointestinal digestion, G. sigillatus protein hydrolysates showed low inhibition between 0 to 50% (depending on the enzymatic hydrolysis conditions used) before simulated gastrointestinal digestion, but after simulated gastrointestinal digestion the inhibition activity increased and reached values between 62 and 69%.23 In other insect species, DPP-IV inhibitory properties were reported in A. assamensis protein hydrolysates (in gastric fluid IC50 = 5063 μg mL−1 and intestinal fluid IC50 = 5221 μg mL−1), but the highest inhibition was found when applying flavourzyme and papain and hydrolyzing at a 2.3% enzyme to substrate ratio for 3 h.30 This may suggest that for A. assamensis, flavourzyme combined with papain shows higher efficiency at generating antidiabetic peptides due to the specific cleavage of hydrophobic amino acid residues. Similar to the antihypertensive properties, antidiabetic properties can also be increased by microwave treatment; a low IC50 value was observed for the microwave plus alcalase treated group (IC50 = 0.27 mg mL−1) when compared to the group subjected to alcalase hydrolysis only (IC50 = 0.65 mg mL−1).21,98 However, a limited number of publications have reported the α-glucosidase inhibitory activity of insect protein hydrolysates or insect-derived peptides, which helps in delaying digestion of carbohydrates, thereby reducing the levels of glucose in blood. Yoon et al. (2019) reported α-glucosidase inhibitory activity for T. molitor, G. bimaculatus and B. mori hydrolysates and found that the most effective inhibitors were those from silkworm pupae treated with alcalase and mealworms treated with flavourzyme coupled to alcalase hydrolysis, which showed approximately 33% and 35% inhibition, respectively.70 But to date the in vivo antidiabetic properties of insect protein hydrolysate have not been assessed.In general, antidiabetic peptides contain 2 to 17 amino acid residues, with most of them being hydrophobic amino acids. However, antidiabetic peptides with hydrophilic amino acids such as ‘Thr, His, Gln, Ser, Lys, Arg’ can also be found. For example, antidiabetic dipeptides presenting ‘Trp/Thr/Met at the N-terminus’ and ‘Ala/Leu/His at the C-terminus’ and peptides containing ‘Pro/Leu/Arg at the C-terminus’ have been identified previously.99 The G. assimilis derived peptides identified as α-glucosidase inhibitors were ‘LAMVEA, LPPPP, ALLVVW, DSYPL and YPGDV’. Thus, these specific traits correspond to the general features of antidiabetic peptides (over 4 amino acids), with hydrophobic amino acids as dominant residues.
3.3. Antioxidant properties
Antioxidant peptides can be released in several ways, including chemical hydrolysis, enzymatic hydrolysis, novel technologies coupled to assisted enzymatic hydrolysis (i.e. ultrasound, pulsed electric fields, etc.), fermentation and other food processing methods (i.e. steaming, boiling, etc.).95 For instance, insect studies have demonstrated the potential of enzymatic hydrolysis to release antioxidant peptides.89,99,100 The release of antioxidant peptides by alcalase from B. mori protein was reported; the enzyme–substrate ratio, pH and temperature were tailored to 7.38%, 7.97 and 60 °C, respectively, to achieve the maximum antioxidant activity (66.1%).69 However, Hall et al. (2018) evaluated the antioxidant activity of G. sigillatus protein hydrolysates, but found that simulated gastrointestinal digestion had little effect on the antioxidant activity, as this was similar before and after digestion. For example, for the ferric ion reducing antioxidant power assay and ABTS assay, cricket protein alcalase hydrolysates showed 516.8 ± 13.5 and 906.6 ± 4.1 μmol TE per mg sample, compared to 689.0 ± 13.8 and 1137.4 ± 2.8 μmol TE per mg sample after gastrointestinal digestion.23 For G. sigillatus, a possible explanation for the stable antioxidant activity is that antioxidant fragments mainly have β-sheet and random coil structures rather than α-helices, although the current study does not include the secondary structure analysis of G. sigillatus antioxidant fragments. Another study showed that G. sigillatus water-soluble proteins were dominated by the α-helix secondary structure, which was hard to break down during digestion, thus, resulting in the negligible generation of peptides with antioxidant properties.101The relationship between antioxidant and antihypertensive properties has also been studied. Vercruysse et al. (2009) showed that the hydrolysates of S. littoralis exerted dual activity as both in vitro ACE inhibition and antioxidant activity were observed, but no relationship was found between ACE inhibition and antioxidant activity.76 The inexistent link between antioxidant and antihypertensive activities was also found in A. diaperinus when hydrolyzed by alcalase coupled to corolase PP.15 However, as for the antidiabetic properties, the in vivo antidiabetic properties of insect protein hydrolysates have not been extensively studied.
The amino acid composition is an important decisive factor for the release of antioxidant peptides with enhanced bioactivity. Peptides with a high presence of hydrophobic amino acids are regarded as peptides with higher radical scavenging potential than peptides with a lower occurrence of hydrophobic amino acids.102 Known antioxidant peptide sequences such as APVAVAHAAVPA and ASVVEKLGDY in A. diaperinus and LAPSTIK in G. sigillatus also align with this feature.97,103 Regarding these known sequences, the high content of hydrophobic amino acids may explain why edible insects have a higher antioxidant activity than the hydrolysates obtained from some animal products or leafy plants after digestion.77 Also, the content of hydrophobic amino acids in insects such as H. illucens, A. domesticus and M. bellicosus is comparable to that of animal products such as milk, beef and lamb, and is higher than for plant materials (Fig. 1).
3.4. Anti-inflammatory properties
The anti-inflammatory properties of insects have not been widely researched. The lipoxygenase (LOX) and cyclooxygenase (COX-2) inhibitory activities are target anti-inflammatory biomarkers. According to limited studies, the anti-inflammatory activities seem to be highly dependent on the hydrolysis conditions.70 For example, G. sigillatus protein hydrolysates showed LOX and COX-2 inhibitory activity with IC50 values of 0.13 and 0.26 μg mL−1, respectively, compared to T. molitor protein hydrolysates (LOX: IC50 = 0.17 μg mL−1; COX-2: IC50 = 0.35 μg mL−1) and S. gregaria protein hydrolysates (LOX: IC50 = 0.18 μg mL−1; COX-2: IC50 = 0.26 μg mL−1).104 However, different results were reported by Yoon et al. (2019).70 Neither T. molitor protein nor T. molitor protein hydrolysates showed anti-inflammatory properties when hydrolysed with flavourzyme and alcalase. Furthermore, heat processing such as boiling and baking can increase the anti-inflammatory activity of the whole insect hydrolysates produced by in vitro digestion. T. molitor, G. sigillatus and S. gregaria showed a significant increase of LOX and COX-2 inhibition after heat treatment, for example, when boiling was applied to G. sigillatus flour, the IC50 value (LOX) decreased to 16.9 μg mL−1 (compared to raw cricket flour IC50 = 20.74 μg mL−1).104Several studies have shown that low molecular weight peptides are more resistant to digestion, and therefore, can easily cross the intestinal barrier and be absorbed in the intestine. Longer peptides such as lunasin, containing 43 amino acids, are also anti-inflammatory peptides, but their mechanism of action is not clear so far.105 A limited number of anti-inflammatory peptides were reported in S. gregaria, including IIAPPER, LAPSTIK and AIGVGAIER.104 Interestingly, these peptides also have antioxidant properties. This may be attributable to the fact that similarly to antioxidant peptides, hydrophobic amino acids are also present in anti-inflammatory peptides. Normally, they are located at the N-terminal position, but more studies are needed to understand the underlying mechanisms.
