 Open Access Article
Open Access ArticleProtein-based nutritional strategies to manage the development of diabetes: evidence and challenges in human studies
Sergio
Montserrat-de la Paz
 ,
Antonio
D. Miguel-Albarreal
,
Teresa
Gonzalez-de la Rosa
,
Antonio
D. Miguel-Albarreal
,
Teresa
Gonzalez-de la Rosa
 ,
Maria C.
Millan-Linares
and
Fernando
Rivero-Pino
,
Maria C.
Millan-Linares
and
Fernando
Rivero-Pino
 *
*
Department of Medical Biochemistry, Molecular Biology, and Immunology, School of Medicine, University of Seville, Av. Sanchez Pizjuan s/n, 41009 Seville, Spain. E-mail: frivero1@us.es
First published on 11th October 2023
Abstract
Type 2 diabetes mellitus (T2DM) is one of the most prevalent diseases in modern society, governed by both genetic and environmental factors, such as nutritional habits. This metabolic disorder is characterized by insulin resistance, which is related to high blood glucose levels, implying negative health effects in humans, hindering the healthy ageing of people. The relationship between food and health is clear, and the ingestion of specific nutrients modulates some physiological processes, potentially implying biologically relevant changes, which can translate into a health benefit. This review aims to summarize human studies published in which the purpose was to investigate the effect of protein ingestion (in native state or as hydrolysates) on human metabolism. Overall, several studies showed how protein ingestion might induce a decrease of glucose concentration in the postprandial state (area under the curve), although it is highly dependent on the source and the dose. Other studies showed no biological effects upon protein consumption, mostly with fish-derived products. In addition, the major challenges and perspectives in this research field are highlighted, suggesting the future directions, towards which scientists should focus on. The dietary intake of proteins has been proven to likely exert a beneficial effect on diabetes-related parameters, which can have a biological relevance in the prevention and pre-treatment of diabetes. However, the number of well-designed human studies carried out to date to demonstrate the effects of specific proteins or protein hydrolysates in vivo is still scarce.
1. Introduction
Proteins are one of the main components of human diets. It has been described how the gastrointestinal digestion of these macromolecules results in the release of peptides after being cleaved by digestive proteases (e.g., trypsin, chymotrypsin, and pancreatin). These released peptides would have an impact on human physiology at numerous levels, by interacting with different targets.1 In the same line, the processing of proteins by enzymatic hydrolysis or fermentation allows the release of peptides prior to oral ingestion, which might contain different sequences compared to the pool of peptides released by human digestion, based on the specificity of the proteases or the microorganisms employed in the production process of these so-called protein hydrolysates.2,3 The length and molecular features of these peptides would differently interact with enzymes, receptors, circulating hormones, etc., having potentially a more pronounced benefit to human health.4On the other hand, the metabolism of carbohydrates, which in food are usually found as polysaccharides or disaccharides, is the process of transforming them into glucose molecules, the most efficient source of energy. This metabolic pathway involves several enzymes and a complex series of metabolic processes.5 Briefly, following the ingestion of the bolus, a group of hormones (incretins), which are mainly gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), are produced by the intestine. These incretins act as endocrine signals to the pancreas, resulting in the release of insulin by the β-cells and the suppression of the release of glucagon in the α-cells.6 As a consequence, the uptake of glucose in the muscles occurs, as well as a lower production of glucose in the liver. The final effect is the decrease in blood glucose, which allows the adequate regulation of postprandial blood glucose levels. The physiological level of incretins is regulated (by their degradation) by the enzyme dipeptidyl peptidase IV (DPP-IV),7 which is mostly found on the luminal surface of enterocytes, meaning that it can interact with the ingested food prior to absorption, and also is found as a circulating enzyme.8 Because of this DPP-IV activity, the circulating GLP-1 half-life is 1 to 2 minutes.9
In the case where there is a lack of ability of the organism to react to the insulin action, or an insufficient production of this hormone, the subject is considered to have insulin resistance. This situation can evolve towards a metabolic disorder called diabetes, and this uncontrol of postprandial glucose level implies high glucose levels in the bloodstream, likely resulting in renal failure, neurological damage and/or cardiovascular disorders.9,10 Type 2 Diabetes Mellitus (T2DM) is one of the most prevalent diseases, affecting more than 537 million people and with estimations of 783 million people becoming affected by 2045.11 This disease can be caused by both genetic and environmental factors, including obesity, because of an imbalance in the concentration of hormones, cytokines and other inflammatory signals.10,12 This metabolic syndrome implies changes in several regulatory pathways in humans. In the following section, some examples are briefly described.
The condition of insulin resistance triggers a defective activity of glucose transporter 4 (GLUT-4), responsible for glucose transfer across the plasma membrane. This transporter is found in the skeletal muscle, as well as in the adipose tissue and myocardium.13 In addition, insulinoma amyloid polypeptide (commonly known as amylin) is commonly found in the pancreatic islets of patients suffering from T2DM.14 Amylin can induce apoptotic cell death in insulin-producing β-cells, an effect that could be relevant in the development of T2DM.15 On the other hand, peptide YY (PYY) is secreted by L cells in the mucosa of the gastrointestinal tract, particularly the colon and rectum, after ingestion. It is believed that its secretion may depend on nerve reflexes, possibly via the vagus nerve. There are other factors that influence the concentration of PYY; for instance, insulin-like growth factor-1 (IGF-1), bombesin, and calcitonin-gene-related peptide increase the amount of PYY and GLP-1 decreases it. It has been proposed that PYY may have significant potential for preservation of β-cell mass and the treatment of diabetes.16 In this sense, PYY has been proposed as a therapeutic gut hormone, although is quickly degraded by the DPP-IV.9 Consequently, the inhibition of DPP-IV, which would have a positive effect on PYY and GLP-1, as previously described, by increasing their half-life, is seen as one of the most promising therapeutic strategies to manage T2DM.
The intake of peptides from protein and protein hydrolysates can play a relevant role in the management of glucose homeostasis, due to their implication at different levels, including positive regulation of incretins (GIP and GLP-1), inhibition of digestive enzymes, inhibition of DPP-IV,17 enhancement of cholecystokinin levels by enteroendocrine cells (a gut hormone regulating food intake),18,19 decrease of body fat, promotion of insulin secretion by pancreatic cells and reduction of glycaemia,20 or inhibition of intestinal glucose uptake, though the underlying mechanisms are not fully defined, as protein digestion products have proved to act as signalling molecules in enteroendocrine cells.21,22 For instance, delayed carbohydrate absorption – which can be achieved by inhibition of digestive hydrolases such as alpha-glucosidase or alpha-amylase – is considered an adequate contributing factor in stimulating GLP-1 secretion, which would ultimately lead to the incretin effect.22,23
Before a patient can be considered to suffer from diabetes, there is a previous disease condition named pre-diabetes. Pre-diabetes is characterized by impaired glucose tolerance, although the relative risks of future stroke are minor.24 On top of that, the prevention and pre-treatment of diabetes mellitus by nutritional interventions following specific dietary guidelines have been demonstrated to be cost-effective, when compared to the treatment of the diseases as such.25 A healthy subject would have less than 100 mg dL−1 in the fasting blood glucose level or below 5.7% in the glycated hemoglobin (A1c) test. Clinically, pre-diabetic patients are those considered to have impaired fasting glucose, characterized by a plasma concentration of 100 to 125 mg dL−1 (5.6–6.9 mmol L−1) or a level of 5.7 to 6.4% in the A1c test. In the case of diabetes, these values would be higher than 126 mg dL−1 and 6.5%, respectively.
