Open Access Article
Open Access ArticleEffect of coenzyme Q10 on cardiac function and survival in heart failure: an overview of systematic reviews and meta-analyses†
Eva
Alarcón-Vieco
,
Irene
Martínez-García
 ,
Irene
Sequí-Domínguez
,
Irene
Sequí-Domínguez
 *,
Eva
Rodríguez-Gutiérrez
,
Nerea
Moreno-Herráiz
and
Carlos
Pascual-Morena
*,
Eva
Rodríguez-Gutiérrez
,
Nerea
Moreno-Herráiz
and
Carlos
Pascual-Morena
Health and Social Research Center, Universidad de Castilla – La, Mancha, 16071, Cuenca, Spain. E-mail: eva.alarcon@alu.uclm.es; irene.mgarcia@uclm.es; irene.sequidominguez@uclm.es; eva.rodriguez@uclm.es; nerea.moreno@alu.uclm.es; carlos.pascual@uclm.es; Tel: +34926053733
First published on 21st June 2023
Abstract
Heart failure (HF) is associated with a deficiency in blood levels of coenzyme Q10 (CoQ10), and its supplementation has been proposed. The aim of this systematic review was to synthesise the available evidence on the effects of CoQ10 on cardiac function and quality of life in HF. A systematic search of Medline, Scopus, Web of Science and the Cochrane Library was conducted from inception until March 2023. Meta-analyses measuring the effect of CoQ10 on cardiac function [i.e., ejection fraction (EF), cardiac output (CO), cardiac index (CI), stroke volume (SV)], quality of life [i.e., mortality, exercise capacity, and New York Heart Association (NYHA) classification], and CoQ10 levels in HF were included. Ten meta-analyses met the inclusion criteria. CoQ10 had an effect on EF in 6 of the 9 studies, with an increase of 1.77% (0.10, 3.44) to 3.81% (1.22, 6.40), while it had an effect on CO, CI and SV in one of the two studies. Moreover, CoQ10 did not improve exercise capacity and only one study showed an effect on NYHA classification, while there was a risk ratio (RR) of 0.69 (0.50, 0.95) to 0.58 (0.35, 0.95) in favour of CoQ10 for mortality and a RR of 0.62 (0.49, 0.78) for hospitalisations. Finally, CoQ10 levels were found to increase by 1.40 μg mL−1 in all studies. CoQ10 showed a possible beneficial effect on heart function, which was associated with a reduction in mortality and hospitalisations. However, more research is needed into the conditions that may optimise CoQ10 therapy.
1. Introduction
Heart failure (HF) is a complex clinical syndrome resulting from structural or functional impairment of ventricular filling or blood ejection.1 In developed countries, the prevalence ranged from 1 to 2% of the adult population, increasing with age to over 10% in people aged over 80 years.1–3 The main causes are chronic arterial hypertension, ischaemic heart disease, toxic damage, genetic alterations, and some arrhythmias.4 HF is characterised by dyspnoea, fluid retention in the lower extremities and fatigue. It may be accompanied by other signs such as increased jugular venous pressure, peripheral oedema and pulmonary crackles.5 As HF progresses, acute decompensation without full recovery becomes more common, which can eventually lead to death.5,6 Usual treatment includes regular aerobic exercise appropriate to the condition, control of cardiovascular risk factors, and drug therapy, particularly angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor antagonists (ARBs) and beta-blockers, which reduce cardiac remodelling and improve heart function.6–11In addition to the above therapeutic approaches, coenzyme Q10 (CoQ10) supplementation has been proposed for patients with HF, as serum and tissue levels are inversely correlated with symptom severity.12,13 CoQ10 is synthesised in the human body and is a fat-soluble substance that can be presented as ubiquinone (oxidised CoQ10) or ubiquinol (reduced CoQ10). It has several roles in the energy production of muscle cells, including those in the heart.12,14,15 CoQ10 is located in the inner membrane of the mitochondria, and participates in the electron transport chain in which the cell obtains the energy in the form of ATP necessary for its growth and structural and functional maintenance.14,16 It also protects cell membranes from free radical damage, acting as a non-enzymatic endogenous antioxidant, in fact, plasma concentrations of CoQ10 and the ubiquinone–ubiquinol ratio are used as a marker of oxidative stress. Tissues with higher energy requirements or high metabolic activity such as the heart, kidney, liver, and muscle contain higher relative concentrations of CoQ10.14–19
Although this is sufficient in normal situations with endogenous synthesis of CoQ10 and a normal diet, supplementation may be required in HF as these patients are CoQ10 deficient.19 Therefore, the aim of this review of systematic reviews and meta-analyses was to synthesise the available evidence on the efficacy of CoQ10 supplementation on heart function, mortality and quality of life in participants with HF.
2. Methods
This review of systematic reviews and meta-analyses of randomised clinical trials was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines and the recommendations of the Cochrane Collaboration Handbook.20,21 The protocol was previously submitted to PROSPERO (CRD42023407503).2.1. Search strategy
A systematic search of the Medline (via Pubmed), Scopus, Web of Science and Cochrane Library databases was carried out from inception to March 2023. The search included the following terms: heart failure, heart disease, cardiovascular disease, cvd, q10, coq10, coenzyme q10, ubiquinone, ubiquinol, ubidecarenone, and meta-analysis, using the Boolean operators AND/OR and the PICO (participants, intervention, comparator and outcome/result) structure, adapted to the nature of this review. A search of the grey literature, including Google Scholar, Open Grey and the Networked Digital Library of Theses and Dissertations databases, and a review of references from previous reviews were also carried out. Where necessary, study authors were contacted. The full search is described in ESI Table S1.†The systematic search was carried out independently by two reviewers (E. A.-V. and C. P.-M.) and disagreements were resolved by consensus or by a third reviewer (I. S.-D.).