4. Bioinformatic (in silico) tools for insect peptides prediction
Bioinformatic (in silico) analysis is based on the use of a peptide database for the identification, selection and prediction of potential bioactive peptides released from proteins during enzymatic hydrolysis.106 The databases available contain various detailed information about peptides, including chemical structures, toxicity, allergenicity, sequences and amino acid contents (Fig. 3). The most common and widely used database is BIOPEP-UMV, while other databases such as UniProtKB and NCBI are mainly used to retrieve known sequences for analysis and comparative and homology purposes.106 Currently, the in silico analysis of insect proteins and insect-derived peptides is mainly used as an aid/guidance for in vitro enzymatic hydrolysis. Most of the studies available simulate the in silico hydrolysis of proteins to mimic individual or sequential enzyme action, and then the selection of the most promising fraction/peptide (obtained from enzymatic hydrolysis) is proposed, and afterwards chemical synthesis of the selected peptide(s) is carried out to verify the activity in vitro. Finally, molecular docking analysis is used to confirm the mechanisms behind specific bioactivities.107 Insect-derived peptides with antihypertensive and/or antidiabetic properties are the most studied ones by in silico analysis. Other peptides with antithrombotic and anti-SARS-CoV-2 properties were also reported with in silico tools.108,109 Currently, most of the insect-derived peptides discussed in section 3 in this review have been identified using the aforementioned databases.Software like ExPASy-peptidecutter can be applied as a prediction tool before in vitro enzymatic hydrolysis. The substrate (protein sequence) and specific enzymes can be selected to simulate the cutting sites and generate the potential peptide profiles.106 For example, eight major proteins from house fly larvae (scientific name not provided) were considered for simulated gastrointestinal digestion while the allergenic proteins from B. mori were simulated for pepsin and bromelain hydrolysis by in silico approaches to select functional peptides.96,107 Besides, A. mellifera peptides (generated by neutrase hydrolysis) with ACE inhibition activity were subjected to in silico digestion to simulate the effectiveness of peptides after human digestion.110
Then the potential mechanisms behind the bioactivities are determined by molecular docking, which is based on simulating the interaction between receptors and ligands (bioactive peptides) or small molecules.111 For insects, molecular docking studies have mainly focused on the purified insect-derived peptides and ACE; the inhibition of ACE is mainly due to hydrogen bond formation and Zn(II) interactions between peptides and ACE. An ACE inhibitor tripeptide (Ala-Ser-Leu) from B. mori was found to complex with ACE through more than fifteen hydrogen bonds, with the main binding sites being between Gln281 and His353 in the ACE S2 pocket and Ala354 in the S1 pocket, but this tripeptide has no interaction with the Zn(II) in ACE.112 A similar study also reported Lys-His-Val as a potent and more effective ACE inhibition peptide, which formed eighteen hydrogen bonds with ACE, and similarly to lisinopril (a well-known ACE inhibitor used to treat high blood pressure), Lys-His-Val showed interactions at Gln281 and His 383.61 Another reported ACE inhibitor, B. mori peptide (GAMVVH), showed competitive coordination with Zn(II) in ACE; the stable structure of ACE was distorted when combining with GAMVVH, besides that, it could also form hydrogen bonds with Ala354, Lys511, and Gln281 in the ACE S1 and S2 pockets, which further helped to stabilize binding.113 For antidiabetic peptides purified from insect sources, they are known to inhibit DPP-IV by mainly forming hydrophobic interactions with the DPP-IV binding pocket. For example, Leu-Pro-Pro-Glu-His-Asp-Trp-Arg (from B. mori) is known to show hydrophobic interactions with Tyr510 and Phe320 in the DPP-IV S1 and S3 pockets, respectively. Similarly, hydrophobic interactions are the main interactions present between Leu-Pro-Ala-Val-Thr-Ile-Arg and the DPP-IV S1 binding pocket.107 Additionally, for the antioxidant B. mori-derived peptide (AKPGVY), it is known to be stabilized by hydrogen bonds, van der Waals interactions and hydrophobic interactions within the human Prxs binding site.114
Moreover, for antidiabetic insect peptides, an in silico modeling method, QSAR (quantitative structure–activity relationships), was applied to predict peptide bioactivities according to their sequence/structure.106 QSAR modeling was first applied to identify the α-glucosidase inhibiting peptides in silkworm (scientific name not available). The four silkworm-derived peptides with strong inhibition activity were Gln-Pro-Gly-Arg (IC50 = 65.8 μmol L−1), Ser-Gln-Ser-Pro-Ala (IC50 = 20 μmol L−1), Gln-Pro-Pro-Thr (IC50 = 560 μmol L−1) and Asn-Ser-Pro-Arg (IC50 = 205 μmol L−1).40 They are known to mainly complex with Lys776 through hydrogen bonds, and Lys776 is potentially the target amino acid for α-glucosidase inhibition peptides, but more studies are required. Nonetheless, in silico analyses are still needed in order to understand and elucidate the possible mechanisms of insect peptides and simulate the interactions between peptides and targeted proteins/ligands (through molecular docking) to predict the behaviour of peptides in regulating enzyme activities such as ACE, DPP-IV, α-glucosidase, inflammatory biomarkers, etc. There is a lack of published studies using bioinformatic tools to determine insect peptides released during digestion, evaluation of peptide stability or changes in biological activity due to different enzyme actions, and allergenicity prediction, among others.
5. Allergenic properties of insect proteins
Possible allergenic responses are becoming a major concern in insect consumption. Although allergens for novel foods are not unique, it is necessary to evaluate the potential risks of edible insect proteins.23,115A major panallergen, tropomyosin (TM), was identified in insects. Vertebrate-TM and invertebrate-TM shared 55% homology in terms of amino acid composition, and invertebrate-TM was not recognized as an allergen at first. However, as tropomyosin was gradually identified as an insect allergen, the cross-reactivity hypothesis among TMs from various species was supported.116 Thus, in order to reveal the potential allergenicity and mechanisms behind tropomyosin present in insects, a “bottom-up” approach reported by Zhao et al. (2023) should be carried out in order to analyze the potential allergic reactions among different insect species (from amino acid sequences to physicochemical properties, protein structures, epitopes and allergic peptides).117 Recently, the TM amino acid sequences of crustaceans and mollusks were compared; interestingly, 65% homology among the amino acid sequences was observed, suggesting cross-reactivity between the two species. The authors reported that allergenic reactions for crustacean allergic individuals could be triggered by some insects.100,118 So, potentially, insect TMs should also share, to higher or lower extents, homology in terms of amino acid sequence with crustaceans and/or mollusks. Research already proved that the cricket-TM shared over 60% homology in terms of amino acid sequence with known shellfish-TM.119 Furthermore, potentially, the allergenicity level of various insect species may depend on the amino acid sequence homology between the different tropomyosin isoforms that have shown highest allergenicity (species are unknown) and specific insect-TMs, as well as the amount of allergen-specific IgE present, the route by which the allergen is introduced and the dose of allergen. Regarding the physicochemical properties, both insect-TMs and invertebrate-TMs are resistant to heat and proteolysis. Although the tropomyosin helical secondary structure collapses when heated above 80 °C, it may reform upon cooling.25,26,120 However, the differences between the physicochemical properties of TMs from different species are not available. Additional studies regarding the epitopes and protein secondary structures of insect allergens are needed, in order to elucidate how these are related to potential allergenicity. For G. sigillatus-TM, 31 peptides were identified as potential linear epitopes, among them, the sequence ‘RSQQDEERM’ was shared by peptides such as ‘RSQQDEERMDQ, RSQQDEERMDQLTNQ and NRSQQDEERMDQLTNQ’.120
Another allergen reported in invertebrates is arginine kinase (AK), an enzyme involved in energy metabolism and muscle mobilization.121 AK is resistant to heat and digestion. A recent study showed limited AK cross reactivity present between crickets and mealworm allergic subjects.122,123 This proved the non-systematic cross-reactivity of AK, thus providing knowledge on the extent to which these different antigens appear similar or different to the immune system. However, the amino acid sequence of AK still shows high homology between different insect species, highlighting the importance of further studies on AK cross reactivity.