In the condition of pre-diabetes, insulin resistance is already present, as well as an impairment of β-cell function. Consequently, the unusual glucose levels in blood might upregulate the markers of chronic inflammation and lead to the production of reactive oxygen species (ROS), which eventually trigger vascular dysfunction. Contrariwise, increased oxidative stress and inflammation might result in insulin resistance and impaired insulin secretion. It is necessary to unravel the mechanisms involved in the development from prediabetes to diabetes, especially how protein and amino acids have an impact on the oxidative and immune status of diabetes.26 Subclinical myocardial injury and a significant prevalence of prediabetes among acute atherosclerotic cardiovascular disease cases show that high-risk prediabetic patients develop atherosclerotic cardiovascular disease before T2DM.27
In addition, there are reports investigating the effect of specific amino acids on the development of metabolic syndrome, as it has been demonstrated that specific functional groups interacting with different targets might lead to impairments. In a nested case-control study including 4754 nondiabetic Japanese subjects who were followed-up for 5 years, the development of T2DM was recorded and the relationship with different parameters was hypothesized. In terms of amino acids, high levels of valine, leucine, isoleucine, phenylalanine, tyrosine, alanine, glutamate, ornithine, and lysine were associated with an increased risk of incident T2DM, in a linear manner. On the contrary, a high glutamine concentration was associated with a decreased risk of incident T2DM.28 Recently, it was reported that phenylalanine alters insulin receptor beta (IRβ) and inactivates insulin signalling and glucose uptake.29 The amount of evidence collected to date is inconclusive and drawing conclusions on the role of specific amino acids is a challenge that needs to be tackled in the near future. On top of that, the existing recommended dietary allowance for protein consumption has been set at 0.8 g per kg per day for young healthy men, while protein consumption of 1.0–1.5 g per kg per day in older adults is able to induce improvements in glycaemic control and muscle mass.30
The number of human studies in which a nutritional intervention is followed to assess whether it has an impact on the plasma levels of key components related to the development of diabetes has increased in recent years, although not all of them allow drawing conclusions because of intrinsic limitations. Nutritional intervention with proteins is relevant for several fields related to nutrition, such as promotion of health, management of diseases or muscle growth, with milk-derived proteins being those most studied, along with fish proteins. Similarly, there is published literature on the effect of the ingestion of peptides (as protein hydrolysates) from these sources, as protein hydrolysates are currently considered as a key component in the food industry, especially in foods for infants and follow-on formula or in the field of sports nutrition. Overall, the effects described in the studies appear promising in terms of modulating human physiology. However, the scientific authorities are promoting the consumption of specific food groups such as vegetables or legumes, while recommending the reduction of foods that contain saturated fats or salt, among others.31 The potential of alternative proteins, such as vegetables, insects or algae, in the prevention or pre-treatment of specific diseases is still to be unravelled.
A systematic review on the nutritional strategies in pre-diabetes32 concluded that until then, ninety-five reports, accounting for more than 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 subjects, had mostly assessed changes in plasma glucose, serum insulin, serum lipid profile, body mass index, and body weight following different types of intervention (e.g., low calorie diet, low glycaemic index diet, specific foods, and a combination of diet and exercise). Overall, these authors mentioned that in more than 50% of the interventions, improvements of the parameters assessed were found, but the variability among humans should be taken into consideration, as specific genetic variants, and some other factors (age, weight, and lifestyle) have also a relevant impact on the management of pre-diabetes. In this line, it has been also reported, for instance, how the quality and quantity of protein intake influence the incidence of T2DM in coronary heart disease patients.33 According to these authors, enriching a diet with plant-based proteins could reduce the risk of developing T2DM, based on analysis in 436 nondiabetic patients, of which 106 developed the disease. However, a detailed assessment of the methodology of each study should be carried out to perform an analysis of the methodology quality and the risk of bias.
000 subjects, had mostly assessed changes in plasma glucose, serum insulin, serum lipid profile, body mass index, and body weight following different types of intervention (e.g., low calorie diet, low glycaemic index diet, specific foods, and a combination of diet and exercise). Overall, these authors mentioned that in more than 50% of the interventions, improvements of the parameters assessed were found, but the variability among humans should be taken into consideration, as specific genetic variants, and some other factors (age, weight, and lifestyle) have also a relevant impact on the management of pre-diabetes. In this line, it has been also reported, for instance, how the quality and quantity of protein intake influence the incidence of T2DM in coronary heart disease patients.33 According to these authors, enriching a diet with plant-based proteins could reduce the risk of developing T2DM, based on analysis in 436 nondiabetic patients, of which 106 developed the disease. However, a detailed assessment of the methodology of each study should be carried out to perform an analysis of the methodology quality and the risk of bias.
In the following sub-sections, nutritional interventions on humans ingesting proteins or peptides are described, classified in protein sources. Reports on healthy subjects as well as subjects suffering from specific conditions (e.g., pre-diabetes and overweight) have been included, as they might have a different response in the regulation of parameters of interest (related to glucose homeostasis), based on the inflammatory status of the different groups.
The bibliographic search was carried out in May 2023. The literature review was done as a comprehensive review, thus not following the systematic review guidelines. The databases employed were Web of Science and Scopus. The keywords employed were ((protein AND diabetes) OR (“protein hydrolysate” AND diabetes)) AND (“oral administration” OR “ingestion” OR “intervention”). No filters for the year of publication were applied but recent articles were prioritised in the selection of articles. Following initial evaluation, milk and fish proteins were chosen as sources, and a third section for “Other sources” was created to highlight the relevance of new sources (e.g., plant-derived proteins). As no standardization of the methodology could be found, the selection of articles was agreed by two independent authors aiming to provide the reader with a full overview of the current literature available (in terms of methodology and type of study).
2. Description of results
2.1. Milk-derived proteins
Milk-derived proteins can be classified mainly into casein and whey. The main difference in terms of ingestion is that the digestion of casein is slower than that of whey, and this has a clearly different effect in terms of impact on the carbohydrate's digestion.34 Several reports have evaluated the efficacy of casein hydrolysates as a managing strategy for the development of diabetes. In terms of comparison of native proteins with their hydrolysates, it has been described that milk protein hydrolysates provoked around 50% more gastric secretion compared to the natural protein, along with an increase of GIP plasma concentrations reported at the beginning of the gastric emptying process.35 In the same line, Koopman et al.36 evaluated the differences between the protein and its hydrolysate upon ingestion (35 g) in elderly men (n = 10, mean age of 64 years old) in the postprandial amino acid bioavailability, and as expected, the hydrolysate led to an accelerated protein digestion and absorption, enhancing its bioavailability and usage in the body. This underlying behaviour might serve as a scientific explanation of the outcomes derived from clinical trials in which modifications of parameters are observed.Regarding specific studies aiming to demonstrate whether the ingestion of milk proteins has an impact on humans, the intake of casein hydrolysate (0.3 g per kg) as the test item has been evaluated in healthy and diabetic male (n = 10), in order to explore postprandial plasma insulin and glucose responses. These authors37 found that the area under the curve (AUC) for plasma insulin increased in the patients with diabetes, while also glucose levels were found to be reduced. However, modification of parameters following acute ingestion of protein is not sufficient evidence to state that the intake of protein might have an impact on diabetes management, as frequency and dose of ingestion have to be taken into consideration. In the same line as this previous report but whey being the test item, Petersen et al.38 reported that in healthy subjects (n = 10, mean age of 44 years old), an acute ingestion of 5, 10, and 20 g of the protein with 50 g of glucose led to a reduction in the AUC of glucose levels, supporting the evidence that protein intake has a positive effect on glucose homeostasis. These studies are also limited by the population size, which is considered small, although it allows suggesting potential differences between healthy and non-healthy subjects.