2.2. Inclusion/exclusion criteria
Inclusion criteria were as follows: (1) population – participants with chronic heart failure; (2) study design – systematic reviews of randomised clinical trials; (3) intervention – CoQ10 supplementation to usual care; (4) outcomes – primary: cardiac function, including ejection fraction (EF), cardiac output (CO), cardiac index (CI), and stroke volume (SV); and secondary: quality of life, including New York Heart Association (NYHA) classification, exercise capacity, mortality, hospitalisations, and CoQ10 blood levels. There were no language restrictions.Exclusion criteria were as follows: (1) systematic reviews without meta-analysis of the outcomes studied; (2) supplementation of coenzyme Q10 with other substances (e.g., other nutraceuticals or phytotherapeutics).
The selection was carried out independently by two reviewers (E. A.-V. and C. P.-M.) and disagreements were resolved by consensus or by a third reviewer (I. S.-D.).
2.3. Data extraction
An ad hoc table was created with the following data extracted from the included studies: (1) reference (authors and year of publication); (2) year of studies included in meta-analysis (minimum-maximum); (3) number of studies included in each meta-analysis; (4) sample size of each meta-analysis; (5) age of participants in studies included in each meta-analysis (minimum–maximum); (6) daily dose of CoQ10 in milligrams; (7) duration of included interventions; (8) outcomes.2.4. Risk of bias assessment
The AMSTAR 2 tool was used to assess the risk of bias.22 The tool uses 16 items to score each study. Depending on the domains involved and whether they are critical or not, each study can receive an overall rating from high to critically low quality review.The risk of bias was assessed independently by two reviewers (E. A.-V. and C. P.-M.) and disagreements were resolved by consensus or by a third reviewer (I. S.-D.).
2.5. Quality of evidence assessment
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) tool was used to assess the quality of evidence.23 This tool rates the evidence for each intervention outcome as high, moderate, low or very low. It takes into account the study design, factors that reduce the quality of the evidence (risk of bias, indirect evidence, publication bias, imprecision) and factors that increase the quality of the evidence (confounding variables that may reduce the observed effect, dose–response gradient, and whether there is a large effect or association).2.6. Data synthesis
An ad hoc table was created to summarise the results for each outcome, and forest plots were used to present them graphically.Continuous variables were expressed as mean difference (MD) or standardised mean difference (SMD) and their confidence intervals (95% CI), while mortality was expressed as risk ratio (RR), odds ratio (OR) or hazard ratio (HR) and its 95% CI. The 95% CI was extracted directly or calculated from the standard error (SE) extracted from the studies or, in exceptional cases, from the p-value.24 SMD effect sizes were considered small, medium or large if they were at or close to 0.2, 0.5 and 0.8 respectively.25
