Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
How can chokeberry (Aronia) (poly)phenol-rich supplementation help athletes? A systematic review of human clinical trials†
Reza
Zare
 ab,
Rachel
Kimble
ab,
Rachel
Kimble
 c,
Ali
Ali Redha
c,
Ali
Ali Redha
 *de,
Giuseppe
Cerullo
*de,
Giuseppe
Cerullo
 f and
Tom
Clifford
f and
Tom
Clifford
 g
g
aMeshkat Sports Complex, Karaj, Alborz Province, Iran
bArses Sports Complex, Karaj, Alborz Province, Iran
cDivision of Sport and Exercise Science, School of Health and Life Sciences, University of the West of Scotland, Blantyre, UK
dThe Department of Public Health and Sport Sciences, University of Exeter Medical School, Faculty of Health and Life Sciences, University of Exeter, Exeter, EX1 2LU, UK. E-mail: aa1249@exeter.ac.uk
eCentre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation (QAAFI), The University of Queensland, Brisbane, QLD 4072, Australia
fDepartment of Biomedical Sciences, University of Padua, 35131, Padova, Italy
gSchool of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, LE11 3TU, UK
First published on 5th June 2023
Abstract
Athletes are increasingly consuming (poly)phenol supplements to modify oxidative stress and/or exercise-induced inflammation, in the hope that this will enhance exercise performance. Chokeberries are rich in (poly)phenols and may therefore influence the health and performance of athletes. The objective of this systematic review was to comprehensively explore the effects of chokeberry supplementation on performance and exercise-induced biomarkers of oxidative stress, inflammation, and haematology in the athletic population. A search was conducted in PubMed, Web of Science, and SCOPUS. Studies were included if the participants were athletes, supplemented with chokeberry or chokeberry-based products, and evaluated sports-related outcomes. A total of ten articles were included in the study. The participants of all the studies were athletes and included rowers, football players, handball players, triathletes, and runners. A qualitative comprehensive summary of the applications of chokeberry supplementation targeting the athletic population has been evaluated. This included the effect of chokeberry supplementation on redox status, exercise-induced inflammation, haematology, iron metabolism, platelet aggregation, metabolic markers, body composition, and exercise performance. Chokeberry (poly)phenol-rich supplementation may be effective in enhancing the redox balance of athletes, yet more evidence is required to provide solid conclusions on its effect on inflammation, platelet function, iron metabolism and exercise performance.
1. Introduction
Black chokeberry (Aronia melanocarpa L.) is a perennial shrub belonging to the Rosaceae family and native to the eastern regions of North America.1 Chokeberries are gaining popularity among consumers due to their high amount of (poly)phenols which are regarded as crucial for chokeberry fruits’ high level of bioactivity.2 Chokeberries are among the best sources of (poly)phenols, rich in anthocyanins, flavonols, flavanols, proanthocyanidins, and phenolic acids.3 The (poly)phenol content of chokeberry fruit is reported to range from 6.3 g per 100 g dry material (DM)4 to 7.8 g per 100 g DM,5 which is approximately 3–8-fold that of red raspberry (Rubus idaeus) and 10-times that of strawberry (Fragaria ananassa).6 The dark blue colour of chokeberry fruit is due to the high concentration of anthocyanins, which represent ∼25% of the total (poly)phenol content.7 Interestingly, it has been reported that almost 40% of the antioxidant activity of chokeberries is related to proanthocyanidins.8 In terms of bioavailability, it has been reported that chokeberry anthocyanins are recovered in blood and urine in nanomolar concentrations, and glucuronidation and methylation are two of the common metabolism pathways of chokeberry anthocyanins.9 Generally, the bioavailability of anthocyanins is affected by the extensive gut microbial metabolism in the colon, leading to the breaking down of the anthocyanin heterocycle which results in a wide range of low molecular weight phenolic metabolites which are more bioavailable and have higher absorption rates.10(Poly)phenols are increasingly researched for their pleiotropic effects on human health. The common (poly)phenol intake in our diet could reach up to 1 g day−1, this is about 10–100 times more than the intake of other antioxidants.11 Most of the health benefits of (poly)phenols are ascribed to their antioxidant and anti-inflammatory activities. Such effects have led to interest from athletic populations. Indeed, antioxidant and anti-inflammatory supplements are purported to enhance exercise and/or accelerate recovery after strenuous exercise.12,13 However, their use by athletes remains controversial; while (poly)phenols regulate various mechanisms associated with exercise performance, antioxidants have also been suggested to blunt training adaptations.14,15 While exercise-induced increases in reactive oxygen species (ROS) production may be crucial for training adaptations,16 when ROS production exceeds the body's antioxidant capacity, the resulting oxidative stress may have a deleterious effect on recovery, performance, and general health.17
Growing evidence suggests that (poly)phenols can upregulate endogenous antioxidant capacity via the nuclear respiratory factor 2 (NRF2) pathway.18 NRF2, plays an important role in mitochondrial biogenesis, and variants of the NRF2 gene have also been associated with endurance performance.19 Another effect of oxidative damage is reduced vasodilatory capacity and blood flow.20 (Poly)phenols have been shown to enhance flow-mediated dilatation and endothelial function in humans by promoting endothelial nitric oxide (NO) synthesis.21 Furthermore, (poly)phenols have shown to reduce the formation of peroxynitrite by inhibiting NADPH oxidase, one of the key sources of superoxide production.22 At the same time, this increases endogenous antioxidant capacity and preserves NO bioavailability. In fact, it has been proposed that (poly)phenols can modulate gene expression in general by increasing the activity of transcription factors, but also by affecting the expression of microRNAs.23 Consequently, (poly)phenol supplementation may counteract fatigue and improve performance by improving the perfusion of the exercising muscle.24 In practice it has been suggested that acute supplementation (300 mg (poly)phenols 1–2 h before exercise) may exhibit ergogenic properties during endurance and repeated sprint exercise.24 On the other hand, chronic supplementation (more than 3 days with >1000 mg (poly)phenols prior to and following exercise) could be helpful to enhance recovery after muscle damage.
Due to chokeberry's high (poly)phenol content several studies have investigated the effects of supplementation with chokeberry fruits or derivates on biomarkers associated with sports and exercise performance and recovery, such as inflammatory status,25 oxidative stress,26–28 and body composition.29 Indeed, oxidative stress and inflammation are important factors closely related to muscle catabolism.30 Athletes performing exhausting exercises, involving heavy training loads, may be affected by sports anaemia due to depletion of iron reserves.31 This is caused by haemolysis, haematuria, and increase in plasma volume as a result of intensive exercise.32 (Poly)phenols may positively impact the cardiovascular status of athletes by inhibiting of platelet function33 and iron metabolism.25 This is achieved by targeting specific thrombogenic pathways.33 Indeed, chokeberry (poly)phenols have been evaluated for their effect on haematology, iron metabolism and platelet aggregation in athletes.26,28,29
Chokeberry fruit and chokeberry-based supplements are principally of interest to athletes due to their high (poly)phenol content in comparison to other sources of (poly)phenols, and therefore potential use as an antioxidant and anti-inflammatory. However, as chokeberry fruit may also impact haematology and iron metabolism, supplementation could alter several aspects of athlete's health status and ultimately enhance their performance. To our knowledge, the effects of black chokeberan ry-based supplementation in healthy people engaged in exercise, and athletes, have not been reviewed comprehensively. Therefore, the aim of this systematic review is to evaluate the biological effects of chokeberry fruit in athletic populations; specifically, the effects on inflammation, oxidative stress, iron metabolism, haematological markers, and performance.