Conventional enzymatic hydrolysis and other processing methods such as microwave assisted-hydrolysis were investigated to lower the immunoreactivity and generate hypoallergenic peptides from insects.21,23,100,124 The decrease in allergenicity can be explained by the changes in the protein secondary structure; α-helices and β-sheets are stable structures and are hard to modify, and therefore, it makes it difficult for antibodies to combine.117 For example, aiming to lower the allergenicity of tropomyosin, 15 min microwave treatment (1000 W, 2.45 GHz) at 125 °C significantly reduced allergenicity by 75%. This decrease is associated with secondary structure modifications, including a decrease in α-helices and turns and an increase in β-sheets. The associated structural changes result in fewer recognition opportunities by the IgE of sensitized subjects.125 In other work, Hall et al. (2020) also reported the differences in the tropomyosin–IgG binding capacity between conventional enzymatic hydrolysis and microwave-assisted hydrolysis. They found that the lowest tropomyosin–IgG binding was obtained with microwave-hydrolyzed G. sigillatus protein (Fig. 4).21 Although the epitopes associated with reactivity are altered by processing and therefore are associated with decreased allergenicity, it is worth noting that new binding sites can be created and then induce potential sensitization and allergic responses.126
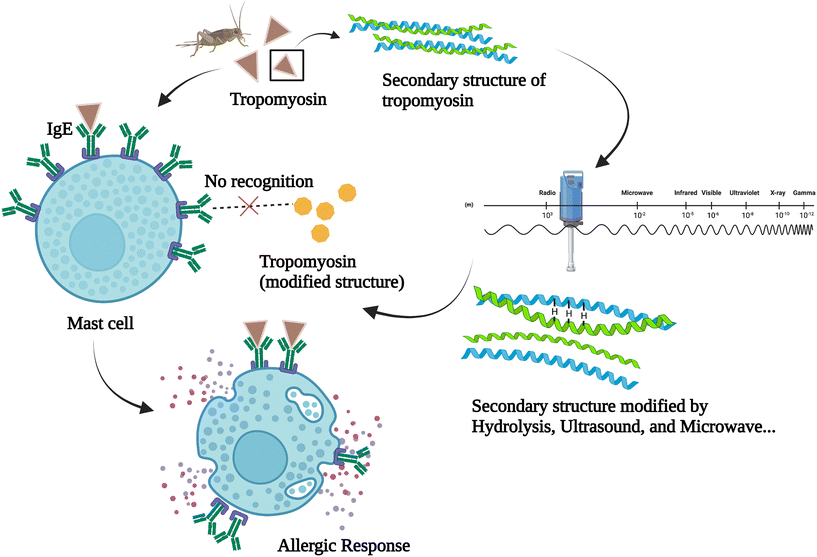 | ||
| Fig. 4 Overview of the procedure to reduce the allergenicity of insect tropomyosin by hydrolysis, microwave and ultrasound treatment. | ||
6. Conclusion and future perspectives
This review has provided relevant evidence that insect proteins can be used as an alternative protein source. From the nutritional perspective, in terms of protein quality, it can be comparable to common protein sources (plant/animal) as the EAA content of well-studied insects is higher than that in plant materials such as peanuts and almonds and similar to that in beef and cow milk. Also, it is worth mentioning that the protein digestibility of edible insects has shown high values in in vitro assays and similar values to those of beef and pork.Insect protein extraction is considered to be an effective method to improve the acceptance of insect intake. Wet fractionation is the most widely used method including traditional alkaline solubilization coupled to isoelectric precipitation or salt extraction and wet fractionation combined with some novel extraction techniques such as ultrasound and microwave treatments. Novel techniques not only have the potential to increase protein quality and the purity of protein ingredients, but also increase the bioactive traits of hydrolysates/peptides. Dry fractionation is less applied and additional studies are required to fully determine its potential to produce insect fractions.
Insects such as B. mori, T. molitor and G. sigillatus have potential for generating bioactive peptides, and more edible insects should be included in further studies to unlock more insect-derived peptides that could benefit human health. The main bioactivities that have been studied and identified in peptides and/or hydrolysates (in vitro) so far are antihypertensive properties, antidiabetic properties and antioxidant properties. Enzyme selection, amino acid composition/sequence, hydrophobicity and the structure of peptide fragments are commonly related to the potential bioactivity of peptides. Only a few anti-inflammatory properties have been researched and most of them have been studied at the protein hydrolysate stage instead of identifying the specific peptides exerting such activities. Also, mechanistic studies on anti-inflammatory insect-derived peptides are still missing, so more in silico studies are needed to reveal the mechanisms behind insect bioactive peptides and their specific inhibition sites of action. It is worth mentioning that current studies on protein identification and peptide bioactivities mainly focus on water-soluble proteins; the bioactivities of other protein groups with different solubility (salt soluble, organic solvents and alkali/acid diluted solutions) could provide key knowledge to characterize and classify insect proteins in more depth and generate insights on novel peptide bioactivities.
To date, the allergenicity of insect proteins is still an urgent matter to study and explore. The side effects of these allergenic reactions can be decreased through enzymatic hydrolysis, and the use of other technologies such as ultrasound and microwave, which have the capacity to disrupt the secondary structure of certain allergens and as a result decrease their recognition by IgE, but it is important to note that new active sites can be generated during processing.126 Also, the cross reactivity mechanisms of other less studied allergens found in insects, such as AK, still need to be elucidated. Edible insects may be safe for human consumption as they have been part of the human diet for many centuries in some countries. However, scientific research is still needed to protect consumer health and increase innovation and cutting-edge research on insect proteins, peptides and bioactive compounds that may exert health benefits for the wider population.
Author contributions
Zidan Ma: writing and editing. Alan Javier Hernández-Álvarez: supervision, reviewing and editing. Martin Mondor and Francisco Goycoolea Valencia: reviewing and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is supported by the University of Leeds, grant number: 95522790.References
- M. Psarianos, G. Dimopoulos, S. Ojha, A. C. M. Cavini, S. Bußler, P. Taoukis and O. K. Schlüter, Effect of pulsed electric fields on cricket (Acheta domesticus) flour: Extraction yield (protein, fat and chitin) and techno-functional properties, Innovative Food Sci. Emerging Technol., 2022, 76, 102908 CrossRef CAS.