Other studies in which the postprandial glucose was reported to be positively modulated by the intake of whey peptides can be found elsewhere,39,40 increasing the evidence of the functionality of these peptides from milk proteins in prevention of diabetes, though long-term studies are needed to state these bioactive properties. In this regard, the concentration–time curves of glucose and insulin were monitored after a supplementation of 1.4 g or 2.8 g of the test item during 6 weeks to prediabetic patients (n = 21). The authors reported a significant impact on the postprandial blood glucose profile with more glycaemic than insulinotropic properties.39 These results reinforce the evidence that milk proteins are useful in managing the postprandial glucose peak, and the authors highlighted the importance of the high content of leucine as responsible for the glucoregulatory properties of the whey protein. Similarly, Saleh et al.41 aimed to demonstrate the antidiabetic effect of casein (17 g per d during 8 days) in patients with mild gestational diabetes (n = 26). Among the parameters assessed (plasma glucose, insulin, and C-peptide levels and the insulin-to-glucose ratio), no insulinotropic effects occurred, but a moderate reduction of plasma glucose levels occurred. The small population size might not account for inter-individual differences, based on the number of external factors that contribute to the health of the subjects and their immunometabolic status.
Considering a larger population in order to reduce the risk of bias, Drummond et al.42 carried out an acute postprandial study (dose of 12 g of casein) in overweight adults and adults with obesity (n = 62, mean age of 54 years old), in which a significant rise in insulin secretion was reported, while glucose levels were reduced. The AUC for the change in glucose was reduced from 181.84 ± 14.6 to 153.87 ± 13.02, but this acute insulinotropic response should be confirmed through long-term studies. In fact, dietary interventions should at least last for 4–8 weeks, depending on the population size and the primary parameters evaluated. Concerning long-term studies, Hovland et al.43 described how the supplementation with milk (mixture of casein and whey, 2.5 g per day) could affect glucose regulation and circulating lipid concentrations in overweight adults (n = 77, mean age of 41 years old), in a randomized, double-blind study for two months. In this study,43 the supplementation led to a decrease (around 7%) of glucose AUC, in addition to a reduction of postprandial insulin C-peptide concentration up to 23%. Other parameters evaluated which were found to be decreased in serum concentrations were some ketone bodies (e.g., acetoacetate and β-hydroxybutyrate). The relevance of this study is higher than that of the short-term studies, although the biological relevance of these changes and their stabilisation should be verified. In addition to that, more factors such as the timing of ingestion (i.e., mealtime) should be considered in the design of the studies.
In this sense, Pekmez et al.44 evaluated in randomized, cross-over meal studies the impact of protein pre-meals on the postprandial plasma metabolome before a fat-rich main meal. This study44 included a dose-dependent evaluation (0, 10 and 20 g of a pre-meal with protein) as well as the effect of different protein sources (whey, casein, or gluten) in metabolic syndrome patients. Plasma samples were evaluated during the first hours after ingestion. The ingestion of whey protein prior to fat led to elevated plasma branched chain and aromatic amino acids, as well as an increase of methionine, which were partially correlated with the glucose and insulin response after the main meal. Both milk proteins led to comparable postprandial amino acid responses. The difference in the protein structure and sequences in this case was not found to be relevant in the amino acid profile.
Concerning other important factors related to the modulation of glucose levels, Fangmann et al.45 reported how the ingestion of casein or of a specific oligopeptide from casein has an in vivo impact on FGF-21 (which is a metabolic modulator implied in the decrease of glucose levels and improvement of insulin sensitivity without hypoglycaemia) in subjects with obesity (n = 40, mean age of 41 years old) in a randomized double-blind cross-over design for 20 weeks (8 of intervention, 4 wash-out, 8 of intervention). The results showed that the concentration of FGF-21 was decreased in these patients with obesity without T2DM after ingesting the protein, but not in the ones suffering from the disease. In addition, the ingestion of the oligopeptide for 8 weeks significantly increased 5% the FGF-21 serum levels. These findings suggest a variance role of the different peptides on FGF-21, thus indicating the need of improvement of the techniques of purification and characterization of peptides from milk proteins. In addition, these results highlighted the need of assessing not only the common parameters (glucose and/or insulin levels), but other variables, as several modulators are involved and their baseline levels, according to the health status, might be different and react differently following specific nutritional interventions.
A review on the clinical application of mealtime whey protein (for its the slowing of gastric emptying and increased secretion of insulin and the incretin peptides) for the treatment of postprandial hyperglycaemia for people with T2DM was recently published by Smith et al.,46 highlighting that the benefits are more evident when the protein is consumed before, rather than with, the meal. Nonetheless, these authors also indicate the need for long-term studies, and the methodological limitations in the acute studies do not allow drawing conclusions.
Overall, the evidence reported during the last years30,47 suggests that both milk proteins and milk protein hydrolysates might have a positive impact on the glucose homeostasis of individuals, depending mainly on their baseline health status (diabetics or not) and the dose ingested. However, as stated, long-term studies in order to state that the glucose levels are indeed lowered and maintained at a considered as healthy status are needed, ideally with proper nutrigenetic characterization. Milk proteins as nutritionally relevant components in managing the glucose peak and the potential development of T2DM have been historically widely explored.48–50 However, there are a number of variants which are genetically determined and might have different effects based on the structural properties of the proteins.51 Further studies considering the time of ingestion, the dose, and the proportion of casein/whey, among other parameters will have an impact based on the speed of gastric emptying. The relevance of understanding the role of milk proteins is based on the fact that this commodity is consumed by more than 6 billion people worldwide according to the Food and Agriculture Organization of the United Nations (FAO).52
2.2. Fish-derived proteins
The relevance of fish consumption by humans has been widely studied, not only because of the quality and quantity of the proteins, but also because of the fatty acid (FA) composition they can have (enriched in omega-3 FAs, proved to be health-promoting, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA))53 or the content of minerals such as zinc, iron or copper.54 According to the FAO, fish accounted for 17% of the total animal protein, and 7% of all proteins consumed globally in recent years. As a consequence, exploring and exploiting this source is crucial in the future to improve the food system resilience (Table 1).| Source and dose | Type of study and duration | Subjects’ characteristic | Changes reported | References |
|---|---|---|---|---|
| Casein hydrolysate (0.3 g per kg) | Acute, randomized, double-blind | Healthy and diabetic patients (n = 11 per group) | AUC for plasma insulin increased in the patients with diabetes, while also glucose levels were found reduced | Manders et al.37 |
| Whey protein (5, 10 and 20 g) | Acute, randomised controlled | Healthy subjects (n = 10) | Reduction on the AUC of glucose levels | Petersen et al.38 |
| Milk protein hydrolysate (1.4 and 2.8 g) | Randomised, cross-over trial, for 6 weeks | Prediabetic patients (n = 21) | Significant impact on postprandial blood glucose profile with more glycaemic than insulinotropic properties | Sartorius et al.39 |
| Whey protein and hydrolysate (0, 0.1, 0.2 and 0.4 g per kg bw) | Placebo-controlled double-blind randomised, acute | Patients with T2DM (n = 10) | Insulin secretion was promoted, leading to reduced plasma glucose in the post-prandial state | Goudarzi et al.40 |
| Casein hydrolysate (17 g) | Single-centre randomised double blind placebo controlled design, for 8 days | Patients with mild gestational diabetes (n = 50) | Moderated reduction of plasma glucose levels | Saleh et al.41 |
| Casein hydrolysate (12 g) | Acute postprandial study | Overweight and adults with obesity (n = 62) | AUC for glucose was decreased, insulin secretion was promoted | Drummond et al.42 |
| Mixture of casein and whey, herring, salmon and cod (2.5 g) | Randomized, double-blind study for eight weeks | Overweight adults (n = 77) | AUC for glucose was decreased for milk proteins and cod | Hovland et al.43 |
| Cod (Gadus morhua) protein hydrolysate (20 mg per kg bw) | Double-blind cross-over trial, acute | Healthy subjects (n = 41) | Postprandial insulin was significantly lower, no effects on glucose nor GLP-1 | Dale et al.,55 |
| Boarfish (Capros aper) (3.5 g) | Randomised controlled intervention crossover study, acute | Healthy subjects (n = 20) | No significant changes | Crowe et al.56 |
| Cod (Gadus morhua) protein hydrolysate (10 to 40 mg per kg bw) | Double-blind cross-over trial, for 7 days | Old adults (range of 60–78 years old, n = 31) | Serum glucose and insulin levels decreased | Jensen et al.57 |
| Cod (Gadus morhua) protein hydrolysate (4 g) | Double-blind cross-over trial, for 8 weeks | Adults with metabolic syndrome (n = 30) | No effect on fasting or postprandial levels of insulin, glucose or GLP-1, lipid profile or body composition | Jensen et al.58 |
| Cod protein (8.1 g) | Randomised double-blind study, for two months | Lean adults (n = 50) | Levels of ketone bodies decreased in the subjects, but not glucose or insulin | Vildmyren et al.59 |
| Salmon protein (5.2 g) | Randomised controlled trial, for two months | Pre-diabetic patients (n = 88) | No significant effect on serum glucose, markers related to glucose tolerance, serum lipids, weight or blood pressure | Hustad et al.60 |
| Salmon peptides (0.5 g) | Randomised, placebo-controlled, double-blind, crossover, pilot clinical trial, for one week | Subjects prone to postprandial glucose elevation (n = 15) | Lower AUC for glucose in subjects without insulin resistance | Takahashi et al.61 |
Dale et al.55 carried out a double-blind cross-over trial to assess the postprandial glucose and GLP-1 concentration changes when healthy subjects (n = 41, mean age of 51 years old) ingested a cod (Gadus morhua) protein hydrolysate. A dose of 20 mg per kg bw did not lead to significant differences compared to the control though postprandial insulin was significantly lower when the fish peptides were ingested compared with the control. Similarly, Crowe et al.56 aimed to evaluate whether boarfish (Capros aper) peptide ingestion could have an effect on postprandial glycemia in healthy subjects (n = 20). By means of a randomised controlled intervention crossover study, participants ingested 3.5 g of the sample, which did not modify the postprandial AUC of insulin or glucose compared to the control. Higher doses of the test items or a more frequent intake of them could be an alternative hypothesis to be tested in new human studies, aiming to observe substantial changes in the parameters assessed. In addition to that, the evaluation of unhealthy subjects is also interesting, as their baseline levels of relevant parameters are different and their responses are likely to be different.