2. Methods
The systematic review was conducted based on the recommendation of The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).34The protocol of this systematic review was registered at the Open Science Framework (https://doi.org/10.17605/OSF.IO/Y832W).
2.1. Literature search
Three electronic databases (PubMed, Web of Science, and SCOPUS) were searched for relevant studies on 17th November 2022 and updated on 14th May 2023. The search strategy was based on including (“chokeberry” OR “Aronia”) AND (“sport” OR “performance” OR “athlete” OR “exercise”). An example of the full electronic search strategy is provided in the ESI.† Two of the reviewers (RZ and AAR) were responsible for independently screening each article's title, abstract, and full text. A third reviewer (GC) arbitrated when needed.2.2. Study selection
The inclusion criteria of this systematic review were: (i) clinical trial (human study), (ii) participants supplemented with chokeberry or chokeberry-based products, (iii) participants were athletes and performed a type of sports activity as a part of a sports team or as independent professional/semi-professional/recreational athletes, or participants who were physically active performing at least 1 h of exercise per day for at least 3 days a week, (iv) study investigated sports and athletic-related outcomes that are linked with exercise performance and biomarkers, and (v) studies were peer-reviewed and written in English. For eligible studies, the PICO considered was as follows: population = athletes or physically active individuals, intervention = chokeberry supplement, comparison = not applicable, and outcomes = inflammation, oxidative stress, iron metabolism, haematological markers, and performance-related outcomes. The search strategy excluded (i) non-clinical studies (including in vitro and in vivo (animals)), (ii) clinical studies that did not use chokeberry-based supplements (iii) clinical studies with inactive or unhealthy participants, (iv) studies evaluating outcomes not relevant to exercise performance and athletic metabolic status or biomarkers, (v) non-English studies, and (vi) comments, editorials, or reviews.2.3. Data extraction
Two of the reviewers (RZ and AAR) independently extracted the data. The characteristics of the included studies are summarized in Table 1. The following information was extracted: background (name of the first author and year of publication), study design, focus topic, characteristics of athletes (number, sports performed, sex, and age), supplementation intervention (number of participants, supplementation form, and supplementation dosage), placebo intervention (number of participants, placebo form, and placebo dosage), study duration, experimental design, and key findings. We did not plan to conduct a meta-analysis due the heterogeneity in study designs, outcomes, and limited studies on this topic.| Authors | Study design | Topic | Athlete group | Training load | Supplemented group | Placebo group | Study duration | Experimental design | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: BMI – body mass index, CAT – catalase, GAE – gallic acid equivalents, GPx – glutathione peroxidase, HCT – haematocrit, HGB – haemoglobin, IL-6 – interleukin 6, IL-10 – interleukin 10, MCH – mean corpuscular haemoglobin, MCHC – mean corpuscular haemoglobin concentration, MCV – mean corpuscular volume, OHdG – 8-hydroxydeoxyguanosine, RBC – red blood cells, SOD – superoxide dismutase, TAC – total antioxidant capacity, TBARS – thiobarbituric acid reactive substances.a Training load quantification was performed using the Objective Load Scale (ECOs). | |||||||||
| Skarpańska-Stejnborn et al., 201425 | Randomised double-blind placebo-controlled trial | Inflammatory status and iron metabolism | Rowers: n = 19 – members of the Polish Rowing Team | Training phase (before first assessment): training volume = 1020 min per week (41% extensive rowing and 21% nonspecific training) | n = 10 | n = 9 | 8 weeks | Participants performed a 2000 m test on a rowing ergometer at the beginning and at the end of the preparatory camp. Blood samples were collected before each exercise test, one minute after completing the test, and after a 24-hour recovery period. The levels of hepcidin, interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-alpha), ferritin, iron, uric acid, myoglobin, total iron-binding capacity, unbound iron-binding capacity, and TAC were measured | Supplementation significantly decreased post-exercise levels of TNF-alpha and significantly increased TACs and iron levels |
| Average duration of training experience was 5.4 ± 1.1 years for the supplemented group and 5.7 ± 1.7 years for the placebo group | Before second assessment: training volume = 880 min per week (53% extensive rowing, 18% intensive rowing, and 11% land training) | Received 150 mL day−1 of chokeberry juice (with 24 mg mL−1 anthocyanin content); anthocyanin dosage = 3600 mg day−1 | Received 150 mL day−1 of placebo drink (containing 6.6% of betaine and 1% of citric acid) | ||||||
| Stevanovic et al., 202042 | Single-blind placebo-controlled crossover trial | Platelet activation and aggregation | Recreational runners: n = 10 males from Belgrade Urban Running Team, mean age, 30.8 years-old | Four practices per week (approximately 40 km of weekly running distance) | n = 5 | n = 5 | One day test, with 1 week washout | Participants received supplementation with breakfast meals. They warmed up and ran a 21.1 km long simulated half-marathon race. Blood samples were collected at baseline, and at 15 min, 1 h, and 24 h after running. Blood samples were analysed for platelet activation markers (P-selectin glycoprotein IIb/IIIa) and platelet-leukocyte (monocyte and neutrophil) aggregates | Supplementation significantly decreased the expression of platelet activation markers (P-selectin and glycoprotein IIb/IIIa) but did not influence platelet aggregation |
| Received 200 mL of (poly)phenol-rich aronia juice (containing 1296.8 mg GAE per 100 mL of phenolic compounds); (poly)phenol dosage = 2600 mg day−1 | Received 200 mL of placebo drink (containing the same amount of vitamin C, but no polyphenols) | ||||||||
| Stankiewicz et al., 202338 | Randomised double-blind placebo-controlled trial | Inflammatory status and iron metabolism | Semi-professional young male football players: n = 22 from MUKS Zawisza Bydgoszcz Soccer Club | No information was provided | n = 10 | n = 12 | 90 days | Participants performed maximal multistage 20 m shuttle run tests at the beginning and at the end of the 90 days supplementation period. Blood samples were collected at different times before and after exercise to measure the levels of IL-6, IL-10, ferritin, myoglobin, hepcidin, 8-OHdG, albumin, and TAC | Supplementation significantly increased distance run, IL-10 and TAC and decreased 8-OHdG and IL-6 |