- M. Laroche, V. Perreault, A. Marciniak, A. Gravel, J. Chamberland and A. Doyen, Comparison of conventional and sustainable lipid extraction methods for the production of oil and protein isolate from edible insect meal, Foods, 2019, 8, 572 CrossRef CAS PubMed.
- L. Yi, C. M. Lakemond, L. M. Sagis, V. Eisner-Schadler, A. van Huis and M. A. van Boekel, Extraction and characterisation of protein fractions from five insect species, Food Chem., 2013, 141, 3341–3348 CrossRef CAS PubMed.
- FE Consultation, Dietary protein quality evaluation in human nutrition, FAO Food Nutr. Pap., 2011, 92, 1–66 Search PubMed.
- E. Zielińska, B. Baraniak, M. Karaś, K. Rybczyńska and A. Jakubczyk, Selected species of edible insects as a source of nutrient composition, Food Res. Int., 2015, 77, 460–466 CrossRef.
- A. Gravel and A. Doyen, The use of edible insect proteins in food: Challenges and issues related to their functional properties, Innovative Food Sci. Emerging Technol., 2020, 59, 102272 CrossRef.
- A. Van Huis, J. Van Itterbeeck, H. Klunder, E. Mertens, A. Halloran, G. Muir and P. Vantomme, Edible insects: future prospects for food and feed security, Food and Agriculture Organization of the United Nations, 2013 Search PubMed.
- R. C. Megido, C. Gierts, C. Blecker, Y. Brostaux, É. Haubruge, T. Alabi and F. Francis, Consumer acceptance of insect-based alternative meat products in Western countries, Food Qual. Prefer., 2016, 52, 237–243 CrossRef.
- Y.-S. Choi, T.-K. Kim, H.-D. Choi, J.-D. Park, J.-M. Sung, K.-H. Jeon, H.-D. Paik and Y.-B. Kim, Optimization of replacing pork meat with yellow worm (Tenebrio molitor L.) for frankfurters, Korean J. Food Sci. Anim. Resour., 2017, 37, 617 CrossRef PubMed.
- E. D. Aguilar-Miranda, M. G. López, C. Escamilla-Santana and A. P. Barba de la Rosa, Characteristics of maize flour tortilla supplemented with ground Tenebrio molitor larvae, J. Agric. Food Chem., 2002, 50, 192–195 CrossRef CAS PubMed.
- H. S. G. Tan, Y. T. Verbaan and M. Stieger, How will better products improve the sensory-liking and willingness to buy insect-based foods?, Food Res. Int., 2017, 92, 95–105 CrossRef PubMed.
- F. Del Valle, M. Mena and H. Bourges, An investigation into insect protein 1, J. Food Process. Preserv., 1982, 6, 99–110 CrossRef CAS.
- B. Purschke, H. Brüggen, R. Scheibelberger and H. Jäger, Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.), Eur. Food Res. Technol., 2018, 244, 269–280 CrossRef CAS.
- B. D. Choi, N. A. Wong and J.-H. Auh, Defatting and sonication enhances protein extraction from edible insects, Korean J. Food Sci. Anim. Resour., 2017, 37, 955 Search PubMed.
- P. Sousa, S. Borges and M. Pintado, Enzymatic hydrolysis of insect Alphitobius diaperinus towards the development of bioactive peptide hydrolysates, Food Funct., 2020, 11, 3539–3548 RSC.
- K. Majumder and J. Wu, Purification and characterisation of angiotensin I converting enzyme (ACE) inhibitory peptides derived from enzymatic hydrolysate of ovotransferrin, Food Chem., 2011, 126, 1614–1619 CrossRef CAS PubMed.
- J. Liu, Z. Yu, W. Zhao, S. Lin, E. Wang, Y. Zhang, H. Hao, Z. Wang and F. Chen, Isolation and identification of angiotensin-converting enzyme inhibitory peptides from egg white protein hydrolysates, Food Chem., 2010, 122, 1159–1163 CrossRef CAS.
- J. Chen, S. Liu, R. Ye, G. Cai, B. Ji and Y. Wu, Angiotensin-I converting enzyme (ACE) inhibitory tripeptides from rice protein hydrolysate: Purification and characterization, J. Funct. Foods, 2013, 5, 1684–1692 CrossRef CAS.
- S. Himaya, D.-H. Ngo, B. Ryu and S.-K. Kim, An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress, Food Chem., 2012, 132, 1872–1882 CrossRef CAS.
- L. Vercruysse, G. Smagghe, G. Herregods and J. Van Camp, ACE inhibitory activity in enzymatic hydrolysates of insect protein, J. Agric. Food Chem., 2005, 53, 5207–5211 CrossRef CAS PubMed.
- F. Hall and A. Liceaga, Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding, J. Funct. Foods, 2020, 64, 103634 CrossRef CAS.
- P. Cohen and M. Goedert, GSK3 inhibitors: development and therapeutic potential, Nat. Rev. Drug Discovery, 2004, 3, 479–487 CrossRef CAS PubMed.
- F. Hall, P. E. Johnson and A. Liceaga, Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein, Food Chem., 2018, 262, 39–47 CrossRef CAS PubMed.
- L. De Marchi, A. Wangorsch and G. Zoccatelli, Allergens from edible insects: Cross-reactivity and effects of processing, Curr. Allergy Asthma Rep., 2021, 21, 1–12 CrossRef PubMed.
- H. Ozawa, K. Umezawa, M. Takano, S. Ishizaki, S. Watabe and Y. Ochiai, Structural and dynamical characteristics of tropomyosin epitopes as the major allergens in shrimp, Biochem. Biophys. Res. Commun., 2018, 498, 119–124 CrossRef CAS PubMed.
- H. Ozawa, S. Watabe and Y. Ochiai, Thermodynamic characterization of muscle tropomyosins from marine invertebrates, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2011, 160, 64–71 CrossRef CAS PubMed.
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Microwaves in organic synthesis. Thermal and non-thermal microwave effects, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- S. Ketnawa and A. M. Liceaga, Effect of microwave treatments on antioxidant activity and antigenicity of fish frame protein hydrolysates, Food Bioprocess Technol., 2017, 10, 582–591 CrossRef CAS.
- B. Julieta Ramos-Elorduy, M. José Manuel Pino and C. Víctor Hugo Martínez, Could grasshoppers be a nutritive meal?, Food Nutr. Sci., 2012, 2012, 17511 Search PubMed.
- P. Sarkar, G. Valacchi and R. K. Duary, Proteome composition and profiling of bioactive peptides of edible Antheraea assamensis pupae by sequential enzymatic digestion and kinetic modeling of in vitro gastrointestinal digestion, Eur. Food Res. Technol., 2021, 248, 1–23 Search PubMed.
- A. J. Hernández-Álvarez, M. G. Nosworthy and M. Mondor, in Plant Protein Foods, Springer, 2022, pp. 343–379, DOI:10.1007/978-3-030-91206-2_12.
- J. Ramos-Elorduy, Insects: a sustainable source of food?, Ecol. Food Nutr., 1997, 36, 247–276 CrossRef.
- J. N. Kinyuru, G. M. Kenji, S. M. Njoroge and M. Ayieko, Effect of processing methods on the in vitro protein digestibility and vitamin content of edible winged termite (Macrotermes subhylanus) and grasshopper (Ruspolia differens), Food Bioprocess Technol., 2010, 3, 778–782 CrossRef CAS.