Jensen et al.57 assessed whether the supplementation with cod peptides in older adults could exert physiological changes on the postprandial glucose metabolism. For this purpose, a double-blind cross-over trial was carried out, in which the patients (n = 31) ingested (from 10 to 40 mg per kg bw) the test item for seven days. It was reported that plasma GLP-1 levels did not change, although serum glucose and insulin levels decreased as the dose was increased. These authors carried out a study with longer duration, in which 4 g of peptides were supplemented for two months, with the same purpose, on adults with metabolic syndrome (n = 30). However, no significant differences were found in postprandial insulin and postprandial glucose of GLP-1.58 On the other hand, Hovland et al.43 reported the effects of the supplementation of 2.5 g per day for 2 months of herring (Clupea harengus), salmon (Salmo salar), or cod (Gadus morhua) in overweight adults (n = 77, mean age of 41 years old). The authors indicated that the ingestion of herring and salmon did not have an impact on glucose and insulin levels, whereas the intake of cod, instead, significantly reduced within-group changes in 90 and 120 min postprandial glucose concentrations, although these changes were not different from the groups ingesting the other species. Ketone bodies (acetoacetate and β-hydroxybutyrate) were reduced at least 24% in the different groups, whereas serum lipid concentrations were not modified in any of the intervention groups. An accurate description of the metabolic status of the patients at the beginning of the study as well as a clear characterization of the test item employed (bioavailability and presence of bioactive peptides) and the effect of the regular diet is needed in order to conclude the reasons leading to differences between studies.
A higher dose was evaluated by Vildmyren et al.,59 who carried out a randomised double-blind study aiming to investigate whether the ingestion of cod protein (8.1 g per day) for two months alters the levels of markers of glucose regulation in lean adults (n = 50, mean age of 28 years old). The authors indicated that fasting glucose or insulin concentrations were not significantly different when the test item was ingested, but the levels of ketone bodies decreased in the subjects, together with an increase of trimethylamine N-oxide concentration in plasma and urine, which are parameters related to impaired glucose metabolism. This study highlights the need of assessing different parameters, as they might provide an insight into the underlying mechanisms by which these nutritional interventions have or do not have a beneficial effect. Similarly, Hustad et al.60 evaluated the effect of salmon protein supplementation (5.2 g per day) for two months in a randomised controlled trial with patients predisposed to develop T2DM. In the postprandial period (for two hours), the glucose levels were not significantly different compared to the control. However, the measurement of more parameters, such as hormones, could have been useful in understanding whether there is an effect on the metabolism. In line with this negative outcome in vivo, Takahashi et al.61 assessed whether the intake of 0.5 g of DPP-IV inhibitory peptides for a week would have a positive impact on postprandial blood glucose and insulin levels in healthy subjects (n = 15), by means of a randomised, placebo-controlled, double-blind, crossover, pilot clinical trial. No biologically relevant changes from the ingestion of the test item in the parameters evaluated occurred. The relevance of these outcomes indicates that the statements indicated in several reports on the health properties of peptides following positive in vitro results are not sufficient.
As observed with the amount of evidence hereby presented,62,63 the results obtained with different fish proteins are somehow contradictory in how these can modulate glucose levels in serum. This is mainly due to the different compositions and structures of the proteins of each species, leading to different peptides upon ingestion, which would have or not have a biological effect, as well as the design of the study. A proper characterization of the protein content of each species as well as their peptidome could be beneficial in terms of understanding why specific species seem to be more likely to exert a quantifiable effect on humans, as the amino acid content and specific molecular features in peptides have been demonstrated to be of high relevance in terms of interacting with receptors or enzymes in the organism.
2.3. Other sources
Sufficient protein consumption is critical to humans and, since the overall demand for protein-containing foods is increasing, the identification of novel high-quality protein sources is required. The urgent need to re-establish our relationship with the environment is linked, now more than ever, to the need to rethink our current food system, improving the resilience and sustainability of the entire food chain. Alternative proteins (e.g., algae, insects, uncommon vegetables, fungi, etc.) are currently seen as adequate sources to be explored and exploited in the upcoming years. Notably, the alternative protein market is expected to grow at a compound annual growth rate of 12.4% between 2022 and 2029 to reach $36.61 billion by 2029. Consequently, the understanding of the biological behaviour of these novel sources is key in order to compose a sustainable food system, promoter of health benefits in humans.For instance, Plat et al.64 evaluated the potential of an egg hydrolysate in a human crossover study in an acute (2 h) and short-term (2 days) condition. The dose ingested by the subjects (n = 40, overweight individuals and individuals with obesity with impaired glucose-tolerance or T2DM) was 5 g per d. In the short-term study, triglycerides in plasma decreased, while high-density lipoprotein cholesterol concentrations increased, suggesting the potential effect that the peptides of egg can have on the glucoregulation, although long-term benefits could not be proved based on the limitations in the methodology. Furthermore, Klementova et al.65 reported how a plant-based meal could increase gastrointestinal hormones and satiety to a higher extent, when compared to a processed-meat meal in T2DM, obese, and healthy men. For this purpose, a three-group (n = 20 per group) randomized crossover study was carried out. In the trial, the acute effect of a meal containing ca. 20% of protein on the hormones (i.e., GLP-1, amylin, and peptide YY) was evaluated during the postprandial period (up to 180 min). An increase in the different hormones was described, depending on the group evaluated. GLP-1 increased in the T2DM and healthy subjects, whereas PYY only increased in the healthy group. According to the authors,65 plasma concentrations of amylin increased in all groups. The difference balance of hormones according to the metabolic status is useful to understand in which target or by which metabolic pathways the diet has an impact and can modulate the development of diseases by affecting risk factors. The relevance of vegetable sources such as buckwheat, fava bean, pea, hemp, and lupin compared to beef in the postprandial state was recently reported.66 Following an acute ingestion (six individual visits, ingesting 30 g of each source's protein) by healthy subjects (n = 10, mean age of 42 years old), the postprandial biomarkers of satiety, gut hormones, amino acid and plant metabolite bioavailability and metabolism were evaluated. Overall, postprandial concentration of plasma amino acids was not significantly different after the ingestion of vegetable sources. However, a significant increase in GLP-1 plasma was found following hemp consumption compared with the other plant-based meals, together with a decrease of plasma ghrelin concentrations. This behaviour was explained by the appearance of metabolites such as 4-hydroxyphenylpyruvic acid, indole 3-pyruvic acid, 5-hydroxytryptophan, genistein and biochanin A with GLP-1, PYY and insulin; 3-hydroxymandelic acid and luteolidin with GLP-1 and ghrelin; and 4-hydroxymandelic acid, benzoic acid and secoisolariciresinol with insulin and ghrelin. This study indicated for the first time the relevance of hemp protein in managing diabetes-related parameters in humans.