Heterogeneity (I2) from the original meta-analyses was included in the forest plots. Heterogeneity was assessed using the I2 statistic. Heterogeneity was classified as not important (I2 < 30%), moderate (I2 = 30–50%), substantial (I2 = 50–75%) or considerable (I2 > 75%).21,26
The statistical programme Stata v15 (StataCorp, College Station, Texas, USA) was used for the graphical presentation of the results.
3. Results
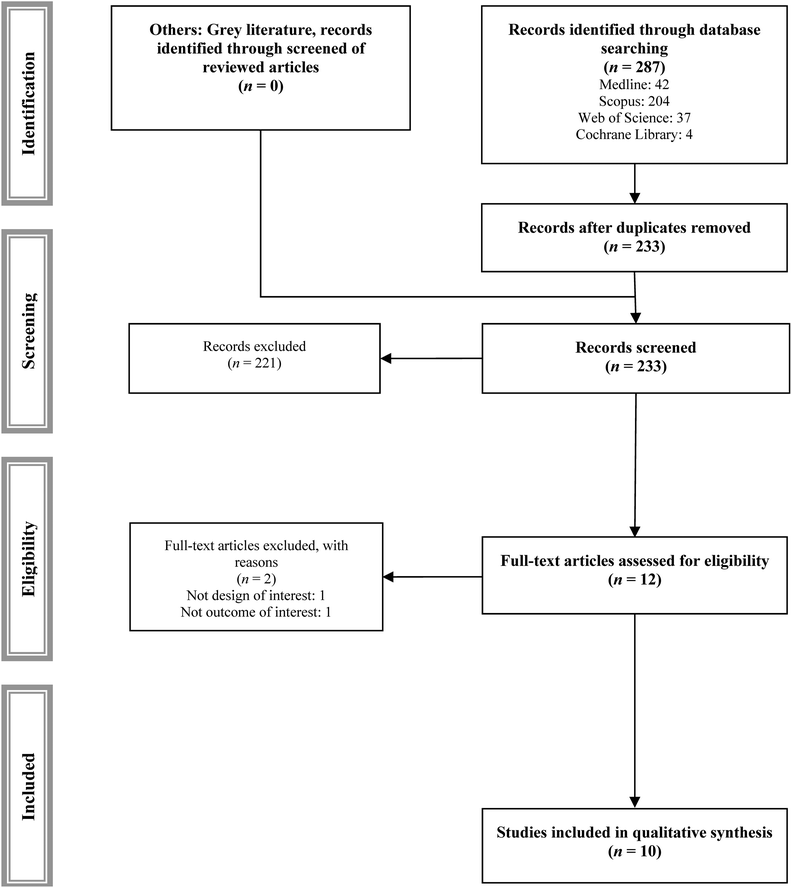
Of the 287 studies identified, 10 studies met the inclusion and exclusion criteria (Table 1),27–36 and 2 studies were excluded with justified reasons (Fig. 1 and ESI Table S2†).37,38| Ref. | Year | N | Sample size | Age | Dosage (mg) | Length | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF | CO | CI | SV | EX | NY | MT | HP | LE | |||||||
| Abbreviations: N, number of trials; EF, ejection fraction; CO, cardiac output; CI, cardiac index; SV, stroke volume; EX, exercise capacity; NY; NYHA classification; MT, mortality; HP, hospitalisations; LE, CoQ10 blood levels. | |||||||||||||||
| Al Saadi T. et al. (2021)27 | 1993–2019 | 11 | 1573 | All | 30–400 | 1–106 | ✓ | — | — | — | ✓ | — | ✓ | ✓ | ✓ |
| Ba Y.-J. et al. (2016)28 | 1992–2015 | 24 | 2883 | 24.4–89.0 | 20–300 | 4–104 | ✓ | — | — | — | ✓ | ✓ | ✓ | — | — |
| Fotino A. D. et al. (2013)29 | 1985–2005 | 13 | 395 | 49.8–68.0 | 60–300 | 2–28 | ✓ | — | — | — | — | ✓ | — | — | ✓ |
| Lei L. et al. (2017)30 | 1992–2015 | 14 | 2149 | 6.0–78.0 | 66–400 | NA | ✓ | — | — | — | ✓ | ✓ | ✓ | - | - |
| Madmani, M. et al. (2013)31 | 1993–2009 | 7 | 914 | All | 60–200 | <52 | ✓ | — | — | — | ✓ | — | — | — | ✓ |
| Rosenfeldt F. et al. (2003)32 | 1985–2003 | 9 | 824 | All | 100–200 | 3–52 | ✓ | — | — | — | — | — | ✓ | — | ✓ |
| Sander S. et al. (2006)33 | 1985–2003 | 11 | 297 | 50.0–67.0 | 60–200 | 4–24 | ✓ | ✓ | ✓ | ✓ | — | — | — | — | — |
| Soja A. et al. (1997)34 | 1986–1995 | 8 | 356 | NA | NA | NA | ✓ | ✓ | ✓ | ✓ | — | — | — | — | — |
| Trongtorsak A. et al. (2017)35 | NA | 16 | 1662 | NA | NA | NA | ✓ | — | — | — | — | — | ✓ | ✓ | ✓ |
| Yu Mareev V. et al. (2022)36 | 2015–2018 | 4 | 1139 | 68.3 | 2 mg kg−1–300 mg | NA | — | — | — | — | — | — | ✓ | — | — |
The publication years of the meta-analyses ranged from 1997 to 2022, and the trials were conducted between 1985 and 2019. The participants in the trials were mostly adults, although some trials included people of all ages. The study interventions consisted of CoQ10 supplementation in addition to usual care for participants in the intervention group and a placebo in the control group. Multiple treatments (e.g., with statins) were also included as long as the control group received the same treatment except for CoQ10. The daily doses of CoQ10 ranged from 20 to 400 mg, and the duration of the interventions ranged from 1 week to 2 years. EF was included in 9 studies, mortality in 6 studies, blood CoQ10 levels in 5 studies, exercise capacity in 4 studies, NYHA classification in 3 studies, and CO, CI, SV and hospitalisations in 2 studies each.