- T. Longvah, K. Mangthya and P. Ramulu, Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae, Food Chem., 2011, 128, 400–403 CrossRef CAS PubMed.
- L. Yi, M. A. Van Boekel, S. Boeren and C. M. Lakemond, Protein identification and in vitro digestion of fractions from Tenebrio molitor, Eur. Food Res. Technol., 2016, 242, 1285–1297 CrossRef CAS.
- K. C. Verhoeckx, S. van Broekhoven, C. F. den Hartog-Jager, M. Gaspari, G. A. de Jong, H. J. Wichers, E. van Hoffen, G. F. Houben and A. C. Knulst, House dust mite (Der p 10) and crustacean allergic patients may react to food containing Yellow mealworm proteins, Food Chem. Toxicol., 2014, 65, 364–373 CrossRef CAS PubMed.
- E. Aruçi, J.-M. Saliou, J.-F. Ferveur and L. Briand, Proteomic Characterization of Drosophila melanogaster Proboscis, Biology, 2022, 11, 1687 CrossRef PubMed.
- Z.-H. Zhou, H.-J. Yang, M. Chen, C.-F. Lou, Y.-Z. Zhang, K.-P. Chen, Y. Wang, M.-L. Yu, F. Yu and J.-Y. Li, Comparative proteomic analysis between the domesticated silkworm (Bombyx mori) reared on fresh mulberry leaves and on artificial diet, J. Proteome Res., 2008, 7, 5103–5111 CrossRef CAS PubMed.
- J. Li, S. H. Hosseini Moghaddam, X. Chen, M. Chen and B. Zhong, Shotgun strategy-based proteome profiling analysis on the head of silkworm Bombyx mori, Amino Acids, 2010, 39, 751–761 CrossRef CAS PubMed.
- P. Zhang, Y. Aso, H. Jikuya, T. Kusakabe, J. M. Lee, Y. Kawaguchi, K. Yamamoto, Y. Banno and H. Fujii, Proteomic Profiling of the Silkworm Skeletal Muscle Proteins during Larval− Pupal Metamorphosis, J. Proteome Res., 2007, 6, 2295–2303 CrossRef CAS PubMed.
- J. R. dos Santos Pinto, E. G. Fox, D. M. Saidemberg, L. D. Santos, A. R. da Silva Menegasso, E. Costa-Manso, E. A. Machado, O. C. Bueno and M. S. Palma, Proteomic view of the venom from the fire ant Solenopsis invicta Buren, J. Proteome Res., 2012, 11, 4643–4653 CrossRef CAS PubMed.
- Y. Pauchet, A. Muck, A. Svatoš, D. G. Heckel and S. Preiss, Mapping the larval midgut lumen proteome of Helicoverpa armigera, a generalist herbivorous insect, J. Proteome Res., 2008, 7, 1629–1639 CrossRef CAS PubMed.
- M. Mishyna, J. K. Keppler and J. Chen, Techno-functional properties of edible insect proteins and effects of processing, Curr. Opin. Colloid Interface Sci., 2021, 56, 101508 CrossRef CAS.
- F. Zhang, Y. Xu, B. Kong, Q. Chen, F. Sun, H. Zhang and Q. Liu, Comparative study of two types of pre-extraction treatment (drying or non-drying) on physicochemical, structural and functional properties of extracted insect proteins from Tenebrio molitor larvae, Curr. Res. Food Sci., 2022, 5, 1570–1580 CrossRef CAS PubMed.
- J. Williams, J. Williams, A. Kirabo, D. Chester and M. Peterson, in Insects as sustainable food ingredients, Elsevier, 2016, pp. 61–84, DOI:10.1016/B978-0-12-802856-8.00003-X.
- I. D. Nwachukwu and R. E. Aluko, Food protein structures, functionality and product development, 2021 Search PubMed.
- E. Zielińska, M. Karaś and B. Baraniak, Comparison of functional properties of edible insects and protein preparations thereof, LWT – Food Sci. Technol., 2018, 91, 168–174 CrossRef.
- S. Bußler, B. A. Rumpold, E. Jander, H. M. Rawel and O. K. Schlüter, Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae, Heliyon, 2016, 2, e00218 CrossRef PubMed.
- K. T. Rudall and W. Kenchington, Arthropod silks: the problem of fibrous proteins in animal tissues, Annu. Rev. Entomol., 1971, 16, 73–96 CrossRef CAS.
- J. E. Kinsella, Functional properties of proteins: possible relationships between structure and function in foams, Food Chem., 1981, 7, 273–288 CrossRef CAS.
- N. Chatsuwan, Y. Puechkamut and P. Pinsirodom, Characterization, Functionality and Antioxidant Activity of Water-Soluble Proteins Extracted from Bombyx mori Linn, Curr. Appl. Sci. Technol., 2018, 18, 83–96 Search PubMed.
- F. G. Hall, O. G. Jones, M. E. O'Haire and A. M. Liceaga, Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates, Food Chem., 2017, 224, 414–422 CrossRef CAS PubMed.
- M. Aremo and O. Olaofe, Functional properties of some Nigerian varieties of legume seed flours and flour concentration effect on foaming and gelation properties, J. Food Technol., 2007, 109–115 Search PubMed.
- A. Aryee, D. Agyei and C. Udenigwe, in Proteins in food processing, Elsevier, 2018, pp. 27–45, DOI:10.1016/B978-0-08-100722-8.00003-6.
- X. Zhao, J. L. Vázquez-Gutiérrez, D. P. Johansson, R. Landberg and M. Langton, Yellow mealworm protein for food purposes-extraction and functional properties, PLoS One, 2016, 11, e0147791 CrossRef PubMed.
- G. R. Ziegler and E. A. Foegeding, in Advances in food and nutrition research, Elsevier, 1990, vol. 34, pp. 203–298 Search PubMed.
- C. Foo, E. Bini, J. Hensman, D. Knight, R. Lewis and D. Kaplan, Role of pH and charge on silk protein assembly in insects and spiders, Appl. Phys. A, 2006, 82, 223–233 CrossRef CAS.
- Y. N. Sreerama, V. B. Sashikala, V. M. Pratape and V. Singh, Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality, Food Chem., 2012, 131, 462–468 CrossRef CAS.
- D. A. T. Sosa and V. Fogliano, Potential of insect-derived ingredients for food applications, in Insect Physiology and Ecology, InTech, Rijeka, 2017, pp. 215–231, DOI:10.5772/67318.
- C. Dai, H. Ma, L. Luo and X. Yin, Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate, Eur. Food Res. Technol., 2013, 236, 681–689 CrossRef CAS.
- J. Jia, Q. Wu, H. Yan and Z. Gui, Purification and molecular docking study of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide from alcalase hydrolysate of ultrasonic-pretreated silkworm pupa (Bombyx mori) protein, Process Biochem., 2015, 50, 876–883 CrossRef CAS.
- A. Gandhi, K. Joshi, K. Jha, V. Parihar, D. Srivastav, P. Raghunadh, J. Kawalkar, S. Jain and R. Tripathi, Studies on alternative solvents for the extraction of oil-I soybean, Int. J. Food Sci. Technol., 2003, 38, 369–375 CrossRef CAS.