| Received 6 g of lyophilised black chokeberry extract (containing 170–210 μmol Trolox per mL of antioxidants) | Received identical-looking rice-flavoured gelatine capsules | ||||||||
| Pilaczynska-Szczesniak et al., 200526 | Randomised double-blind placebo-controlled trial | Oxidative stress | Rowers: n = 19 – members of the Polish Rowing Team | No precise information was provided other than being during a 1-month training camp between the preparation period and the competition period | n = 9 | n = 10 | 1 month | An incremental rowing exercise test was performed before and after the supplementation period. Blood samples were collected before each exercise test, 1 min after the test, and following a 24 h recovery period. Redox parameters (SOD, GPx, and TBARS), HGB, create kinase activity, and lactate levels in blood were measured | Supplementation decreased the level of glutathione peroxidase activity determined after 1 min after the exercise test, and decreased superoxide dismutase activity significantly following the 24 h recovery period in comparison to placebo |
| Average duration of training experience was 6.0 ± 1.0 years for the supplemented group and 8.0 ± 2.4 years for the placebo group | Received 150 mL day−1 of chokeberry juice (with 23 mg mL−1 anthocyanin content); anthocyanin dosage = 3450 mg day−1 | Received 150 mL day−1 of placebo drink (containing 6.6% of betaine and 1% of citric acid) | |||||||
| Stankiewicz et al., 202128 | Randomised double-blind placebo-controlled trial | Oxidative stress | Young semi-professional football players: male; n = 20; mean age, 15.8 years-old | Training total time: 510–540 min per week (including low, medium, and high-intensity exercise) | n = 12 | n = 8 | 7 weeks | Participants received supplementation before and after performing the beep test. Blood samples were collected before, immediately after, 3 h, and 24 h after the beep test. Levels of thiobarbituric acid reactive products, 8-hydroxy-2′-deoxyguanosine, TAC, iron, hepcidin, ferritin, myoglobin, and albumin, and morphological blood parameters (RBC, HGB, HCT, MCV, MCH, MCHC, and lactic acid) were measured | Supplementation did not significantly affect the beep test results. Besides, it did not significantly affect the morphological, biochemical, or performance parameters evaluated in this study |
| Received 200 mL day−1 of chokeberry juice (with 165.3 mg per 100 mL anthocyanin content); anthocyanin dosage = 330 mg day−1 | Received 200 mL day−1 of placebo drink (containing 6.6% of betaine and 1% of citric acid) | ||||||||
| Petrovic et al., 201627 | Randomised double-blind placebo-controlled trial | Fatty acid profiles and lipid peroxidation | Elite handball players: n = 32; age range, 16–20 years old | Regular training regimen before the study: 1.5 h day−1 (combination of aerobic, conditioning, and strength exercise) | n = 18 (8 males, 10 females) | n = 8 (7 males, 7 females) | 4 weeks | Participants consumed chokeberry juice consumption during a preparatory training period (between two competition seasons) that involved a combination of aerobic, conditioning, and strength exercise, once a day for 1.5 h. Lipid status (including lipid peroxidation), glucose, TBARS, and percentages of fatty acids were determined at baseline and after the intervention period | Supplementation decreased the levels of C18:1n-9 and C18:3n-3 in men, but not in females. However, placebo-controlled groups had reduced proportions of mono- (C16:1n-7, C18:1n-7) and polyunsaturated fatty acids (PUFAs: C18:3n-3, C20:5n-3, and C22:4n-6) in males, as well as n-6 PUFAs and total PUFAs in females after consumption |
| Campus training: 3 h day−1 (same combination of exercises but with higher intensity) | Received 100 mL day−1 of chokeberry juice (containing 586.7 ± 3.3 mg GAE per 100 mL of phenolic compounds and 29 mg of vitamin C); (poly)phenol dosage = 590 mg GAE day−1 | Received 100 mL day−1 of placebo drink (containing the same amount of vitamin C, but no polyphenols) | |||||||
| Cikiriz et al., 202129 | Not accurately defined | Oxidative stress (redox status) and body composition | Handball players: n = 16; age range, 16–24 years old | No information was provided | n = 16 | Not applicable | 12 weeks | Body composition (BMI, body fat percentage, total body fat, total body muscle, body mineral and protein content, amount and distribution of water within the body) and aerobic power (VO2max – by physical load test) of participants were measured. Blood samples were collected before (basal), immediately after the physical load test (peak), and 10 minutes after the end of the physical load test (recovery) to measure the levels of superoxide anion radical (O2-), hydrogen peroxide (H2O2), nitric oxide (NO), TBARS, activity of nonenzymatic antioxidant [reduced glutathione (GSH)] and activity of the enzymatic defence system [CAT and SOD]. Lipid components, blood cells, glucose, urea, creatinine and HGB of basal blood were measured as well | Supplementation significantly decreased the levels of prooxidants (TBARS and nitrites) and increased catalase activity. Supplementation also decreased body fat and body fat percent. It increased the levels of high-density lipoprotein, red blood cells and haemoglobin significantly. Besides, it decreased the level of leukocytes |
| Received 30 mL day−1 of chokeberry aqueous extract | |||||||||
| García-Flores et al., 201639 | Randomised double-blind placebo-controlled crossover trial | Oxidative stress: DNA catabolism | Elite triathletes: n = 16 (10 male and 6 female); age range, 19–21 years old | Before control-baseline: 37.5 ± 5.5a | n = 8 | n = 8 | 45 days | Participants received supplementation of 45 days. At the end of the control baseline stage, control training stage, supplementation/placebo intake stage, and control post-training stage blood and urine samples were collected from participants to measure DNA oxidation catabolites in plasma and urine isoprostane (8-iso-PGF2α) | Supplementation maintained the plasmatic concentration of guanosine-3′,5′-cyclic monophosphate, significantly decreasing the concentration of 8-hydroxyguanine, while significantly increasing the concentration of 8-nitroguanosine. The concentration of urinary 8-iso-PGF2α was decreased significantly |