3.1. Systematic review
Fig. 2–4 show the effect of the intervention in the different included studies for the different outcomes.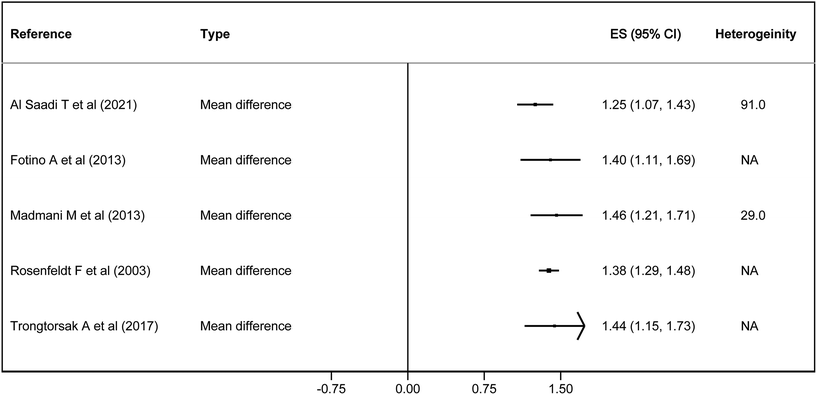 | ||
| Fig. 4 Forest plot of the effect of coenzyme Q10 on coenzyme Q10 blood levels, estimated as mean difference. | ||
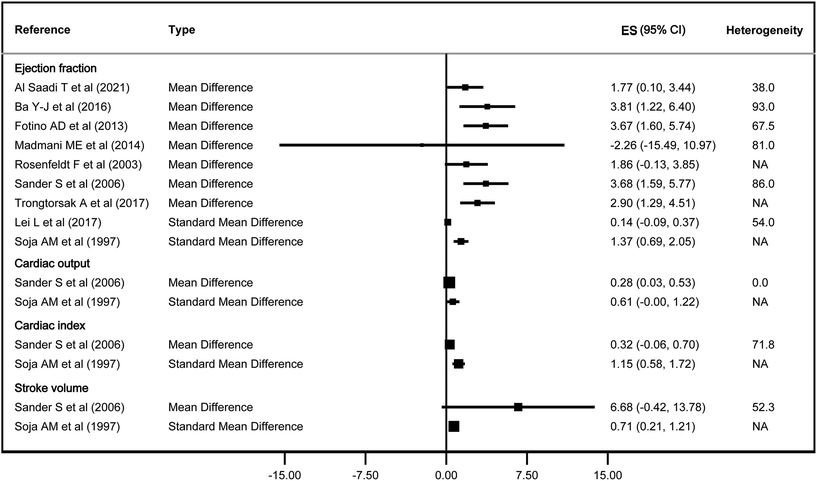
For cardiac function, CoQ10 increased EF by 1.77 to 3.81% in 5 out of 7 studies that estimated it using MD, while it had an effect of 1.37 in 1 out of 2 studies that estimated it using SMD, whereas for CI and SV it only had an effect in one of the two studies that estimated these parameters, with an SMD of 1.15 and 0.71, respectively. It did not have a statistically significant effect on CO (Fig. 2).
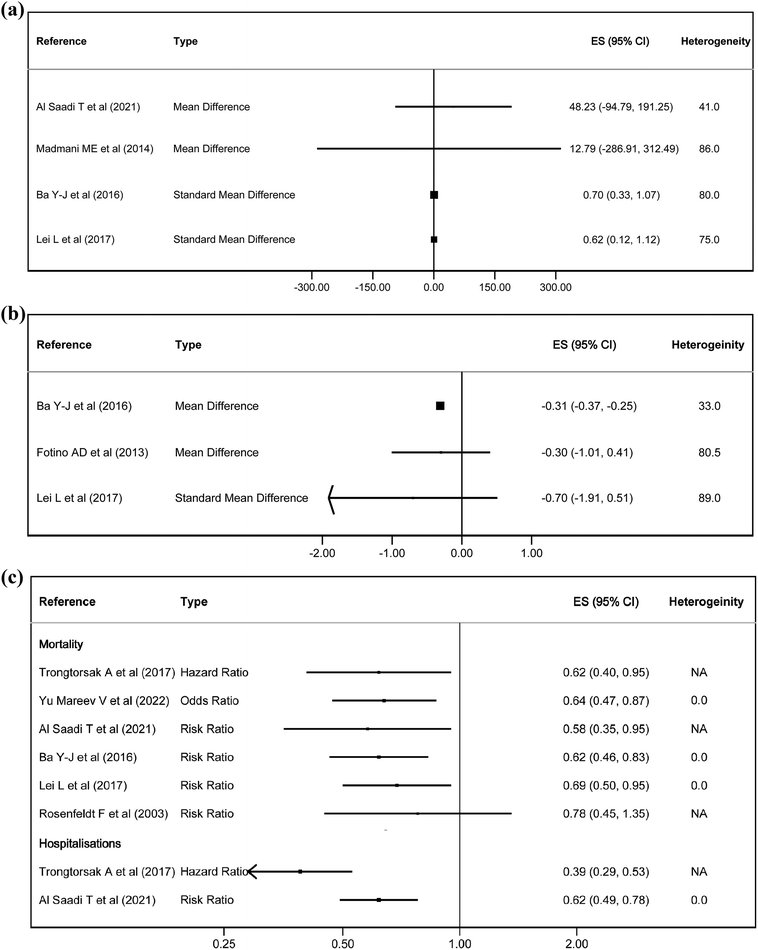
In terms of quality of life, two out of four studies showed some effect on exercise capacity, with an SMD of 0.62–0.70. In the NYHA classification, there was only an effect in one study with −0.31 points. On the other hand, it had a statistically significant effect on mortality in 5 studies, with an RR of 0.69–0.58, an OR of 0.64 and an HR of 0.62. It also reduced hospitalisations, with an RR of 0.62 and a HR of 0.39 (Fig. 3). Finally, CoQ10 increased blood CoQ10 levels from 1.25 to 1.46 μg ml−1 in all the studies (Fig. 4).
Heterogeneity was generally substantial or considerable for most studies and outcomes, except for mortality and hospitalisations, where it was 0%.