- R. Dennell, A study of an insect cuticle: the larval cuticle of Sarcophaga falculata Pand.(Diptera), Proc. R. Soc. London, Ser. B, 1946, 133, 348–373 CAS.
- Y. P. Huang, T. Komiya and K. Maruyama, Actin isoforms of insect muscles: Two-dimensional electrophoresis studies, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1984, 79, 511–514 CrossRef.
- M. M. Rahman, B. Byanju and B. P. Lamsal, Protein, lipid, and chitin fractions from insects: Method of extraction, functional properties, and potential applications, Crit. Rev. Food Sci. Nutr., 2023, 1–17 Search PubMed.
- M. Mishyna, J.-J. I. Martinez, J. Chen and O. Benjamin, Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera), Food Res. Int., 2019, 116, 697–706 CrossRef CAS PubMed.
- M. H. Sipponen, O. E. Mäkinen, K. Rommi, R.-L. Heiniö, U. Holopainen-Mantila, S. Hokkanen, T. K. Hakala and E. Nordlund, Biochemical and sensory characteristics of the cricket and mealworm fractions from supercritical carbon dioxide extraction and air classification, Eur. Food Res. Technol., 2018, 244, 19–29 CrossRef CAS.
- F. Toldrá, M. Reig, M.-C. Aristoy and L. Mora, Generation of bioactive peptides during food processing, Food Chem., 2018, 267, 395–404 CrossRef PubMed.
- R. Yang, X. Zhao, Z. Kuang, M. Ye, G. Luo, G. Xiao, S. Liao, L. Li and Z. Xiong, Optimization of antioxidant peptide production in the hydrolysis of silkworm (Bombyx mori L.) pupa protein using response surface methodology, J. Food Agric. Environ., 2013, 11, 952–956 CAS.
- S. Yoon, N. A. Wong, M. Chae and J.-H. Auh, Comparative characterization of protein hydrolysates from three edible insects: Mealworm larvae, adult crickets, and silkworm pupae, Foods, 2019, 8, 563 CrossRef CAS PubMed.
- D. J. Daroit and A. Brandelli, In vivo bioactivities of food protein-derived peptides–a current review, Curr. Opin. Food Sci., 2021, 39, 120–129 CrossRef CAS.
- K. Singh and R. Jayasomu, Bombyx mori–A Review of its Potential as a Medicinal Insect, Pharm. Biol., 2002, 40, 28–32 CrossRef.
- F. M. de Matos, J. T. J. G. de Lacerda, G. Zanetti and R. J. S. de Castro, Production of black cricket protein hydrolysates with α-amylase, α-glucosidase and angiotensin I-converting enzyme inhibitory activities using a mixture of proteases, Biocatal. Agric. Biotechnol., 2022, 39, 102276 CrossRef CAS.
- A. J. da Silva Lucas, L. M. de Oliveira, M. Da Rocha and C. Prentice, Edible insects: An alternative of nutritional, functional and bioactive compounds, Food Chem., 2020, 311, 126022 CrossRef PubMed.
- L. M. Real Hernandez and E. Gonzalez de Mejia, Enzymatic production, bioactivity, and bitterness of chickpea (Cicer arietinum) peptides, Compr. Rev. Food Sci. Food Saf., 2019, 18, 1913–1946 CrossRef CAS PubMed.
- L. Vercruysse, G. Smagghe, T. Beckers and J. Van Camp, Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis, Food Chem., 2009, 114, 38–43 CrossRef CAS.
- E. Zielińska, M. Karaś and A. Jakubczyk, Antioxidant activity of predigested protein obtained from a range of farmed edible insects, Int. J. Food Sci. Technol., 2017, 52, 306–312 CrossRef.
- C. S. Teixeira, C. Villa, J. Costa, I. M. Ferreira and I. Mafra, Edible Insects as a Novel Source of Bioactive Peptides: A Systematic Review, Foods, 2023, 12, 2026 CrossRef CAS PubMed.
- L. Vercruysse, G. Smagghe, T. Matsui and J. Van Camp, Purification and identification of an angiotensin I converting enzyme (ACE) inhibitory peptide from the gastrointestinal hydrolysate of the cotton leafworm, Spodoptera littoralis, Process Biochem., 2008, 43, 900–904 CrossRef CAS.
- J. H. Lee, T.-K. Kim, H. I. Yong, J. Y. Cha, K.-M. Song, H. G. Lee, J.-G. Je, M.-C. Kang and Y.-S. Choi, Peptides inhibiting angiotensin-I-converting enzyme: Isolation from flavourzyme hydrolysate of Protaetia brevitarsis larva protein and identification, Food Chem., 2023, 399, 133897 CrossRef CAS PubMed.
- S. Charoensiddhi, C. Franco, P. Su and W. Zhang, Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction, J. Appl. Phycol., 2015, 27, 2049–2058 CrossRef CAS.
- E. Nguyen, O. Jones, Y. H. B. Kim, S. Martin-Gonzalez and A. M. Liceaga, Impact of microwave-assisted enzymatic hydrolysis on functional and antioxidant properties of rainbow trout Oncorhynchus mykiss by-products, Fish. Sci., 2017, 83, 317–331 CrossRef CAS.
- E. R. Coscueta, M. M. Amorim, G. B. Voss, B. B. Nerli, G. A. Picó and M. E. Pintado, Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis, Food Chem., 2016, 198, 36–44 CrossRef CAS PubMed.
- P. Jäkälä and H. Vapaatalo, Antihypertensive peptides from milk proteins, Pharmaceuticals, 2010, 3, 251–272 CrossRef PubMed.
- H. Fujita and M. Yoshikawa, LKPNM: a prodrug-type ACE-inhibitory peptide derived from fish protein, Immunopharmacology, 1999, 44, 123–127 CrossRef CAS PubMed.
- M. Miguel, I. Recio, J. Gomez-Ruiz, M. Ramos and R. López-Fandiño, Angiotensin I–converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis, J. Food Prot., 2004, 67, 1914–1920 CrossRef CAS PubMed.
- W. D. Devi, R. Bonysana, K. Kapesa, A. K. Rai, P. K. Mukherjee and Y. Rajashekar, Potential of edible insects as source of functional foods: biotechnological approaches for improving functionality, Syst. Microbiol. Biomanuf., 2022, 2, 461–472 CrossRef CAS.
- R. A. Herman, C.-H. Yan, J.-Z. Wang, X.-M. Xun, C.-K. Wu, Z.-N. Li, E. Ayepa, S. You, L.-C. Gong and J. Wang, Insight into the silkworm pupae: Modification technologies and functionality of the protein and lipids, Trends Food Sci. Technol., 2022, 129, 408–420 CrossRef CAS.
- D. Martínez-Maqueda, B. Miralles, I. Recio and B. Hernández-Ledesma, Antihypertensive peptides from food proteins: a review, Food Funct., 2012, 3, 350–361 RSC.
- L. Vercruysse, J. Van Camp, N. Morel, P. Rouge, G. Herregods and G. Smagghe, Ala-Val-Phe and Val-Phe: ACE inhibitory peptides derived from insect protein with antihypertensive activity in spontaneously hypertensive rats, Peptides, 2010, 31, 482–488 CrossRef CAS PubMed.
- J. Jia, H. Ma, W. Zhao, Z. Wang, W. Tian, L. Luo and R. He, The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein, Food Chem., 2010, 119, 336–342 CrossRef CAS.