| Before control-training: 1008 ± 105 | Received 200 mL day−1 of aronia–citrus juice (95% citrus juice with 5% of Aronia melanocarpa juice); anthocyanin dosage = 50 mg day−1 | Received 200 mL day−1 of placebo drink (containing water, authorized red dye, flavouring agent, and sweetener) | |||||||
| Before first assessment: 923 ± 119 | |||||||||
| Washout period: 0 ± 0 | |||||||||
| Before second assessment: 923 ± 119 | |||||||||
| Before control-post training: 552 ± 45 | |||||||||
| García-Flores et al., 201640 | Randomised double-blind placebo-controlled crossover trial | Oxidative stress: lipid peroxidation and neural membrane degradation | Young adult triathletes: n = 16; age range, 19–21 years old | Before control-baseline: 37.5 ± 5.5a | n = 8 | n = 8 | 45 days | Participants received supplementation of 45 days. Twenty-four-hour urine samples were collected from the participants at the end of the control baseline stage, control training stage, supplementation/placebo intake stage, and control post-training stage to measure oxidative stress markers (F4-neuroprostanes and F2-dihomo-isoprostanes) linked with central nervous system | Supplementation decreased lipid peroxidation associated with neuronal membrane degradation (reflected by a decrease in the concentration of 10-epi-10-F4t-neuroprostane and 10-F4t-neuroprostane). It also decreased the concentration of 17-epi-17-F2t-dihomo-isoprostane |
| Before control-training: 1008 ± 105 | Received 200 mL day−1 of aronia–citrus juice (95% citrus juice with 5% of Aronia melanocarpa juice); anthocyanin dosage = 50 mg day−1 | Received 200 mL day−1 of placebo drink (containing water, authorized red dye, flavouring agent, and sweetener) | |||||||
| Before first assessment: 923 ± 119 | |||||||||
| Washout period: 0 ± 0 | |||||||||
| Before second assessment: 923 ± 119 | |||||||||
| Before control-post training: 552 ± 45 | |||||||||
| García-Flores et al., 201841 | Randomised double-blind placebo-controlled crossover trial | Oxidative stress: lipid peroxidation and inflammation | Elite triathletes: n = 16 (10 male and 6 female); age range, 19–21 years old | Before control-baseline: 37.5 ± 5.5a | n = 8 | n = 8 | 45 days | Participants received supplementation of 45 days. Twenty-four-hour urine samples were collected from the participants at the end of the control baseline stage, control training stage, supplementation/placebo intake stage, and control post-training stage to measure urinary oxylipins (isoprostanes (IsoPs), leukotrienes (LTs), prostaglandins (PGs), and thromboxanes (TXs)) | Supplementation decreased 2,3-dinor-11β-prostaglandin F2α and 11-dehydro-thromboxane B2, while increasing prostaglandin E2,15-keto-F2t-isoprostane, 15-epi-15-E2t-isoprostane, leukotriene E4, and 20-OH-P prostaglandin E2 levels |
| Before control-training: 1008 ± 105 | Received 200 mL day−1 of aronia–citrus juice (95% citrus juice with 5% of Aronia melanocarpa juice); anthocyanin dosage = 50 mg day−1 | Received 200 mL day−1 of placebo drink (containing water, authorized red dye, flavouring agent, and sweetener) | |||||||
| Before first assessment: 923 ± 119 | |||||||||
| Washout period: 0 ± 0 | |||||||||
| Before second assessment: 923 ± 119 | |||||||||
| Before control-post training: 552 ± 45 | |||||||||
2.4. Risk of bias assessment
Two of the reviewers (RZ and AAR) independently assessed the risk of bias for each included study according to the revised Cochrane tool for assessing the risk of bias in randomised trials (RoB 2 tool),35 and non-randomised studies of interventions (ROBINS-I tool).36 A third reviewer (GC) arbitrated when needed. For randomised studies, the assessment was based on (1) bias arising from the randomisation process, (2) bias due to deviations from the intended intervention, (3) bias due to missing outcome data, (4) bias in the measurement of the outcome, and (5) bias in the selection of the reported results. For crossover studies, the risk of bias arising from period and carryover effects was also considered. Each domain was assessed and assigned either a low risk of bias, high risk of bias or some concerns with respect to risk of bias. For non-randomised studies, the assessment also evaluated the bias due to confounding in addition to the domains stated for randomised studies. Visualising of the risk of bias assessment was performed using robvis online tool.373. Results
3.1. Study selection
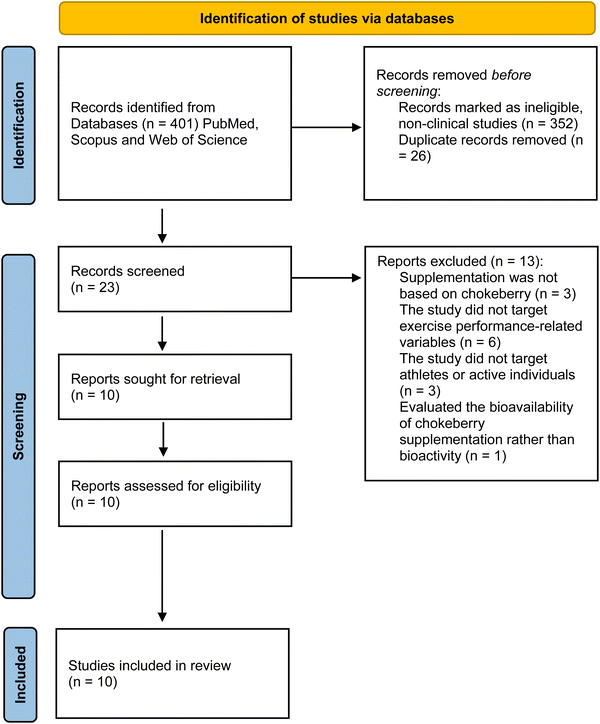
The review identified 401 records by searching the three databases, from which 352 non-clinical articles were identified and removed before the screening. After removing duplicates, 23 articles remained and were screened by title, abstract, and keywords by two of the reviewers (RZ and AAR) independently. A third reviewer (GC) arbitrated when needed. Thirteen articles were excluded for different reasons: supplementation was not based on chokeberry (n = 3), the study did not target exercise performance-related variables (targeted diseases such as cancer (n = 3), hypercholesterolemia (n = 1), bowel disease (n = 1), intermittent claudication (n = 1)), the study did not target athletes or active individuals (n = 3), or the study evaluated the bioavailability of chokeberry supplementation rather than bioactivity (n = 1). Ten articles were assessed for eligibility, and all ten were eligible and included in the qualitative synthesis of the current systematic review. The details of the study selection process are shown in Fig. 1.3.2. Characteristics of the included studies
Among the included studies, five studies were randomised double-blind placebo-controlled trials,25–28,38 three studies were randomised double-blind placebo-controlled crossover trials,39–41 one study was single-blind placebo-controlled crossover trial,42 and one study was a non-randomised non-blind trial.29 The participants of all the studies were athletes and this included rowers (n = 38),25,26 football players (n = 42),28,38 handball players (n = 48),27,29 triathletes (n = 48),39–41 and runners (n = 10).42In terms of supplementation form and dosage of the chronic studies four of nine chronic studies used pure chokeberry juice25–28 for a period of 4–8 weeks. Three of these studies quantified the anthocyanin content of the juice such that the dose of chokeberry anthocyanins was 330–3600 mg day−1.25,26,28 Three studies used a two-ingredient fruit juice containing chokeberry (5%) and citrus (95%).39–41 The total anthocyanin content of this juice was 50 mg day−1. Two studies used extracts, of which, one used dry chokeberry extract38 and another one used aqueous chokeberry extract29 – the anthocyanin content of these extracts was not determined. The only acute study in this review used a chokeberry juice with a (poly)phenol dose of 2600 mg.
3.3. General findings
The comprehensive search revealed that chokeberries in the field of sports science has mostly been evaluated for its effects on oxidative stress, inflammation, iron metabolism, and platelet aggregation.3.4. Risk of bias assessment
A total of eight out of ten studies were randomised controlled trials, while two were non-randomised. Most of the randomised controlled trials were associated with a low risk of bias (Fig. 2), except one study28 which was associated with some concerns with respect to bias in measurement of outcome. No clear information was provided regarding the awareness of outcome assessors of the intervention received, yet it was probably not influenced by knowledge of intervention since researchers were blinded to group assignment. The two non-randomised studies29,42 were associated with a moderate risk of bias due to moderate bias in the classification of interventions and measurement of outcomes (Fig. 3). The bias in the classification of intervention was linked with the lack of information about how the intervention status could have been affected by the knowledge of the outcome.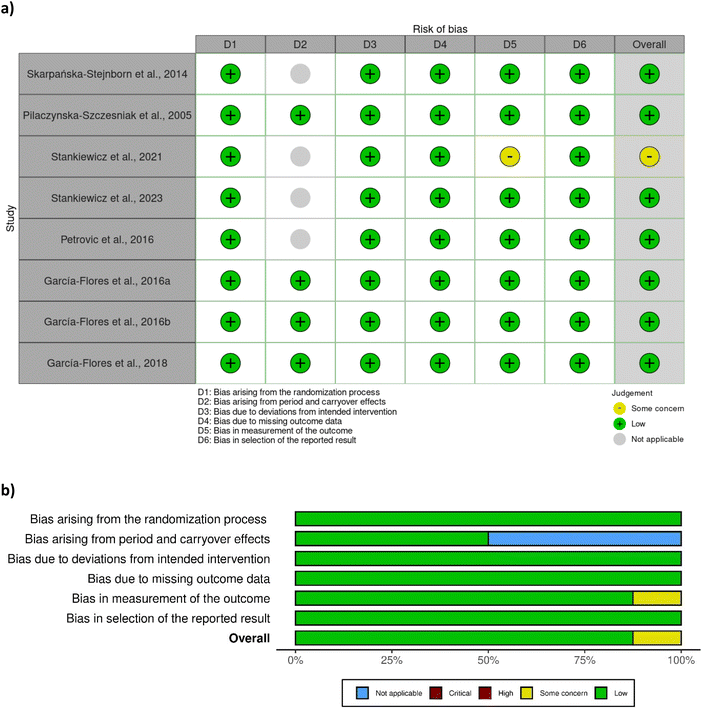 | ||
| Fig. 2 Assessment of bias of the randomised studies according to RoB 2 tool – (a) traffic light plot and (b) summary plot. | ||
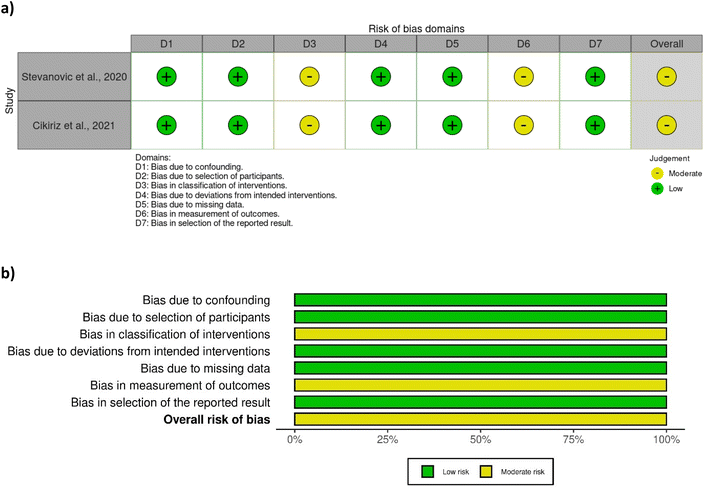 | ||
| Fig. 3 Assessment of bias of the non-randomised studies according to ROBINS-I tool – (a) traffic light plot and (b) summary plot. | ||
4. Discussion
This is the first study to systematically evaluate the impact of chokeberry (Aronia) supplementation on inflammation, oxidative stress, iron metabolism, and platelet activation in athletes. The current review identified 10 human studies that fit the criteria; there was some evidence that chokeberry could alter redox status, however, there were limited studies on the other outcomes precluding any firm conclusions to be drawn from the available evidence.The studies in the current review were conducted on athletes from several different disciplines; rowers, footballers, handballers, triathletes, and runners. It is well documented that high-intensity and/or prolonged exercise evoke a pro-oxidative and proinflammatory response.30,43,44 Although the generation of ROS and reactive nitrogen species (RNS) are important signalling molecules in physiological processes, excess generation of the most volatile ROS and RNS (e.g., peroxynitrite), as is common after high strenuous exercise, can overwhelm the endogenous antioxidant defence, causing oxidative damage.45 As oxidative damage to proteins, lipids, and DNA can affect force production and exercise performance, there is a significant interest in whether dietary supplements with antioxidant properties can enhance performance.30
Chokeberries contain bioactive compounds that act as natural antioxidants (e.g., vitamin C and carotenoids46), although their antioxidative potential is predominantly attributed to their (poly)phenolics.47 In accordance, several studies included in this review demonstrated that chokeberry supplementation reduced markers of exercise-induced lipid peroxidation, even in those with vitamin C-matched placebos,29,38–41 and increased TAC following exercise.25,38 In addition, chokeberry supplementation modified antioxidant enzymes. In one study, GPx activity immediately and SOD activity 24 h following an incremental rowing exercise test were lower compared to a placebo after 4 weeks.26 In another study, following 12 weeks of supplementation catalase was increased and GSH was decreased immediately and 10 min after a physical load test relative to baseline.29 Although not included in the current review due to the non-athletic population Chung et al., (2023) also reported the ability of chokeberry to modulate the glutathione defence system by increasing GSH availability and GPx activity immediately and 30 minutes post–exercise.48 These data could suggest (i) less reliance on the endogenous antioxidant defence systems due to the antioxidative capacity of the chokeberry or (ii) upregulation of endogenous antioxidant production via the NRF2 antioxidant response element pathway after longer-term chokeberry administration.49 For example, chokeberry supplementation has been shown to activate NRF2 by degrading its repressor, Kelch-like ECH-associated protein 1, leading to an increase in expression of antioxidant enzymes in mice.50 However, due to the limitations of the markers used to assess redox status in vivo51 and the non-controlled study design of ref. 29, these findings should be approached with caution. Nevertheless, based on available studies, these findings suggest chokeberries have the potential to influence redox status and therefore performance during exercise that are negatively affected by the excess generation of ROS and RNS.
After an intense exercise bout, the increase in RONS is generally accompanied by further secondary inflammatory-mediated damage.24,52,53 In addition to antioxidant activities, the anti-inflammatory properties of (poly)phenols are well documented.54 Specifically, (poly)phenols have been shown to interact with cellular enzymes and signalling pathways involved in the inflammatory process.55 However, as compared to other (poly)phenol-rich fruits the anti-inflammatory effects of chokeberry in exercise paradigms have been examined to a much lesser extent.56 In the current review, only two studies included inflammatory markers.25,38 Both studies found that supplementation with chokeberry reduced markers of inflammation (TNF-α and IL-6) and Stankiewicz et al., 2023 found an increase in anti-inflammatory cytokine IL-10.38 While more studies are needed to corroborate the findings of ref. 25, the potential anti-inflammatory actions of chokeberries could have applications in exercise recovery by counteracting any inflammatory-related damage to skeletal muscle. As the oxidative and inflammatory response to exercise is now widely accepted as playing a role in driving training adaptations, athletes should be cautious of interfering with these processes when adaptations are a priority (e.g., during pre-season). Notwithstanding, there is limited evidence that polyphenol supplements disrupt training adaptations.24,52,53,57
Additionally, exercise-induced oxidative stress and inflammation can have deleterious physiological effects such as enhanced platelet activation and anaemia.58,59 In one study, acute chokeberry supplementation was shown to decrease the expression of platelet activation markers (P-selectin and glycoprotein IIb/IIIa) but did not influence platelet aggregation following a simulated half-marathon race.42 This may be beneficial to those unaccustomed to prolonged exercise in which acute stress can increase platelet hyperactivity and have negative consequences on the cardiovascular system.60 In addition, anthocyanins, a major chokeberry (poly)phenol, have been shown to protect erythrocytes from oxidative damage61 and regulate iron metabolism by inhibiting the expression of hepcidin.62 The role of chokeberries in iron metabolism after exercise was reported in three studies with one showing increased iron levels 24 hours, but not immediately following, a 2000 m row25 and the other no effect after a beep test.28 However, the two studies25,38 demonstrated no effect on levels of hepcidin and the authors suggested that the dynamics of serum iron are determined by a phase of training rather than supplementation, thus the effects of chokeberry on iron metabolism are not well evidenced.
A key strength of this study is that it is the first review to systematically and comprehensively review studies supplementing chokeberry in athletes, according to PRISMA guidelines. Nevertheless, there a several limitations that should be acknowledged. Firstly, we were not able to conduct a meta-analysis due the heterogeneity in study design, population, training status/types and supplementation regimes. Secondly, we only found a small number of studies for each of the study outcomes, which limits our interpretation of the findings due to conflicting findings and the ability to draw evidence-based recommendations for athletes based on the current literature. For example, it is not possible to suggest the recommended dose of chokeberry supplementation. Although fruit juice was the most common form of chokeberry supplementation, the amount of anthocyanins in all the pure juice supplements was not analysed or reported. Future studies should conduct an analysis of the anthocyanin and polyphenol content of the study supplement batch to help aid with critical analysis of the study outcomes and recommended dose of chokeberry for athletes. Finally, a limitation of the evidence included in this systematic review was the mixed quality, including methodological rigour due to non-randomised or single-blinded studies being included. Although this was done to gain a better insight into the current literature, the findings should be interpreted cautiously as there is a need for further well-controlled investigations in this area.
5. Conclusion
Taken together, the findings from the 10 studies in this review suggest that chokeberry supplementation could alter redox balance in athletes. However, there were insufficient studies examining the effects of chokeberry supplements on other outcomes of interest (inflammation, platelet function, iron metabolism and performance) to draw any firm conclusions. Further large-scale, well-controlled investigations are needed to clarify the potential benefits of chokeberry supplementation on these biological markers in athletes.Author contributions
RZ and AAR: concept, planning, design, literature screening and assessment of risk of bias. RZ, RK, AAR: data analysis and interpretation. RZ, RK, AAR, GC: writing the manuscript. RZ, RK, AAR and TC: revision of the manuscript.Conflicts of interest
All authors declare that they have no conflict of interest relevant to the content of this review.Acknowledgements
The graphical abstract has been created using icons and figures obtained from BioRender.com.References
- I. D. Ochmian, J. Grajkowski and M. Smolik, Comparison of Some Morphological Features, Quality and Chemical Content of Four Cultivars of Chokeberry Fruits (Aronia melanocarpa), Not. Bot. Horti Agrobot. Cluj-Napoca, 2012, 40, 253–260 CrossRef CAS.
- T. Oniszczuk, G. Widelska, A. Oniszczuk, K. Kasprzak, A. Wójtowicz, M. Olech, R. Nowak, K. W. Kulesza, G. Jóźwiak and M. W. Hajnos, Influence of Production Parameters on the Content of Polyphenolic Compounds in Extruded Porridge Enriched with Chokeberry Fruit (Aronia melanocarpa (Michx.) Elliott), Open Chem., 2019, 17, 166–176 CAS.
- A. Sidor, A. Drożdżyńska and A. Gramza-Michałowska, Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors - An overview, Trends Food Sci. Technol., 2019, 89, 45–60 CrossRef CAS.
- M. Teleszko and A. Wojdyło, Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves, J. Funct. Foods, 2015, 14, 736–746 CrossRef CAS.
- J. Oszmiański and A. Wojdylo, Aronia melanocarpa phenolics and their antioxidant activity, Eur. Food Res. Technol., 2005, 221, 809–813 CrossRef.
- T. Jurikova, J. Mlcek, S. Skrovankova, D. Sumczynski, J. Sochor, I. Hlavacova, L. Snopek and J. Orsavova, Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases, Molecules, 2017, 22, 944 CrossRef PubMed.
- T. Kaloudi, D. Tsimogiannis and V. Oreopoulou, Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components, Molecules, 2022, 27, 4375 CrossRef CAS PubMed.
- P. Denev, M. Ciz, M. Kratchanova and D. Blazheva, Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities, Food Chem., 2019, 284, 108–117 CrossRef CAS PubMed.
- P. N. Denev, C. G. Kratchanov, M. Ciz, A. Lojek and M. G. Kratchanova, Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: in vitro and in vivo Evidences and Possible Mechanisms of Action: A Review, Compr. Rev. Food Sci. Food Saf., 2012, 11, 471–489 CrossRef CAS.
- R. Mattioli, A. Francioso, L. Mosca and P. Silva, Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases, Molecules, 2020, 25, 3809 CrossRef CAS PubMed.
- A. Ali Redha, S. Anusha Siddiqui, R. Zare, D. Spadaccini, S. Guazzotti, X. Feng, N. A. Bahmid, Y. S. Wu, F. Z. Ozeer and R. E. Aluko, Blackcurrants: A Nutrient-Rich Source for the Development of Functional Foods for Improved Athletic Performance, Food Rev. Int., 2022, 1–23, DOI:10.1080/87559129.2022.2162076.
- E. Moslemi, P. Dehghan and M. Khalafi, Effectiveness of supplementation with date seed (Phoenix dactylifera) as a functional food on inflammatory markers, muscle damage, and BDNF following high-intensity interval training: a randomized, double-blind, placebo-controlled trial, Eur. J. Nutr., 2023 DOI:10.1007/s00394-023-03125-9.
- E. Moslemi, P. Dehghan, M. Khani, P. Sarbakhsh and B. Sarmadi, The effects of date seed (Phoenix dactylifera) supplementation on exercise-induced oxidative stress and aerobic and anaerobic performance following high-intensity interval training sessions: a randomised, double-blind, placebo-controlled trial, Br. J. Nutr., 2022, 1–12, DOI:10.1017/S0007114522002124.
- M. T. Dutra, W. R. Martins, A. L. A. Ribeiro and M. Bottaro, The Effects of Strength Training Combined with Vitamin C and E Supplementation on Skeletal Muscle Mass and Strength: A Systematic Review and Meta-Analysis, J. Sports Med., 2020, 2020, 3505209 Search PubMed.