3.2. Risk of bias assessment
According to the AMSTAR 2 tool, 2 out of 10 studies (20.0%) had an overall rating of low, while the rest were rated as critically low. The most affected domains were those related to the explicit statement that the methodology was determined before the review was conducted and the lack of information on the funding of the studies included in the meta-analyses. The assessment of risk of bias is detailed in ESI Table S3.†3.3. Quality of evidence assessment
According to the GRADE tool, CoQ10 levels, mortality and hospitalisations had moderate certainty, while EF had low certainty. The remaining outcomes had very low certainty. The most affected domains were risk of bias, inconsistency and imprecision. The quality of the evidence is detailed in ESI Table S4.†4. Discussion
4.1. Main findings
Our review of systematic reviews and meta-analyses synthesised the evidence for the efficacy of CoQ10 supplementation in HF. The findings suggest that CoQ10 may improve cardiac function (EF, SV, CO, and CI), with statistically significant results being obtained in more than half of the studies that measured these outcomes, although the effect was limited. However, the effect on NYHA classification and exercise capacity was inconsistent. Conversely, CoQ10 supplementation could significantly reduce mortality and hospitalisations and increase blood levels of CoQ10.4.2. Interpretation
With regard to cardiac function, there is some evidence that CoQ10 could improve EF and perhaps closely related outcomes such as SV, CO and CI. EF improved between 1.7 and 3.8%, except in one meta-analysis due to the inclusion of only 2 trials. This effect is smaller than, for example, carvedilol or metoprolol, which increase EF by 5 and 7.4% respectively.39 This improvement in EF could be due to an improvement in mitochondrial ATP production, a reduction in cellular and mitochondrial reactive oxygen species, and a reduction in peripheral vascular resistance.40–44 The latter may explain the finding in one of the included meta-analyses that the people who responded best were those who had not been treated with ACEIs.33 There is also the possibility that patients with more advanced HF (EF < 30%) may respond less well to supplementation with the commonly used doses of CoQ10.29 This would explain the atypical positive effect of the oldest meta-analysis, which shows an SMD of 1.37, which is higher than what would be expected in the other meta-analyses, probably because this meta-analysis included trials with predominantly participants with EF > 30%, whereas later trials included patients with worse cardiac function.34The effect on mortality and hospitalisations was more interesting. Only the oldest study did not show a statistically significant reduction in mortality, while the two studies that measured hospitalisations also showed a reduction. This reduction in mortality could, with due caution, be comparable to that observed with other drugs, such as beta-blockers used in some patients with compensated HF.45 Although causality is difficult to establish, an inverse association has been observed between serum CoQ10 levels and mortality and symptom severity, which is consistent with the results of our review, in which an increase in CoQ10 levels was observed in all studies. Since there is altered synthesis of ATP, reactive oxygen species and the electron transport chain in HF, as mentioned above, CoQ10 may benefit by reversing these alterations.46 In addition, CoQ10 supplementation may also improve lipid and glycaemic parameters, endothelial function, lower systolic blood pressure, and reduce the proinflammatory state by reducing C-reactive protein and tumour necrosis factor alpha.47–53
Finally, the results for NYHA classification and exercise capacity were conflicting. However, some effect on NYHA classification cannot be ruled out, as the study with the largest number of trials found a positive effect.28 It should also be considered that this outcome could behave like EF, with the participants with worse heart function having the least effect. The same reasoning could be applied to exercise capacity. However, the use of different tests to assess exercise capacity and statistical analyses make it difficult to interpret the data.
Our study has several clinical and research implications. First, one of the biggest challenges in this area is to determine which supplements may be beneficial, which may not be beneficial, or which may be harmful in HF, especially considering that they are sold without any kind of regulation. In this sense, although not our original aim, CoQ10 appears to be safe in the treatment of HF. Second, and related to the above, more research is needed into the conditions under which its administration may or may not be effective (i.e., use of ubiquinone or ubiquinol, co-administration with other treatments that could interfere with its effect, etc.). For example, some authors suggest that the effect of ubiquinol is greater than ubiquinone, at least in advanced HF, because of greater bioavailability. However, most trials use ubiquinone.14,19,54 The optimal dose is also unclear, although the evidence suggests that the maximum effective dose would be 200 mg, unless future studies establish a narrower dose range.27 Third, and related to the above, trials are needed that thoroughly evaluate the effect of CoQ10 in combination with the use of the most commonly used drugs in HF (i.e., ACEIs, ARBs, beta-blockers). Although it is possible that the effect of CoQ10 is reduced with some of these drugs, because the effect of CoQ10 is reduced, it cannot be a substitute for usual care. However, the benefit of CoQ10 does not seem to be limited to cardiac function, but could have an effect on mortality, an important outcome in this disease.
4.3. Limitations
Our review had the following limitations. Fist, some of the results included a limited number of meta-analyses, which limits the interpretation of the results. Second, the risk of bias was high in all studies. This is not uncommon as the requirements for systematic reviews have evolved. Third, different instruments were used to measure outcomes and it cannot be excluded that this affected the estimates obtained. Fourth, some meta-analyses did not assess the risk of bias of the included trials and/or did not discuss their possible impact on the results obtained, so the results could be biased. Fifth, the interventions in each meta-analysis varied in terms of participants, CoQ10 dose and duration, which limited the interpretation of the results. Sixth, the heterogeneity reported by the authors was high for some outcomes. Seventh, the baseline health conditions of the participants could have been very different, as it is not specified whether the participants had other pathologies in addition to HF.5. Conclusions
CoQ10 supplementation may have some benefits for heart function because of its role in the electron transport chain and its antioxidant effect. Thus, our study suggests that this supplementation tends to be beneficial for some outcomes of cardiac function, particularly EF and mortality, because these were the outcomes studied and there was more evidence of a positive effect. This is because CoQ10 may have more benefits than just improving heart function, such as improving some lipid, metabolic and inflammatory parameters. Due to the limitations of our study and the limitations of the included studies, the most appropriate interventions and optimal doses based on clinical stage are unknown, so further research is needed on dose, presentation of CoQ10, and length of interventions to determine which may be more optimal.Author contributions
Conceptualisation: E. A.-V.; methodology: E. A.-V. and C. P.-M.; data curation and investigation: E. A.-V. and C. P.-M.; formal analysis: E. A.-V., I. M.-G., I. S.-D., and E. R.-G.; validation and visualisation: E. A.-V., I. M.-G., and E. R.-G.; writing – original draft preparation: E. A.-V., and C. P.-M.; writing – review and editing: E. A.-V., I. S.-D., N. M.-H., and C. P.-M.; supervision: C. P.-M.; funding acquisition: C. P.-M.; project administration: C. P.-M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This study was supported by the University of Castilla-La Mancha, of which the authors are members.References
- J. J. V. McMurray and M. A. Pfeffer, Heart failure, Lancet, 2005, 365, 1877–1889 CrossRef PubMed.
- B. Ziaeian and G. C. Fonarow, Epidemiology and aetiology of heart failure, Nat. Rev. Cardiol., 2016, 13, 368–378 CrossRef PubMed.
- D. M. Lloyd-Jones, M. G. Larson, E. P. Leip, A. Beiser, R. B. D'Agostino, W. B. Kannel, J. M. Murabito, R. S. Vasan, E. J. Benjamin and D. Levy, Lifetime risk for developing congestive heart failure: the Framingham Heart Study, Circulation, 2002, 106, 3068–3072 CrossRef PubMed.
- A. L. Bui, T. B. Horwich and G. C. Fonarow, Epidemiology and risk profile of heart failure, Nat. Rev. Cardiol., 2011, 8, 30–41 CrossRef PubMed.
- M. King, J. Kingery and B. Casey, Diagnosis and evaluation of heart failure, Am. Fam. Physician, 2012, 85, 1161–1168 Search PubMed.
- E. J. Brennan, Chronic heart failure nursing: integrated multidisciplinary care, Br. J. Nurs., 2018, 27, 681–688 CrossRef PubMed.
- N. P. Nikitin, R. de Silva and J. G. F. Cleland, The utility of a comprehensive cardiac magnetic resonance examination for the evaluation of patients with heart failure, Heart, 2004, 90, 1166 CrossRef CAS PubMed.
- P. Rossignol, A. F. Hernandez, S. D. Solomon and F. Zannad, Heart failure drug treatment, Lancet, 2019, 393, 1034–1044 CrossRef CAS PubMed.
- T. A. McDonagh, M. Metra, M. Adamo, R. S. Gardner, A. Baumbach, M. Böhm, H. Burri, J. Butler, J. Čelutkienė, O. Chioncel, J. G. F. Cleland, A. J. S. Coats, M. G. Crespo-Leiro, D. Farmakis, M. Gilard, S. Heymans, A. W. Hoes, T. Jaarsma, E. A. Jankowska, M. Lainscak, C. S. P. Lam, A. R. Lyon, J. J. V. McMurray, A. Mebazaa, R. Mindham, C. Muneretto, M. F. Piepoli, S. Price, G. M. C. Rosano, F. Ruschitzka and A. K. Skibelund, ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure, Eur. Heart J., 2021, 42, 3599–3726 CrossRef CAS PubMed.
- V. L. Roger, Heart Failure, Internationa Encyclopedia of Public Health, Elsevier, 2017, 520–526 Search PubMed.
- M. Jessup and S. Brozena, Heart failure, N. Engl. J. Med., 2003, 348, 2007–2018 CrossRef PubMed.
- D. Alf, M. E. Schmidt and S. C. Siebrecht, Ubiquinol supplementation enhances peak power production in trained athletes: a double-blind, placebo controlled study, J. Int. Soc. Sports Nutr., 2013, 10, 24 CrossRef CAS PubMed.
- C. Kawashima, Y. Matsuzawa, M. Konishi, E. Akiyama, H. Suzuki, R. Sato, H. Nakahashi, S. Kikuchi, Y. Kimura, N. Maejima, N. Iwahashi, K. Hibi, M. Kosuge, T. Ebina, K. Tamura and K. Kimura, Ubiquinol Improves Endothelial Function in Patients with Heart Failure with Reduced Ejection Fraction: A Single-Center, Randomized Double-Blind Placebo-Controlled Crossover Pilot Study, Am. J. Cardiovasc. Drugs, 2020, 20, 363–372 CrossRef CAS PubMed.
- A. Di Lorenzo, G. Iannuzzo, A. Parlato, G. Cuomo, C. Testa, M. Coppola, G. D′ambrosio, D. A. Oliviero, S. Sarullo, G. Vitale, C. Nugara, F. M. Sarullo and F. Giallauria, Clinical evidence for Q10 coenzyme supplementation in heart failure: From energetics to functional improvement, J. Clin. Med., 2020, 9(5), 1266 CrossRef PubMed.
- V. I. Zozina, S. Covantev, O. A. Goroshko, L. M. Krasnykh and V. G. Kukes, Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem, Curr. Cardiol. Rev., 2018, 14, 164–174 CrossRef CAS PubMed.
- J. Jankowski, K. Korzeniowska, A. Cieślewicz and A. Jabłecka, Coenzyme Q10 - A new player in the treatment of heart failure?, Pharmacol. Rep., 2016, 68, 1015–1019 CrossRef CAS PubMed.
- J. Garrido-Maraver, M. D. Cordero, M. Oropesa-Avila, A. F. Vega, M. de la Mata, A. D. Pavon, E. Alcocer-Gomez, C. P. Calero, M. V. Paz, M. Alanis, I. de Lavera, D. Cotan and J. A. Sanchez-Alcazar, Clinical applications of coenzyme Q10, Front. Biosci., 2014, 19, 619–633 CrossRef CAS PubMed.
- A. Sharma, G. C. Fonarow, J. Butler, J. A. Ezekowitz and G. M. Felker, Coenzyme Q10 and Heart Failure: A State-of-the-Art Review, Circ.: Heart Failure, 2016, 9, e002639 CAS.
- S. Oleck and H. O. Ventura, Coenzyme Q10 and Utility in Heart Failure: Just Another Supplement?, Curr. Heart Failure Rep., 2016, 13, 190–195 CrossRef CAS PubMed.
- D. Moher, A. Liberati, J. Tetzlaff, D. G. Altman, D. Altman, G. Antes, D. Atkins, V. Barbour, N. Barrowman, J. A. Berlin, J. Clark, M. Clarke, D. Cook, R. D’Amico, J. J. Deeks, P. J. Devereaux, K. Dickersin, M. Egger, E. Ernst, P. C. Gøtzsche, J. Grimshaw, G. Guyatt, J. Higgins, J. P. A. Ioannidis, J. Kleijnen, T. Lang, N. Magrini, D. McNamee, L. Moja, C. Mulrow, M. Napoli, A. Oxman, B. Pham, D. Rennie, M. Sampson, K. F. Schulz, P. G. Shekelle, D. Tovey and P. Tugwell, Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement, PLoS Med., 2009, 6(7), e1000097 CrossRef PubMed.
- J. P. Higgins and S. Green, Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Serie, John Wiley and Sons, 2008 Search PubMed.
- B. J. Shea, B. C. Reeves, G. Wells, M. Thuku, C. Hamel, J. Moran, D. Moher, P. Tugwell, V. Welch, E. Kristjansson and D. A. Henry, AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both, Br. Med. J., 2017, 358, j4008 CrossRef PubMed.
- G. Guyatt, A. D. Oxman, E. A. Akl, R. Kunz, G. Vist, J. Brozek, S. Norris, Y. Falck-Ytter, P. Glasziou, H. DeBeer, R. Jaeschke, D. Rind, J. Meerpohl, P. Dahm and H. J. Schünemann, GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables, J. Clin. Epidemiol., 2011, 64, 383–394 CrossRef PubMed.
- D. G. Altman and J. M. Bland, How to obtain the confidence interval from a P value, Br. Med. J., 2011, 343, d2090 CrossRef PubMed.
- J. Cohen, A power primer, Psychol. Bull., 1992, 112, 155–159 CAS.
- J. P. T. Higgins and S. G. Thompson, Quantifying heterogeneity in a meta-analysis, Stat. Med., 2002, 21, 1539–1558 CrossRef PubMed.
- T. Al Saadi, Y. Assaf, M. Farwati, K. Turkmani, A. Al-Mouakeh, B. Shebli, M. Khoja, A. Essali and M. E. Madmani, Coenzyme Q10 for heart failure, Cochrane Database Syst. Rev., 2021, 2, CD008684 Search PubMed.
- Y.-J. Ba, M. Yang, Y. Sun, Z. Li, G. Cheng, S. Zhang and M.-J. Zou, Efficacy of coenzyme Q10 as adjuvant therapy in heart failure: A meta-analysis, Chin. J. Evid.-Based Med., 2016, 16, 311–318 Search PubMed.
- A. D. Fotino, A. M. Thompson-Paul and L. A. Bazzano, Effect of coenzyme Q10 supplementation on heart failure: a meta-analysis, Am. J. Clin. Nutr., 2013, 97, 268–275 CrossRef CAS PubMed.
- L. Lei and Y. Liu, Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials, BMC Cardiovasc. Disord., 2017, 17, 196 CrossRef PubMed.
- M. E. Madmani, A. Yusuf Solaiman, K. Tamr Agha, Y. Madmani, Y. Shahrour, A. Essali and W. Kadro, Coenzyme Q10 for heart failure, Cochrane Database Syst. Rev., 2014, 6, CD008684 Search PubMed.
- F. Rosenfeldt, D. Hilton, S. Pepe and H. Krum, Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure, Biofactors, 2003, 18, 91–100 CrossRef CAS PubMed.
- S. Sander, C. I. Coleman, A. A. Patel, J. Kluger and C. M. White, The impact of coenzyme Q10 on systolic function in patients with chronic heart failure, J. Card. Failure, 2006, 12, 464–472 CrossRef CAS PubMed.
- A. M. Soja and S. A. Mortensen, Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials, Mol. Aspects Med., 1997, 18 Suppl, S159–S168 CrossRef CAS PubMed.
- A. Trongtorsak, K. Kongnatthasate, P. Susantitaphong, V. Kittipibul and A. Ariyachaipanich, Effect of coenzyme Q10 on left ventricular remodeling and mortality in patients with heart failure: a meta-analysis, J. Am. Coll. Cardiol., 2017, 69, 707 CrossRef.
- V. Y. Mareev, Y. V. Mareev and Y. L. Begrambekova, [Coenzyme Q-10 in the treatment of patients with chronic heart failure and reduced left ventricular ejection fraction: systematic review and meta-analysis], Kardiologiia, 2022, 62, 3–14 CrossRef PubMed.
- M. Banach, C. Serban, S. Ursoniu, J. Rysz, P. Muntner, P. P. Toth, S. R. Jones, M. Rizzo, S. P. Glasser, G. F. Watts, R. S. Blumenthal, G. Y. H. Lip, D. P. Mikhailidis and A. Sahebkar, Lipid and B. P. M. C. (LBPMC) Group, Statin therapy and plasma coenzyme Q10 concentrations - A systematic review and meta-analysis of placebo-controlled trials, Pharmacol. Res., 2015, 99, 329–336 CrossRef CAS PubMed.
- S. Pepe, S. F. Marasco, S. J. Haas, F. L. Sheeran, H. Krum and F. L. Rosenfeldt, Coenzyme Q10 in cardiovascular disease, Mitochondrion, 2007, 7, S154–S167 CrossRef CAS PubMed.
- L. C. van Campen, F. C. Visser and C. A. Visser, Ejection fraction improvement by beta-blocker treatment in patients with heart failure: an analysis of studies published in the literature, J. Cardiovasc. Pharmacol., 1998, 32(Suppl 1), S31–S35 CAS.
- K. Folkers, Perspectives from research on vitamins and hormones, J. Chem. Educ., 1984, 61, 747–756 CrossRef CAS.
- J. M. Hodgson and G. F. Watts, Can coenzyme Q10 improve vascular function and blood pressure? Potential for effective therapeutic reduction in vascular oxidative stress, Biofactors, 2003, 18, 129–136 CrossRef CAS PubMed.
- M. F. McCarty, Coenzyme Q versus hypertension: does CoQ decrease endothelial superoxide generation?, Med. Hypotheses, 1999, 53, 300–304 CrossRef CAS PubMed.
- P. Forsmark-Andrée, C. P. Lee, G. Dallner and L. Ernster, Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles, Free Radicals Biol. Med., 1997, 22, 391–400 CrossRef PubMed.
- R. Ferrari, G. Guardigli, D. Mele, G. F. Percoco, C. Ceconi and S. Curello, Oxidative stress during myocardial ischaemia and heart failure, Curr. Pharm. Des., 2004, 10, 1699–1711 CrossRef CAS PubMed.
- F. A. McAlister, N. Wiebe, J. A. Ezekowitz, A. A. Leung and P. W. Armstrong, Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure, Ann. Intern. Med., 2009, 150, 784–794 CrossRef PubMed.
- K. J. Filipiak, S. Surma, M. Romańczyk and B. Okopień, Heart Failure-Do We Need New Drugs or Have Them Already? A Case of Coenzyme Q10, J. Cardiovasc. Dev. Dis., 2022, 9(5), 161 CrossRef CAS PubMed.
- M. V. Jorat, R. Tabrizi, N. Mirhosseini, K. B. Lankarani, M. Akbari, S. T. Heydari, R. Mottaghi and Z. Asemi, The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials, Lipids Health Dis., 2018, 17, 230 CrossRef CAS PubMed.
- B.-J. Lee, Y.-C. Lin, Y.-C. Huang, Y.-W. Ko, S. Hsia and P.-T. Lin, The relationship between coenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronary artery disease, Sci. World J., 2012, 2012, 792756 Search PubMed.
- L. Gao, Q. Mao, J. Cao, Y. Wang, X. Zhou and L. Fan, Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials, Atherosclerosis, 2012, 221, 311–316 CrossRef CAS PubMed.
- A. F. G. Cicero, F. Fogacci and A. Colletti, Commentary to: “The Effects of Coenzyme Q10 Supplementation on Blood Pressures Among Patients with Metabolic Diseases: A Systematic Review and Meta-analysis of Randomized Controlled Trials”, High Blood Pressure Cardiovasc. Prev., 2018, 25, 51–52 CrossRef PubMed.
- P. V. Dludla, P. Orlando, S. Silvestri, F. Marcheggiani, I. Cirilli, T. M. Nyambuya, V. Mxinwa, K. Mokgalaboni, B. B. Nkambule, R. Johnson, S. E. Mazibuko-Mbeje, C. J. F. Muller, J. Louw and L. Tiano, Coenzyme Q10 supplementation improves adipokine levels and alleviates inflammation and lipid peroxidation in conditions of metabolic syndrome: A meta-analysis of randomized controlled trials, Int. J. Mol. Sci., 2020, 21(9), 3247 CrossRef CAS PubMed.
- J. Zhai, Y. Bo, Y. Lu, C. Liu and L. Zhang, Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis, PLoS One, 2017, 12, e0170172 CrossRef PubMed.
- L. Fan, Y. Feng, G.-C. Chen, L.-Q. Qin, C.-L. Fu and L.-H. Chen, Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials, Pharmacol. Res., 2017, 119, 128–136 CrossRef CAS PubMed.
- I. Cirilli, E. Damiani, P. V. Dludla, I. Hargreaves, F. Marcheggiani, L. E. Millichap, P. Orlando, S. Silvestri and L. Tiano, Role of Coenzyme Q(10) in Health and Disease: An Update on the Last 10 Years (2010–2020), Antioxidants, 2021, 10, 8, DOI:10.3390/antiox10081325.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo01255g |
| This journal is © The Royal Society of Chemistry 2023 |