- B. Zhang, J. Liu, H. Wen, F. Jiang, E. Wang and T. Zhang, Structural requirements and interaction mechanisms of ACE inhibitory peptides: molecular simulation and thermodynamics studies on LAPYK and its modified peptides, Food Sci. Hum. Wellness, 2022, 11, 1623–1630 CrossRef CAS.
- M. Mirzaei, S. Mirdamadi, M. Safavi and N. Soleymanzadeh, The stability of antioxidant and ACE-inhibitory peptides as influenced by peptide sequences, LWT – Food Sci. Technol., 2020, 130, 109710 CrossRef CAS.
- Y. Nakamura, N. Yamamoto, K. Sakai, A. Okubo, S. Yamazaki and T. Takano, Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk, J. Dairy Sci., 1995, 78, 777–783 CrossRef CAS PubMed.
- M. Mardani, K. Badakné, J. Farmani and R. E. Aluko, Antioxidant peptides: Overview of production, properties, and applications in food systems, Compr. Rev. Food Sci. Food Saf., 2023, 22, 46–106 CrossRef PubMed.
- J.-A. Koh, J.-H. Ong, F. Abd Manan, K.-Y. Ee, F.-C. Wong and T.-T. Chai, Discovery of bifunctional anti-DPP-IV and anti-ACE peptides from housefly larval proteins after in silico gastrointestinal digestion, Biointerface Res. Appl. Chem., 2022, 12, 4929–4944 CAS.
- E. Zielińska, M. Karaś, B. Baraniak and A. Jakubczyk, Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects, Eur. Food Res. Technol., 2020, 246, 1361–1369 CrossRef.
- F. Rivero-Pino, F. J. Espejo-Carpio, R. Pérez-Gálvez, A. Guadix and E. M. Guadix, Effect of ultrasound pretreatment and sequential hydrolysis on the production of Tenebrio molitor antidiabetic peptides, Food Bioprod. Process., 2020, 123, 217–224 CrossRef CAS.
- A. Brai, C. I. Trivisani, C. Vagaggini, R. Stella, R. Angeletti, G. Iovenitti, V. Francardi and E. Dreassi, Proteins from Tenebrio molitor: An interesting functional ingredient and a source of ACE inhibitory peptides, Food Chem., 2022, 393, 133409 CrossRef CAS PubMed.
- T. David-Birman, G. Raften and U. Lesmes, Effects of thermal treatments on the colloidal properties, antioxidant capacity and in vitro proteolytic degradation of cricket flour, Food Hydrocolloids, 2018, 79, 48–54 CrossRef CAS.
- L. A. Santiago, O. M. Fadel and G. M. Tavares, How does the thermal-aggregation behavior of black cricket protein isolate affect its foaming and gelling properties?, Food Hydrocolloids, 2021, 110, 106169 CrossRef CAS.
- A. Araiza-Calahorra, M. Mondor, C. Boesch, C. Orfila, F. M. Goycoolea and A. J. Hernández-Álvarez, in Current advances for development of functional foods modulating inflammation and oxidative stress, Elsevier, 2022, pp. 137–164 Search PubMed.
- L. Tejada, L. Buendía-Moreno, I. Hernández, A. Abellán, J. M. Cayuela, E. Salazar and E. Bueno-Gavilá, Bioactivities of Mealworm (Alphitobius diaperinus L.) Larvae Hydrolysates Obtained from Artichoke (Cynara scolymus L.) Proteases, Biology, 2022, 11, 631 CrossRef CAS PubMed.
- E. Zielińska, B. Baraniak and M. Karaś, Identification of antioxidant and anti–inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria), Int. J. Food Sci. Technol., 2018, 53, 2542–2551 CrossRef.
- R. Aleksis, K. Jaudzems, R. Muceniece and E. Liepinsh, Lunasin is a redox sensitive intrinsically disordered peptide with two transiently populated α-helical regions, Peptides, 2016, 85, 56–62 CrossRef CAS PubMed.
- M. Manzoor, J. Singh and A. Gani, Exploration of bioactive peptides from various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies, Food Chem., 2022, 373, 131395 CrossRef CAS PubMed.
- F. Luo, Y. Fu, L. Ma, H. Dai, H. Wang, H. Chen, H. Zhu, Y. Yu, Y. Hou and Y. Zhang, Exploration of dipeptidyl peptidase-IV (DPP-IV) inhibitory peptides from silkworm pupae (Bombyx mori) proteins based on in silico and in vitro assessments, J. Agric. Food Chem., 2022, 70, 3862–3871 CrossRef CAS PubMed.
- F. Chen, H. Jiang, Y. Lu, W. Chen and G. Huang, Identification and in silico analysis of antithrombotic peptides from the enzymatic hydrolysates of Tenebrio molitor larvae, Eur. Food Res. Technol., 2019, 245, 2687–2695 CrossRef CAS.
- J. Ong, C. Liang, W. Wong, F. Wong and T. Chai, Multi-target anti-sars-cov-2 peptides from mealworm proteins: An in silico study, Malays. J. Biochem. Mol. Biol., 2021, 83–91 Search PubMed.
- X. Yang, K. Chen, H. Liu, Y. Zhang and Y. Luo, Purification and identification of peptides with high angiotensin-I converting enzyme (ACE) inhibitory activity from honeybee pupae (Apis mellifera) hydrolysates with in silico gastrointestinal digestion, Eur. Food Res. Technol., 2019, 245, 535–544 CrossRef CAS.
- A. Iwaniak, M. Darewicz, D. Mogut and P. Minkiewicz, Elucidation of the role of in silico methodologies in approaches to studying bioactive peptides derived from foods, J. Funct. Foods, 2019, 61, 103486 CrossRef CAS.
- Q. Wu, J. Jia, H. Yan, J. Du and Z. Gui, A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: biochemical characterization and molecular docking study, Peptides, 2015, 68, 17–24 CrossRef CAS PubMed.
- M. Tao, H. Sun, L. Liu, X. Luo, G. Lin, R. Li, Z. Zhao and Z. Zhao, Graphitized porous carbon for rapid screening of angiotensin-converting enzyme inhibitory peptide GAMVVH from silkworm pupa protein and molecular insight into inhibition mechanism, J. Agric. Food Chem., 2017, 65, 8626–8633 CrossRef CAS PubMed.
- S. Khammuang, R. Sarnthima and K. Sanachai, Purification and identification of novel antioxidant peptides from silkworm pupae (Bombyx mori) protein hydrolysate and molecular docking study, Biocatal. Agric. Biotechnol., 2022, 42, 102367 CrossRef CAS.
- R. J. S. de Castro, A. Ohara, J. G. dos Santos Aguilar and M. A. F. Domingues, Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges, Trends Food Sci. Technol., 2018, 76, 82–89 CrossRef.
- F. Papia, C. Bellia and C. G. Uasuf, A panallergen that causes a wordwide allergic problem, Allergy Asthma Proc., 2021, 42, 145–151 CrossRef PubMed.
- S. Zhao, F. Pan, S. Cai, J. Yi, L. Zhou and Z. Liu, Secrets behind Protein Sequences: Unveiling the Potential Reasons for Varying Allergenicity Caused by Caseins from Cows, Goats, Camels, and Mares Based on Bioinformatics Analyses, Int. J. Mol. Sci., 2023, 24, 2481 CrossRef CAS PubMed.