The effects of legume consumption on the markers of glycaemic control in individuals with and without DM were systematically reviewed and eighteen studies were included, although not specifically the protein role was the objective of the study. These authors indicated that only the studies with T2DM patients reported substantial effects on fasting blood glucose, glycosylated haemoglobin, fasting blood insulin and/or postprandial glucose, while the quality of the evidence was low.67
There are proteins from sources which have been evaluated at in vitro levels, but no clinical trials regarding the specific role of their protein were found, such as fungi.68 It is expected that clinical trials employing alternative proteins will be carried out soon and will pave the way to employ these protein sources as ingredients in the food industry. Promoting the use of alternative protein sources can contribute to the achievement of Sustainable Development Goals (SDGs), in particular the zero hunger goal (SDG 2) aiming to end hunger ensuring food safety, while taking advantage of environmentally friendly sources or using food waste as a source of dietary compounds (SDG 13-climate change or SDG 15-terrestrial ecosystems, forestry, and biodiversity).69
3. Gaps and perspectives
As reviewed before, there are many clinical trials aiming to demonstrate the potential positive effects of ingesting proteins and peptides from specific sources on pre-diabetic patients, as well as the effect on healthy subjects or patients suffering from diabetes. However, there are still many research challenges to be addressed, which should be investigated in the near future. In Fig. 1, a global picture of the topic hereby described is depicted, indicating the main factors to be considered in the nutritional interventions.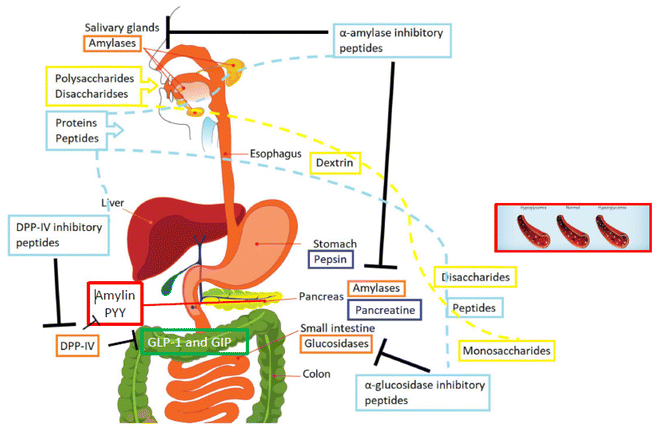 | ||
| Fig. 1 Factors affecting human health and the potential development of diabetes (modified from Rivero-Pino et al.10 (2020)). | ||
Recently, Barbaresko et al.70 systematically summarized the evidence on diet and all-cause mortality in individuals with T2DM, and from 107 studies, these authors concluded that higher intake of fish, whole grain, fiber, and omega-3 polyunsaturated FAs was inversely associated with all-cause mortality in individuals with T2DM. However, they also highlighted that there is limited evidence for other dietary factors, and thus, more research is needed. This raises the question of how different mixtures and concentrations of non-protein components, such as fat, carbohydrates, and micronutrients, interact and affect the glucoregulatory properties of proteins in different populations, linked to the individual's phenotypic and nutrigenomic status (e.g., age, weight, gender, activity level, disease status, etc.).71
The relevance of the mealtime (when)44 and the frequency (how often)39,40 in the ingestion of protein to regulate the glycaemic index of subjects has been demonstrated, and consequently, a modelling of the digestion process and the gastric emptying of components is crucial in understanding the underlying mechanisms by which and how protein exerts the glucoregulatory properties. The mealtime, consequently, is a parameter to be taken into account when designing studies related to postprandial response of glucose levels, as well as for long-term studies.
Clinical trials are expensive and not all the variables are always controllable, so the results may not be reliable nor applicable to a wider population, and consequently, the design (including the power calculation of the sample size) and the statistical analysis have to be clearly defined and justified.72,73 The most reliable type of human studies to demonstrate claimable effects are randomised controlled trials (i.e., at low risk of bias). The duration of the studies reported in the literature is sometimes very short (i.e., acute ingestion, or just a few days). Ideally, studies of at least 4–8 weeks are recommended in order to establish relevant changes.74 Considering that nutritional interventions should be safe for human beings, adverse effects have to be recorded, based on the conditions of use. A clear demonstration of health benefits should be carried out by multi-center analyses, and the nutritional interventions must be realistic, meaning that the dose ingested needed to observe a health benefit is plausible for human consumption in long term, considering the specificity and magnitude of the effect.75 In the published reports, the number of parameters analysed is not always sufficient, since most of the studies usually rely their conclusions on the quantification of blood glucose and insulin levels, whereas the concentration of hormones in subjects suffering from different metabolic conditions and subjected to specific nutritional interventions could be useful to unravel how proteins affect the development or delay of diabetes.
Considering the intake of proteins or their hydrolysates, the main difference is observed at the bioavailability level, as the peptides ingested are different and will be metabolized in a different way. In addition, a different higher uptake rate of amino acids has been demonstrated when ingesting hydrolysates compared to native proteins.76 In general, most of the studies hereby reported on the effect of supplementation with food-derived peptides or protein on the regulation of glucose homeostasis are lacking quality experimental design. A more exhaustive assessment of the toxicokinetic parameters as well as glucose-metabolism related parameters is needed to confirm the physiological changes ensuing. The metabolism of healthy subjects is not necessarily representative of the status of patients with impaired metabolism. Studies with pre-diabetic patients are more useful to unravel the underlying mechanisms by which the ingestion of protein has an impact on the oxidative and immunological status, and how the different parameters are regulated. Furthermore, differences among populations due to metabotyping must be considered, because intervariability can occur.10
In this line, Curran et al.77 pointed the necessity for additional precise nutrition testing, as they observed that the potential of casein peptides to improve glycaemic function was found exclusively in some of the subjects analysed. Similarly, evidence regarding how amino acids affect the development of T2DM has been shown to be a bit inconsistent. Different responses have been found when evaluating different populations (e.g., European or Asiatic).28 Nevertheless, the pre-diabetic population is not homogeneous, and phenotyping it into specific categories can be advantageous for primary prevention, follow-up, and long-term risk assessment.27
4. Conclusions
Human studies are essential in order to demonstrate and unravel the underlying mechanisms by which specific proteins and products thereof can have an impact on glucose homeostasis and manage the development of diabetes. In this review, a recent summary of nutritional interventions with protein and protein hydrolysates of diverse nature (milk, fish, vegetables, etc.), mentioning the most relevant outcomes as defined by the authors (e.g., postprandial glucose and insulin resistance), has been described. The main parameters to be considered in nutritional interventions are the dose of the test item ingested, the duration of the trial and the target population (mostly their health status), although mealtime has been also recently described as relevant in understanding the postprandial response. Overall, the literature published to date shows a tendency to believe that the intake of some proteins has a beneficial effect in diabetes care, in spite of the contradictory results found for some sources. Nonetheless, some limitations of the studies hereby reported were also identified, highlighting the need for well-designed studies, in terms of hypothesis and methodology. Further research on the topic is needed, in order to help promoting a more sustainable food system, as well as to suggest dietary patterns that are proved to be health-promoting in humans to avoid the development of diseases.Author contributions
All authors have contributed to the writing – review and editing and agree to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was supported by a grant from the Andalusian Government (2021/CTS-1074). Maria C. Millan-Linares acknowledges the financial grant US-1381492 supported by Andalusian Plan for Research, Development, and Innovation 2020 from the European Regional Development Fund (ERDF). Fernando Rivero-Pino acknowledges his postdoctoral contract supported by TED2021-130521A-I00 (Ministry of Science and Innovation, Government of Spain) into the Recovery, Transformation, and Resilience Plan funding by NextGenerationEU.References
- R. E. Aluko, Food protein-derived peptides: Production, isolation, and purification, Elsevier Ltd., 2nd edn, 2017 Search PubMed.
- R. Nasri, O. Abdelhedi, M. Nasri and M. Jridi, Fermented protein hydrolysates: biological activities and applications, Curr. Opin. Food Sci., 2022, 43, 120–127 CrossRef CAS.
- D. E. Cruz-Casas, C. N. Aguilar, J. A. Ascacio-Valdés, R. Rodríguez-Herrera, M. L. Chávez-González and A. C. Flores-Gallegos, Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides, Food Chem.: Mol. Sci., 2021, 3, 100047 CAS.
- F. Rivero-Pino, Bioactive food-derived peptides for functional nutrition: Effect of fortification, processing and storage on peptide stability and bioactivity within food matrices, Food Chem., 2023, 406, 135046 CrossRef CAS.
- F. Hosseini, A. Jayedi and T. A. Khan, et al. Dietary carbohydrate and the risk of type 2 diabetes: an updated systematic review and dose–response meta-analysis of prospective cohort studies, Sci. Rep., 2022, 12, 2491 CrossRef CAS.
- N. E. Nasr and K. M. Sadek, Role and mechanism(s) of incretin-dependent therapies for treating diabetes mellitus, Environ. Sci. Pollut. Res., 2022, 29, 18408–18422 CrossRef CAS.
- A. A. Tahrani, C. J. Bailey, S. Del Prato and A. H. Barnett, Management of type 2 diabetes: new and future developments in treatment, Lancet, 2011, 378, 182–197 CrossRef CAS.
- J. Caron, D. Domenger, P. Dhulster, R. Ravallec and B. Cudennec, Protein digestion-derived peptides and the peripheral regulation of food intake, Front. Endocrinol., 2017, 8, 85 CrossRef.
- I. Chan-Zapata, C. Sandoval-Castro and M. R. Segura-Campos, Proteins and peptides from vegetable food sources as therapeutic adjuvants for the type 2 diabetes mellitus, Crit. Rev. Food Sci. Nutr., 2022, 62, 2673–2682 CrossRef CAS PubMed.
- F. Rivero-Pino, F. J. Espejo-Carpio and E. M. Guadix, Antidiabetic Food-Derived Peptides for Functional Feeding: Production, Functionality and In Vivo Evidences, Foods, 2020, 9, 883 CrossRef PubMed.
- International Diabetes Federation, IDF Diabetes Atlas, 10th edn, 2021 Search PubMed.
- P. Patil, S. Mandal, S. K. Tomar and S. Anand, Food protein-derived bioactive peptides in management of type 2 diabetes, Eur. J. Nutr., 2015, 54, 863–880 CrossRef CAS PubMed.
- T. Wang, J. Wang, X. Hu, X. Huang and G.-X. Chen, Current understanding of glucose transporter 4 expression and functional mechanisms, World J. Biol. Chem., 2020, 11, 76–98 CrossRef PubMed.
- L. Hieronymus and S. Griffin, Role of Amylin in Type 1 and Type 2 Diabetes, Diabetes Educ., 2015, 41, 47S–56S CrossRef PubMed.
- A. Lorenzo, B. Razzabonl, G. C. Weirt and B. A. Yankner, Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus, Nature, 1994, 368, 756–760 CrossRef CAS PubMed.
- R. A. Lafferty, P. R. Flatt and N. Irwin, Established and emerging roles peptide YY (PYY) and exploitation in obesity-diabetes, Curr. Opin. Endocrinol., Diabetes Obes., 2021, 28, 253–261 CrossRef CAS PubMed.
- J. Yan, J. Zhao, R. Yang and W. Zhao, Bioactive peptides with antidiabetic properties: a review, Int. J. Food Sci. Technol., 2019, 54, 1909–1919 CrossRef CAS.
- M. K. N. B. Sufian, T. Hira, K. Miyashita, T. Nishi, K. Asano and H. Hara, Pork peptone stimulates cholecystokinin secretion from enteroendocrine cells and suppresses appetite in rats, Biosci. Biotechnol. Biochem., 2006, 70, 1869–1874 CrossRef CAS PubMed.
- T. Nishi, H. Hara, K. Asano and F. Tomita, The soybean β-conglycinin β 51-63 fragment suppresses appetite by stimulating cholecystokinin release in rats, J. Nutr., 2003, 133, 2537–2542 CrossRef CAS PubMed.
- L. Fleury, B. Deracinois, C. Dugardin, A. B. Nongonierma, R. J. FitzGerald, C. Flahaut, B. Cudennec and R. Ravallec, In Vivo and In Vitro Comparison of the DPP-IV Inhibitory Potential of Food Proteins from Different Origins after Gastrointestinal Digestion, Int. J. Mol. Sci., 2022, 23, 8365 CrossRef CAS PubMed.
- M. Santos-Hernández, B. Miralles, L. Amigo and I. Recio, Intestinal Signaling of Proteins and Digestion-Derived Products Relevant to Satiety, J. Agric. Food Chem., 2018, 66, 10123–10131 CrossRef.
- J. Caron, B. Cudennec, D. Domenger, Y. Belguesmia, C. Flahaut, M. Kouach, J. Lesage, J. F. Goossens, P. Dhulster and R. Ravallec, Simulated GI digestion of dietary protein: Release of new bioactive peptides involved in gut hormone secretion, Food Res. Int., 2016, 89, 382–390 CrossRef CAS PubMed.
- A. T. Hutchison, C. Feinle-Bisset, P. C. E. Fitzgerald, S. Standfield, M. Horowitz, P. M. Clifton and N. D. Luscombe-Marsh, Comparative effects of intraduodenal whey protein hydrolysate on antropyloroduodenal motility, gut hormones, glycemia, appetite, and energy intake in lean and obese men, Am. J. Clin. Nutr., 2015, 102, 1323–1331 CrossRef CAS PubMed.
- M. Lee, J. L. Saver, K. S. Hong, S. Song, K. H. Chang and B. Ovbiagele, Effect of pre-diabetes on future risk of stroke: Meta-analysis, Br. Med. J., 2012, 344, 1–11 Search PubMed.
- M. Mata-Cases, B. Rodríguez-Sánchez, D. Mauricio, J. Real, B. Vlacho, J. Franch-Nadal and J. Oliva, The association between poor glycemic control and health care costs in people with diabetes: A population-based study, Diabetes Care, 2020, 43, 751–758 CrossRef PubMed.
- K. Luc, A. Schramm-Luc, T. J. Guzik and T. P. Mikolajczyk, Oxidative stress and inflammatory markers in prediabetes and diabetes, J. Physiol. Pharmacol., 2019, 70, 809–824 CAS.
- E. Barbu, M. R. Popescu, A. C. Popescu and S. M. Balanescu, Phenotyping the prediabetic population—a closer look at intermediate glucose status and cardiovascular disease, Int. J. Mol. Sci., 2021, 22, 6864 CrossRef CAS PubMed.
- S. Chen, S. Akter, K. Kuwahara, Y. Matsushita, T. Nakagawa, M. Konishi, T. Honda, S. Yamamoto, T. Hayashi, M. Noda and T. Mizoue, Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study, Sci. Rep., 2019, 9, 1–9 CrossRef.
- Q. Zhou, W. W. Sun, J. C. Chen, H. L. Zhang, J. Liu, Y. Lin, P. C. Lin, B. X. Wu, Y. P. An, L. Huang, W. X. Sun, X. W. Zhou, Y. M. Li, Y. Y. Yuan, J. Y. Zhao, W. Xu and S. M. Zhao, Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRβ, Nat. Commun., 2022, 13, 1–19 Search PubMed.
- K. M. Beaudry and M. C. Devries, Nutritional Strategies to Combat Type 2 Diabetes in Aging Adults: The Importance of Protein, Front. Nutr., 2019, 6, 1–10 CrossRef PubMed.
- EFSA NDA Panel, Scientific advice related to nutrient profiling for the development of harmonised mandatory front-of-pack nutrition labelling and the setting of nutrient profiles for restricting nutrition and health claims on foods, EFSA J., 2022, 20, 7259 Search PubMed.
- J. W. Yau, S. M. Thor and A. Ramadas, Nutritional strategies in prediabetes: A scoping review of recent evidence, Nutrients, 2020, 12, 1–24 CrossRef PubMed.
- S. de la Cruz-Ares, F. M. Gutiérrez-Mariscal, J. F. Alcalá-Díaz, G. M. Quintana-Navarro, A. Podadera-Herreros, M. P. Cardelo, J. D. Torres-Peña, A. P. Arenas-De Larriva, P. Pérez-Martínez, J. Delgado-Lista, E. M. Yubero-Serrano and J. López-Miranda, Quality and quantity of protein intake influence incidence of type 2 diabetes mellitus in coronary heart disease patients: From the cordioprev study, Nutrients, 2021, 13, 1217 CrossRef CAS PubMed.
- J. F. Lesgards, Benefits of Whey Proteins on Type 2 Diabetes Mellitus Parameters and Prevention of Cardiovascular Diseases, Nutrients, 2023, 15, 1294 CrossRef CAS PubMed.
- J. A. L. Calbet and J. J. Holst, Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans, Eur. J. Nutr., 2004, 43, 127–139 CrossRef CAS PubMed.
- R. Koopman, N. Crombach, A. P. Gijsen, S. Walrand, J. Fauquant, A. K. Kies, S. Lemosquet, W. H. Saris, Y. Boirie and L. J. van Loon, Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein, Am. J. Clin. Nutr., 2009, 90, 106–115 CrossRef CAS PubMed.
- R. J. F. Manders, R. Koopman, W. E. Sluijsmans, R. van den Berg, K. Verbeek, W. H. Saris, A. J. Wagenmakers and L. J. van Loon, Co-Ingestion of a Protein Hydrolysate with or without Additional Leucine Effectively Reduces Postprandial Blood Glucose Excursions in Type 2 Diabetic Men, J. Nutr., 2006, 136, 1294–1299 CrossRef CAS.
- B. L. Petersen, L. S. Ward, E. D. Bastian, A. L. Jenkins, J. Campbell and V. Vuksan, A whey protein supplement decreases post-prandial glycemia, Nutr. J., 2009, 8, 47 CrossRef PubMed.
- T. Sartorius, A. Weidner, T. Dharsono, A. Boulier, M. Wilhelm and C. Schön, Postprandial Effects of a Proprietary Milk Protein Hydrolysate Containing Bioactive Peptides in Prediabetic Subjects, Nutrients, 2019, 11, 1700 CrossRef CAS PubMed.
- M. Goudarzi and A. Madadlou, Influence of whey protein and its hydrolysate on prehypertension andpostprandial hyperglycaemia in adult men, Int. Dairy J., 2013, 33, 62–66 CrossRef CAS.
- L. Saleh, N. L. Schrier, M. J. Bruins, E. A. P. Steegers, A. H. van den Meiracker and W. Visser, Effect of oral protein hydrolysate on glucose control in patients with gestational diabetes, Clin. Nutr., 2018, 37, 878–883 CrossRef CAS PubMed.
- E. Drummond, S. Flynn, H. Whelan, A. B. Nongonierma, T. A. Holton, A. Robinson, T. Egan, G. Cagney, D. C. Shields, E. R. Gibney, P. Newsholme, C. Gaudel, J. C. Jacquier, N. Noronha, R. J. Fitzgerald and L. Brennan, Casein Hydrolysate with Glycemic Control Properties: Evidence from Cells, Animal Models, and Humans, J. Agric. Food Chem., 2018, 66, 4352–4363 CrossRef CAS PubMed.
- I. H. Hovland, I. S. Leikanger, O. Stokkeland, K. H. Waage, S. A. Mjøs, K. A. Brokstad, A. McCann, P. M. Ueland, R. Slizyte, A. Carvajal, G. Mellgren, T. Remman, I. Høgøy and O. A. Gudbrandsen, Effects of low doses of fish and milk proteins on glucose regulation and markers of insulin sensitivity in overweight adults: a randomised, double blind study, Eur. J. Nutr., 2020, 59, 1013–1029 CrossRef CAS PubMed.
- C. T. Pekmez, A. Bjørnshave, G. Pratico, K. Hermansen and L. O. Dragsted, Pre-meal protein intake alters postprandial plasma metabolome in subjects with metabolic syndrome, Eur. J. Nutr., 2020, 59, 1881–1894 CrossRef CAS PubMed.
- D. Fangmann, C. Geisler, K. Schlicht, K. Hartmann, J. Köpke, A. Tiede, U. Settgast, K. Türk, D. M. Schulte, K. Altmann, I. Clawin-Rädecker, P. C. Lorenzen, S. Schreiber, K. Schwarz and M. Laudes, Differential effects of protein intake versus intake of a defined oligopeptide on FGF-21 in obese human subjects in vivo, Clin. Nutr., 2021, 40, 600–607 CrossRef CAS PubMed.
- K. Smith, K. A. Bowden Davies, E. J. Stevenson and D. J. West, The Clinical Application of Mealtime Whey Protein for the Treatment of Postprandial Hyperglycaemia for People With Type 2 Diabetes: A Long Whey to Go, Front. Nutr., 2020, 7, 587843 CrossRef PubMed.
- A. H. Frid, M. Nilsson, J. J. Holst and I. M. E. Björck, Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects, Am. J. Clin. Nutr., 2005, 82, 69–75 CrossRef CAS PubMed.
- J. T. Jonker, M. A. Wijngaarden, J. Kloek, Y. Groeneveld, C. Gerhardt, R. Brand, A. K. Kies, J. A. Romijn and J. W. A. Smit, Effects of low doses of casein hydrolysate on post-challenge glucose and insulin levels, Eur. J. Intern. Med., 2011, 22, 245–248 CrossRef CAS PubMed.
- B. F. Geerts, M. G. J. Van Dongen, B. Flameling, M. M. Moerland, M. L. D. Kam, A. F. Cohen, J. A. Romijn, C. C. Gerhardt, J. Kloek and J. Burggraaf, Hydrolyzed casein decreases postprandial glucose concentrations in T2DM patients irrespective of leucine content, J. Diet. Suppl., 2011, 8, 280–292 CrossRef CAS PubMed.
- R. J. F. Manders, D. Hansen, A. H. G. Zorenc, P. Dendale, J. Kloek, W. H. M. Saris and L. J. C. Van Loon, Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients, J. Med. Food, 2014, 17, 758–763 CrossRef CAS PubMed.
- S. Kaskous, A1- and A2-Milk and Their Effect on Human Health, J. Food Eng. Technol., 2020, 9, 15–21 CrossRef.
- FAO and GDP, Climate change and the global dairy cattle sector – The role of the dairy sector in a low-carbon future, Rome, 2018, p. 36. Licence: CC BY-NC-SA-3.0 IGO.
- N. E. Rahmani-Manglano, I. González-Sánchez, P. J. García-Moreno, F. J. Espejo-Carpio, C. Jacobsen and E. M. Guadix, Development of fish oil-loaded microcapsules containing whey protein hydrolysate as film-forming material for fortification of low-fat mayonnaise, Foods, 2020, 9, 545 CrossRef CAS PubMed.
- S. Balami, A. Sharma and R. Karn, Significance Of Nutritional Value Of Fish For Human Health, Malays. J. Halal Res., 2019, 2, 32–34 CrossRef.
- H. F. Dale, C. Jensen, T. Hausken, E. Lied, J. G. Hatlebakk, I. Brønstad, D. A. L. Hoff, G. A. Lied, D. A. Lihaug Hoff and G. A. Lied, Effect of a cod protein hydrolysate on postprandial glucose metabolism in healthy subjects: A double-blind cross-over trial, J. Nutr. Sci., 2018, 7, e33 CrossRef PubMed.
- W. Crowe, C. M. McLaughlin, P. J. Allsopp, M. M. Slevin, P. A. Harnedy, Y. Cassidy, J. Baird, M. Devaney, R. J. Fitzgerald, F. P. M. O’Harte and E. M. McSorley, The effect of boarfish protein hydrolysate on postprandial glycaemic response and satiety in healthy adults, Proc. Nutr. Soc., 2018, 77, E105 CrossRef.
- C. Jensen, H. F. Dale, T. Hausken, E. Lied, J. G. Hatlebakk, I. Brønstad, G. A. Lied and D. A. L. Hoff, Supplementation with cod protein hydrolysate in older adults: A dose range cross-over study, J. Nutr. Sci., 2019, 4–11 Search PubMed.
- C. Jensen, H. F. Dale, T. Hausken, J. G. Hatlebakk, I. Brønstad, G. A. Lied and D. A. L. Hoff, Supplementation with low doses of a cod protein hydrolysate on glucose regulation and lipid metabolism in adults with metabolic syndrome: A randomized, double-blind study, Nutrients, 2020, 12, 1–15 Search PubMed.
- I. Vildmyren, A. Halstensen, A. McCann, Ø. Midttun, P. M. Ueland, Å. Oterhals and O. A. Gudbrandsen, Effect of Cod Residual Protein Supplementation on Markers of Glucose Regulation in Lean Adults: A Randomized Double-Blind Study, Nutrients, 2020, 12, 1445 CrossRef CAS PubMed.
- K. S. Hustad, I. Ottestad, M. Hjorth, K. T. Dalen, T. Sæther, N. A. Sheikh, M. Strømnes, S. M. Ulven and K. B. Holven, No effect of salmon fish protein on 2-h glucose in adults with increased risk of type 2 diabetes: A randomised controlled trial, Br. J. Nutr., 2021, 126, 1304–1313 CrossRef CAS PubMed.
- Y. Takahashi, A. Kamata, M. Nishimura and J. Nishihira, Effect of one-week administration of dipeptidyl peptidase-IV inhibitory peptides from chum salmon milt on postprandial blood glucose level: A randomised, placebo-controlled, double-blind, crossover, pilot clinical trial, Food Funct., 2021, 12, 8544–8551 RSC.
- C.-F. Zhu, G.-Z. Z. Li, H.-B. Bin Peng, F. Zhang, Y. Chen and Y. Li, Treatment with marine collagen peptides modulates glucose and lipid metabolism in chinese patients with type 2 diabetes mellitus, Appl. Physiol., Nutr., Metab., 2010, 35, 797–804 CrossRef CAS PubMed.
- E.-Q. Xia, S.-S. Zhu, M.-J. He, F. Luo, C.-Z. Fu and T. Bin Zou, Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect, Mar. Drugs, 2017, 15, 88 CrossRef PubMed.
- J. Plat, N. Severins and R. P. Mensink, Improvement of pulse wave velocity and metabolic cardiovascular risk parameters through egg protein hydrolysate intake: A randomized trial in overweight or obese subjects with impaired glucose tolerance or type 2 diabetes, J. Funct. Foods, 2019, 52, 418–423 CrossRef CAS.
- M. Klementova, L. Thieme, M. Haluzik, R. Pavlovicova, M. Hill, T. Pelikanova and H. Kahleova, A plant-based meal increases gastrointestinal hormones and satiety more than an energy-and macronutrient-matched processed-meat meal in T2D, obese, and healthy men: A three-group randomized crossover study, Nutrients, 2019, 11, 1–10 CrossRef PubMed.
- M. Neacsu, N. J. Vaughan, S. Multari, E. Haljas, L. Scobbie, G. J. Duncan, L. Cantlay, C. Fyfe, S. Anderson, G. Horgan, A. M. Johnstone and W. R. Russell, Hemp and buckwheat are valuable sources of dietary amino acids, beneficially modulating gastrointestinal hormones and promoting satiety in healthy volunteers, Eur. J. Nutr., 2022, 61, 1057–1072 CrossRef CAS PubMed.
- D. Bielefeld, S. Grafenauer and A. Rangan, The effects of legume consumption on markers of glycaemic control in individuals with and without diabetes mellitus: A systematic literature review of randomised controlled trials, Nutrients, 2020, 12, 1–18 CrossRef.
- M. Z. Shamim, A. K. Mishra, T. Kausar, S. Mahanta, B. Sarma, V. Kumar, P. K. Mishra, J. Panda, K. H. Baek and Y. K. Mohanta, Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment, Molecules, 2023, 28, 2837 CrossRef CAS PubMed.
- E. Piercy, W. Verstraete, P. R. Ellis, M. Banks, J. Rockström, P. Smith, O. C. Witard, J. Hallett, C. Hogstrand, G. Knott, A. Karwati, H. F. Rasoarahona, A. Leslie, Y. He and M. Guo, A sustainable waste-to-protein system to maximise waste resource utilisation for developing food- and feed-grade protein solutions, Green Chem., 2022, 25, 808–832 RSC.
- J. Barbaresko, A. Lang, E. Szczerba, C. Baechle, J. Beckhaus, L. Schwingshackl, M. Neuenschwander and S. Schlesinger, Dietary Factors and All-Cause Mortality in Individuals With Type 2 Diabetes: A Systematic Review and Meta-analysis of Prospective Observational Studies, Diabetes Care, 2023, 46, 469–477 CrossRef CAS.
- K. B. Comerford and G. Pasin, Emerging evidence for the importance of dietary protein source on glucoregulatory markers and type 2 diabetes: Different effects of dairy, meat, fish, egg, and plant protein foods, Nutrients, 2016, 8, 446 CrossRef.
- E. Fountzilas, A. M. Tsimberidou, H. H. Vo and R. Kurzrock, Clinical trial design in the era of precision medicine, Genome Med., 2022, 14, 1–27 CrossRef PubMed.
- C. C. Serdar, M. Cihan, D. Yücel and M. A. Serdar, Sample size, power and effect size revisited: Simplified and practical approachin pre-clinical, clinical and laboratory studies, Biochem. Med., 2021, 31, 1–27 Search PubMed.
- F. Rivero-Pino, Á. Villanueva, S. Montserrat-de-la-Paz, S. Sanchez-Fidalgo and M. C. Millán-Linares, Evidence of Immunomodulatory Food-Protein Derived Peptides in Human Nutritional Interventions: Review on the Outcomes and Potential Limitations, Nutrients, 2023, 15, 2681 CrossRef CAS.
- P. J. Aggett, Dose-response relationships in multifunctional food design: Assembling the evidence, Int. J. Food Sci. Nutr., 2012, 63, 37–42 CrossRef PubMed.
- P. Jimenez-Barrios, L. Sánchez-Rivera, D. Martínez-Maqueda, Y. Le Gouar, D. Dupont, B. Miralles and I. Recio, Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model, Nutrients, 2023, 15, 1065 CrossRef CAS PubMed.
- A. M. Curran, K. Horner, V. O'Sullivan, A. B. Nongonierma, S. Le Maux, E. Murphy, P. Kelly, R. J. Fitzgerald and L. Brennan, Variable Glycemic Responses to Intact and Hydrolyzed Milk Proteins in Overweight and Obese Adults Reveal the Need for Precision Nutrition, J. Nutr., 2019, 149, 88–97 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