- T. L. Merry and M. Ristow, Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training?, J. Physiol., 2016, 594, 5135–5147 CrossRef CAS PubMed.
- J. Bouviere, R. S. Fortunato, C. Dupuy, J. P. Werneck-de-Castro, D. P. Carvalho and R. A. Louzada, Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle, Antioxidants, 2021, 10, 537 CrossRef CAS PubMed.
- A. J. Cheng, B. Jude and J. T. Lanner, Intramuscular mechanisms of overtraining, Redox Biol., 2020, 35, 101480 CrossRef CAS PubMed.
- V. Somerville, C. Bringans and A. Braakhuis, Polyphenols and Performance: A Systematic Review and Meta-Analysis, Sports Med., 2017, 47, 1589–1599 CrossRef PubMed.
- Y. Kitaoka, The Role of Nrf2 in Skeletal Muscle on Exercise Capacity, Antioxidants, 2021, 10, 1712 CrossRef CAS PubMed.
- T. Tofas, D. Draganidis, C. K. Deli, K. Georgakouli, I. G. Fatouros and A. Z. Jamurtas, Exercise-Induced Regulation of Redox Status in Cardiovascular Diseases: The Role of Exercise Training and Detraining, Antioxidants, 2019, 9, 13 CrossRef PubMed.
- K. Labonté, C. Couillard, A. Motard-Bélanger, M.-E. Paradis, P. Couture and B. Lamarche, Acute Effects of Polyphenols from Cranberries and Grape Seeds on Endothelial Function and Performance in Elite Athletes, Sports, 2013, 1, 55–68 CrossRef.
- T. Maraldi, Natural compounds as modulators of NADPH oxidases, Oxid. Med. Cell. Longevity, 2013, 2013, 271602 Search PubMed.
- E. Cione, C. La Torre, R. Cannataro, M. C. Caroleo, P. Plastina and L. Gallelli, Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation, Molecules, 2019, 25, 63 CrossRef PubMed.
- J. Bowtell and V. Kelly, Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance, Sports Med., 2019, 49, 3–23 CrossRef PubMed.
- A. Skarpańska-Stejnborn, P. Basta, J. Sadowska and Ł. Pilaczyńska-Szczeńniak, Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers, J. Int. Soc. Sports Nutr., 2014, 11, 48 CrossRef PubMed.
- L. Pilaczynska-Szczesniak, A. Skarpanska-Steinborn, E. Deskur, P. Basta and M. Horoszkiewicz-Hassan, The influence of chokeberry juice supplementation on the reduction of oxidative stress resulting from an incremental rowing ergometer exercise, Int. J. Sport Nutr. Exercise Metab., 2005, 14, 48–58 Search PubMed.
- S. Petrovic, A. Arsic, M. Glibetic, N. Cikiriz, V. Jakovljevic and V. Vucic, The effects of polyphenol-rich chokeberry juice on fatty acid profiles and lipid peroxidation of active handball players: results from a randomized, double-blind, placebo-controlled study, Can. J. Physiol. Pharmacol., 2016, 94, 1058–1063 CrossRef CAS PubMed.
- B. Stankiewicz, M. Cieslicka, S. Kujawski, E. Piskorska, T. Kowalik, J. Korycka and A. Skarpanska-Stejnborn, Effects of antioxidant supplementation on oxidative stress balance in young footballers- a randomized double-blind trial, J. Int. Soc. Sports Nutr., 2021, 18, 44 CrossRef CAS PubMed.
- N. Cikiriz, I. Milosavljevic, B. Jakovljevic, S. Bolevich, J. Jeremic, T. N. Turnic, M. Mitrovic, I. Srejovic, S. Bolevich and V. Jakovljevic, The influences of chokeberry extract supplementation on redox status and body composition in handball players during competition phase, Can. J. Physiol. Pharmacol., 2021, 99, 42–47 CrossRef CAS PubMed.
- S. K. Powers, R. Deminice, M. Ozdemir, T. Yoshihara, M. P. Bomkamp and H. Hyatt, Exercise-induced oxidative stress: Friend or foe?, J. Sport Health Sci., 2020, 9, 415–425 CrossRef PubMed.
- W.-N. Kong, G. Gao and Y.-Z. Chang, Hepcidin and sports anemia, Cell Biosci., 2014, 19 CrossRef PubMed.
- G. Lippi and F. Sanchis-Gomar, Epidemiological, biological and clinical update on exercise-induced hemolysis, Ann. Transl. Med., 2019, 7, 270 CrossRef CAS PubMed.
- B. Ed Nignpense, K. A. Chinkwo, C. L. Blanchard and A. B. Santhakumar, Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation?, Int. J. Mol. Sci., 2019, 21, 146 CrossRef PubMed.
- M. J. Page, J. E. McKenzie, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, J. M. Tetzlaff, E. A. Akl, S. E. Brennan, R. Chou, J. Glanville, J. M. Grimshaw, A. Hrobjartsson, M. M. Lalu, T. Li, E. W. Loder, E. Mayo-Wilson, S. McDonald, L. A. McGuinness, L. A. Stewart, J. Thomas, A. C. Tricco, V. A. Welch, P. Whiting and D. Moher, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, Br. Med. J., 2021, 372, n71 CrossRef PubMed.
- J. A. C. Sterne, J. Savovic, M. J. Page, R. G. Elbers, N. S. Blencowe, I. Boutron, C. J. Cates, H. Y. Cheng, M. S. Corbett, S. M. Eldridge, J. R. Emberson, M. A. Hernan, S. Hopewell, A. Hrobjartsson, D. R. Junqueira, P. Juni, J. J. Kirkham, T. Lasserson, T. Li, A. McAleenan, B. C. Reeves, S. Shepperd, I. Shrier, L. A. Stewart, K. Tilling, I. R. White, P. F. Whiting and J. P. T. Higgins, RoB 2: a revised tool for assessing risk of bias in randomised trials, Br. Med. J., 2019, 366, l4898 CrossRef PubMed.
- J. A. Sterne, M. A. Hernan, B. C. Reeves, J. Savovic, N. D. Berkman, M. Viswanathan, D. Henry, D. G. Altman, M. T. Ansari, I. Boutron, J. R. Carpenter, A. W. Chan, R. Churchill, J. J. Deeks, A. Hrobjartsson, J. Kirkham, P. Juni, Y. K. Loke, T. D. Pigott, C. R. Ramsay, D. Regidor, H. R. Rothstein, L. Sandhu, P. L. Santaguida, H. J. Schunemann, B. Shea, I. Shrier, P. Tugwell, L. Turner, J. C. Valentine, H. Waddington, E. Waters, G. A. Wells, P. F. Whiting and J. P. Higgins, ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions, Br. Med. J., 2016, 355, i4919 CrossRef PubMed.
- L. A. McGuinness and J. P. T. Higgins, Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments, Res. Synth. Methods, 2021, 12, 55–61 CrossRef PubMed.
- B. Stankiewicz, M. Cieslicka, J. Mieszkowski, A. Kochanowicz, B. Niespodzinski, A. Szwarc, T. Waldzinski, J. Reczkowicz, E. Piskorska, M. Petr, A. Skarpanska-Stejnborn and J. Antosiewicz, Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial, Nutrients, 2023, 15, 975 CrossRef CAS PubMed.
- L. A. Garcia-Flores, S. Medina, R. Cejuela-Anta, J. M. Martinez-Sanz, A. Abellan, H. G. Genieser, F. Ferreres and A. Gil-Izquierdo, DNA catabolites in triathletes: effects of supplementation with an aronia-citrus juice (polyphenols-rich juice), Food Funct., 2016, 7, 2084–2093 RSC.
- L. A. Garcia-Flores, S. Medina, C. Oger, J. M. Galano, T. Durand, R. Cejuela, J. M. Martinez-Sanz, F. Ferreres and A. Gil-Izquierdo, Lipidomic approach in young adult triathletes: effect of supplementation with a polyphenols-rich juice on neuroprostane and F2-dihomo-isoprostane markers, Food Funct., 2016, 7, 4343–4355 RSC.
- L. A. Garcia-Flores, S. Medina, C. Gomez, C. E. Wheelock, R. Cejuela, J. M. Martinez-Sanz, C. Oger, J. M. Galano, T. Durand, A. Hernandez-Saez, F. Ferreres and A. Gil-Izquierdo, Aronia-citrus juice (polyphenol-rich juice) intake and elite triathlon training: a lipidomic approach using representative oxylipins in urine, Food Funct., 2018, 9, 463–475 RSC.
- V. Stevanovic, A. Pantovic, I. Krga, M. Zekovic, I. Sarac, M. Glibetic and N. Vidovic, Aronia juice consumption prior to half-marathon race can acutely affect platelet activation in recreational runners, Appl. Physiol., Nutr., Metab., 2020, 45, 393–400 CrossRef CAS PubMed.
- F. He, J. Li, Z. Liu, C. C. Chuang, W. Yang and L. Zuo, Redox Mechanism of Reactive Oxygen Species in Exercise, Front. Physiol., 2016, 7, 486 Search PubMed.
- G. S. Metsios, R. H. Moe and G. D. Kitas, Exercise and inflammation, Best Pract. Res., Clin. Rheumatol., 2020, 34, 101504 CrossRef PubMed.
- K. Fisher-Wellman and R. J. Bloomer, Acute exercise and oxidative stress: a 30 year history, Dyn. Med., 2009, 8, 1 CrossRef PubMed.
- S. E. Kulling and H. M. Rawel, Chokeberry (Aronia melanocarpa) - A review on the characteristic components and potential health effects, Planta Med., 2008, 74, 1625–1634 CrossRef CAS PubMed.
- A. Sidor and A. Gramza-Michalowska, Black Chokeberry Aronia melanocarpa L.-A Qualitative Composition, Phenolic Profile and Antioxidant Potential, Molecules, 2019, 24, 3710 CrossRef CAS PubMed.
- J. W. Chung, J. E. Kim, Y. E. Nam, W. S. Kim, I. Lee, S. V. Yim and O. Kwon, Eight-week supplementation of Aronia berry extract promoted the glutathione defence system against acute aerobic exercise-induced oxidative load immediately and 30 min post-exercise in healthy adults: a double-blind, randomised controlled trial, J. Hum. Nutr. Diet., 2023 DOI:10.1111/jhn.13150.
- M. Ciocoiu, L. Badescu, A. Miron and M. Badescu, The involvement of a polyphenol-rich extract of black chokeberry in oxidative stress on experimental arterial hypertension, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 912769 Search PubMed.
- Z. Wang, Y. Liu, X. Zhao, S. Liu, Y. Liu and D. Wang, Aronia melanocarpa Prevents Alcohol-Induced Chronic Liver Injury via Regulation of Nrf2 Signaling in C57BL/6 Mice, Oxid. Med. Cell. Longevity, 2020, 2020, 4054520 Search PubMed.
- T. Henning and D. Weber, Redox biomarkers in dietary interventions and nutritional observation studies - From new insights to old problems, Redox Biol., 2021, 41, 101922 CrossRef CAS PubMed.
- T. Bongiovanni, F. Genovesi, M. Nemmer, C. Carling, G. Alberti and G. Howatson, Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: current knowledge, practical application and future perspectives, Eur. J. Appl. Physiol., 2020, 120, 1965–1996 CrossRef PubMed.
- D. J. Owens, C. Twist, J. N. Cobley, G. Howatson and G. L. Close, Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions?, Eur. J. Sport Sci., 2018, 19, 71–85 CrossRef PubMed.
- N. Yahfoufi, N. Alsadi, M. Jambi and C. Matar, The Immunomodulatory and Anti-Inflammatory Role of Polyphenols, Nutrients, 2018, 10, 1618 CrossRef PubMed.
- M. Sobhani, M. H. Farzaei, S. Kiani and R. Khodarahmi, Immunomodulatory; Anti-inflammatory/antioxidant Effects of Polyphenols: A Comparative Review on the Parental Compounds and Their Metabolites, Food Rev. Int., 2020, 37, 759–811 CrossRef.
- K. Doma, D. Gahreman and J. Connor, Fruit supplementation reduces indices of exercise-induced muscle damage: a systematic review and meta-analysis, Eur. J. Sport Sci., 2021, 21, 562–579 CrossRef PubMed.
- G. Martinez-Negrin, J. P. Acton, S. P. Cocksedge, S. J. Bailey and T. Clifford, The effect of dietary (poly)phenols on exercise-induced physiological adaptations: A systematic review and meta-analysis of human intervention trials, Crit. Rev. Food Sci. Nutr., 2022, 62, 2872–2887 CrossRef CAS PubMed.
- C. Di Massimo, P. Scarpelli and M. G. Tozzi-Ciancarelli, Possible involvement of oxidative stress inexercise-mediated platelet activation, Clin. Hemorheol. Microcirc., 2004, 30, 313–316 CAS.
- P. Peeling, B. Dawson, C. Goodman, G. Landers and D. Trinder, Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones, Eur. J. Appl. Physiol., 2008, 103, 381–391 CrossRef CAS PubMed.
- I. Singh, H. Quinn, M. Mok, R. J. Southgate, A. H. Turner, D. Li, A. J. Sinclair and J. A. Hawley, The effect of exercise and training status on platelet activation: do cocoa polyphenols play a role?, Platelets, 2006, 17, 361–367 CrossRef CAS PubMed.
- D. Bonarska-Kujawa, H. Pruchnik and H. Kleszczynska, Interaction of selected anthocyanins with erythrocytes and liposome membranes, Cell. Mol. Biol. Lett., 2012, 17, 289–308 CAS.
- F. Li, Z.-H. Liu, X. Tian, T. Liu, H.-L. Wang and G. Xiao, Black soybean seed coat extract protects Drosophila melanogaster against Pb toxicity by promoting iron absorption, J. Funct. Foods, 2020, 75, 104201 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo00336a |
| This journal is © The Royal Society of Chemistry 2023 |