- M. Abdelmoteleb, L. K. Palmer, J. Marsh, P. Johnson and R. E. Goodman, Bioinformatics and Proteomics Evaluations of Potential IgE Cross-Reactive Proteins in Novel Edible Insects and Shrimp for Food Safety, J. Allergy Clin. Immunol., 2019, 143, AB237 CrossRef.
- J. C. Ribeiro, L. M. Cunha, B. Sousa-Pinto and J. Fonseca, Allergic risks of consuming edible insects: A systematic review, Mol. Nutr. Food Res., 2018, 62, 1700030 CrossRef PubMed.
- F. G. Hall and A. M. Liceaga, Isolation and proteomic characterization of tropomyosin extracted from edible insect protein, Food Chem.: Mol. Sci., 2021, 3, 100049 CAS.
- C.-L. Yao, P.-F. Ji, P. Kong, Z.-Y. Wang and J.-H. Xiang, Arginine kinase from Litopenaeus vannamei: cloning, expression and catalytic properties, Fish Shellfish Immunol., 2009, 26, 553–558 CrossRef CAS PubMed.
- F. Francis, V. Doyen, F. Debaugnies, G. Mazzucchelli, R. Caparros, T. Alabi, C. Blecker, E. Haubruge and F. Corazza, Limited cross reactivity among arginine kinase allergens from mealworm and cricket edible insects, Food Chem., 2019, 276, 714–718 CrossRef CAS PubMed.
- S. de Gier and K. Verhoeckx, Insect (food) allergy and allergens, Mol. Immunol., 2018, 100, 82–106 CrossRef CAS PubMed.
- P. Phiriyangkul, C. Srinroch, C. Srisomsap, D. Chokchaichamnankit and P. Punyarit, Effect of food thermal processing on allergenicity proteins in Bombay locust (Patanga succincta), Int. J. Food Eng., 2015, 1, 23–28 Search PubMed.
- X. Dong, J. Wang and V. Raghavan, Impact of microwave processing on the secondary structure, in vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei) proteins, Food Chem., 2021, 337, 127811 CrossRef CAS PubMed.
- N. Sun, Y. Liu, K. Liu, S. Wang, Q. Liu and S. Lin, Gastrointestinal fate of food allergens and its relationship with allergenicity, Compr. Rev. Food Sci. Food Saf., 2022, 21, 3376–3404 CrossRef CAS PubMed.
- A. Osasona and O. Olaofe, Nutritional and functional properties of Cirina forda larva from Ado-Ekiti, Nigeria, Afr. J. Food Sci., 2010, 4, 775–777 CAS.
- B. Assielou, E. A. Due, M. D. Koffi, S. Dabonne and P. L. Kouame, Oryctes owariensis larvae as good alternative protein source: Nutritional and functional properties, Annu. Res. Rev. Biol., 2015, 1–9, DOI:10.9734/ARRB/2015/19093.
- O. Omotoso, Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina forda (Westwood)(Lepidoptera: Saturniidae), J. Zhejiang Univ., Sci., B, 2006, 7, 51–55 CrossRef CAS PubMed.
- N. Udomsil, S. Imsoonthornruksa, C. Gosalawit and M. Ketudat-Cairns, Nutritional values and functional properties of house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus), Food Sci. Technol. Res., 2019, 25, 597–605 CrossRef CAS.
- R. V. Amarender, K. Bhargava, A. T. Dossey and S. Gamagedara, Lipid and protein extraction from edible insects–Crickets (Gryllidae), LWT – Food Sci. Technol., 2020, 125, 109222 CrossRef CAS.
- A. K. Ndiritu, J. N. Kinyuru, G. M. Kenji and P. N. Gichuhi, Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate, J. Food Meas. Charact., 2017, 11, 2013–2021 CrossRef.
- B. Purschke, H. Tanzmeister, P. Meinlschmidt, S. Baumgartner, K. Lauter and H. Jäger, Recovery of soluble proteins from migratory locust (Locusta migratoria) and characterisation of their compositional and techno-functional properties, Food Res. Int., 2018, 106, 271–279 CrossRef CAS PubMed.
- T.-K. Kim, H. I. Yong, H. H. Chun, M.-A. Lee, Y.-B. Kim and Y.-S. Choi, Changes of amino acid composition and protein technical functionality of edible insects by extracting steps, J. Asia-Pac. Entomol., 2020, 23, 298–305 CrossRef.
- C. Azagoh, F. Ducept, R. Garcia, L. Rakotozafy, M.-E. Cuvelier, S. Keller, R. Lewandowski and S. Mezdour, Extraction and physicochemical characterization of Tenebrio molitor proteins, Food Res. Int., 2016, 88, 24–31 CrossRef CAS PubMed.
- A. R. F. Mba, E. David-Briand, M. Viau, A. Riaublanc, G. Kansci and C. Genot, Protein extraction yield, lipid composition, and emulsifying properties of aqueous extracts of Rhynchophorus phoenicis larvae extracted at pH 3.0 to 10.0, Future Foods, 2021, 4, 100037 CrossRef.
- T.-K. Kim, H. I. Yong, C. H. Jeong, S. G. Han, Y.-B. Kim, H.-D. Paik and Y.-S. Choi, Technical functional properties of water-and salt-soluble proteins extracted from edible insects, Food Sci. Anim. Resour., 2019, 39, 643 CrossRef PubMed.
- P. Kurdi, P. Chaowiwat, J. Weston and C. Hansawasdi, Studies on Microbial Quality, Protein Yield, and Antioxidant Properties of Some Frozen Edible Insects, Int. J. Food Sci., 2021, 2021, 5580976 Search PubMed.
- B. K. Mintah, R. He, A. A. Agyekum, M. Dabbour, M. K. Golly and H. Ma, Edible insect protein for food applications: Extraction, composition, and functional properties, J. Food Process Eng., 2020, 43, e13362 Search PubMed.
- L. S. Queiroz, M. Regnard, F. Jessen, M. A. Mohammadifar, J. J. Sloth, H. O. Petersen, F. Ajalloueian, C. M. C. Brouzes, W. Fraihi and H. Fallquist, Physico-chemical and colloidal properties of protein extracted from black soldier fly (Hermetia illucens) larvae, Int. J. Biol. Macromol., 2021, 186, 714–723 CrossRef CAS PubMed.
- Q.-Y. Wu, J.-Q. Jia, G.-X. Tan, J.-L. Xu and Z.-Z. Gui, Physicochemical properties of silkworm larvae protein isolate and gastrointestinal hydrolysate bioactivities, Afr. J. Biotechnol., 2011, 10, 6145–6153 CAS.
- W. Wang, N. Wang, Y. Zhou, Y. Zhang, L. Xu, J. Xu, F. Feng and G. He, Isolation of a novel peptide from silkworm pupae protein components and interaction characteristics to angiotensin I-converting enzyme, Eur. Food Res. Technol., 2011, 232, 29–38 CrossRef CAS.
- W. Wang, N. Wang and Y. Zhang, in Food and Nutrition Sciences, 2014, vol. 5 Search PubMed.
- X. Li, Y. Li, X. Huang, J. Zheng, F. Zhang and J. Kan, Identification and characterization of a novel angiotensin I-converting enzyme inhibitory peptide (ACEIP) from silkworm pupa, Food Sci. Biotechnol., 2014, 23, 1017–1023 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |