Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterization of microplastics in water bottled in different packaging by Raman spectroscopy
Isabella
Gambino
a,
Cosimino
Malitesta
 a,
Francesco
Bagordo
b,
Tiziana
Grassi
a,
Alessandra
Panico
a,
Francesco
Bagordo
b,
Tiziana
Grassi
a,
Alessandra
Panico
 *a,
Silvia
Fraissinet
a,
Antonella
De Donno
a and
Giuseppe Egidio
De Benedetto
c
*a,
Silvia
Fraissinet
a,
Antonella
De Donno
a and
Giuseppe Egidio
De Benedetto
c
aDepartment of Biological and Environmental Science and Technologies, University of Salento, Lecce, Italy. E-mail: isabella.gambino@unisalento.it; alessandra.panico@unisalento.it; cosimino.malitesta@unisalento.it; tiziana.grassi@unisalento.it; alessandra.panico@unisalento.it; silvia.fraissinet@unisalento.it; antonella.dedonno@unisalento.it
bDepartment of Pharmacy Pharmaceutical Science, University of Bari Aldo Moro, Bari, Italy. E-mail: francesco.bagordo@uniba.it
cDepartment of Cultural Heritage, University of Salento, Lecce, Italy. E-mail: giuseppe.debenedetto@unisalento.it
First published on 25th October 2023
Abstract
Raman spectroscopy was applied to quantitatively and qualitatively characterize microplastics (MPs) in bottled water packaged in three different materials: polyethylene terephthalate (PET), recycled PET (rPET) and glass. The results showed a low mean concentration of MPs in all samples, with higher values in glass bottles (8.65 ± 5.39 p L−1) than in PET (5.09 ± 3.28 p L−1) or rPET (3.33 ± 1.34 p L−1) bottles. Through the use of a method capable of detecting smaller particles, MPs of 20–100 μm were dominant and fragments were the most abundant particle shape. PET was the prevalent polymer in PET bottles underlying the possible contribution of packaging in MP contamination, while polyethylene (PE) with additives prevailed in rPET and glass bottles, suggesting a contribution from the cap. A standardized protocol would allow comparable data to be obtained and allow an objective assessment of exposure, in view of plans to monitor contaminants of emerging concern (including MPs) under recent European legislation.
Water impactMicroplastics are present in natural water sources and food. Ingestion of contaminated drinking water is one of the main methods of exposure for humans. A sensitive and shared analytical protocol is required to detect microplastics in water, also in light of recent European legislation that indicates microplastics as contaminants of emerging concern should be monitored for protecting human health. |
Introduction
Plastic has become the most widely used material ever and its production amounts to 8.3 million metric tonnes since 1950.1 Its properties (i.e., low cost, durability, light weight, etc.) made plastic a suitable material for many applications. These traits make plastics popular and render them ubiquitous in the environment. In 2015, 66–99 million tonnes of plastics ended up in the environment.2 Once in the environment, due to degradation processes larger particles can be fragmented into smaller particles commonly called microplastics (MPs).3 MPs are generally classified as particles smaller than 5 mm. The lower boundary size of microplastics is still not fixed, but fragments smaller than 1 μm are usually called nanoplastics (NPs).4 In addition, a further definition classifies them into primary microplastics, or “microplastics by design”, utilized in cosmetics (i.e., scrubs) or in abrasive pastes, and secondary microplastics. The latter arise from the fragmentation of larger items and represent the dominant source of plastic in the environment.5 Their shapes include beads, fragments, fibers, and films.6A great number of studies concerning the presence of MPs are available for marine7 and terrestrial ecosystems.8 Recently, studies also reported MPs in natural water sources, such as surface water9 and groundwater,10 drawing attention to the presence of MPs in drinking water. Studies on drinking water have grown significantly over the last decade.11 Moreover, MPs have been reported in food, such as milk,12 table salt,13 honey,12 and seafood,14 and some authors have demonstrated that MPs can be transported through the food chain even at the top trophic level, including humans.15–19 However, it is not known whether human exposure to MPs can lead to adverse effects and, if so, under what conditions (concentrations level, shapes, etc.) Studies on animal models and on human cell lines in vitro have reported cellular uptake of MPs and found that cellular toxicity depends on the concentration, shape, size and chemical composition of the particles.20,21 Humans are exposed daily to a wide range of sources of MPs, and ingestion of contaminated food is one of the main ones.14,19,22 MP ingestion through drinking water could represent a cause of concern, considering the direct and long-term exposure of the entire population and the increase in consumption of bottled water, especially in PET packaging.23,24 Only a limited number of studies about MPs in bottled water is available in the literature.11 To date the main analytical techniques used for the analysis of MPs in bottled water are spectroscopic ones, such as Raman spectroscopy, FTIR (Fourier transformation-infrared), and SEM/EDS (scanning electron microscopy).11 Among them, Raman spectroscopy allows the identification of particles down to 1 μm in size.25 This is of paramount importance given that small particles can be internalized and absorbed in organisms.26 The presence of MPs in bottled water needs to be investigated in depth in order to increase and improve data on the occurrence of MPs and to gather information on human exposure through food.27 The aim of this research was to apply Raman spectroscopy to qualitatively and quantitatively investigate the presence of MPs in water bottled in packaging made of three different materials: polyethylene terephthalate (PET), recycled PET (rPET), and glass.
Materials and methods
Samples
A total of 130 water bottles made of three different materials (thirty-five 2-liter PET single-use bottles, thirty-five 2-liter rPET bottles with 30% to 50% recycled PET, sixty 1-liter returnable glass bottles) were purchased from Italian commercial outlets on a single day for each type of packaging. The contents of the bottles were unified, according to brand and market, to make twenty ten-liter samples, as recommended by Koelmans et al.5 Overall, 7 samples from PET bottles, 7 from rPET bottles, and 6 from glass bottles were analyzed.Quality assurance/quality control (QA/QC) procedures to avoid air particle contamination
To avoid air particle contamination, all the processes were carried out under a fume hood with a laminar air flow. During sample processing, 100% cotton clothes and face masks were worn. Before running the analyses, as reported by Oßmann et al.,28 the labels were removed from the external surface of the bottles and then they were washed with detergent and rinsed with ultrapure water (Farmalabor R2106274) and pre-filtrated on a white 0.45 μm mixed cellulose ester (MCE) membrane (Membrane Solutions, diameter Ø 47 mm, Lot. 280653820). The bottles were left to dry under the laminar flow cabinet. Only glass or stainless steel laboratory devices were used. They were thoroughly washed before the processes. The washing procedures consisted of different steps: after energic washing with water and detergent, the devices were cleaned with 10% HCl (hydrochloric acid 37%, Carlo Erba, Lot. V7N446017N), rinsed with ultrapure water and finally with 50% ethanol (absolute alcohol A15o1, Honeywell, Lot. K2160, Mat. No. 10641691).Filtration
MP extraction was performed by filtering on a steel filtration ramp apparatus connected to a vacuum pump. During the filtration process, the funnel was covered with a glass Petri dish and opened only for refilling since its filtration volume was 500 ml. Bottled water samples were filtered through a 1.2 μm polycarbonate (PC) membrane (Merck Ref. RTTP04700, Isopore membrane – diameter Ø 47 mm). Once the entire volume of the samples (10 L) had been filtered, the filter was gently removed with steel tweezers, placed in a glass Petri dish, put in an oven for 1 hour at 50 °C and then stored for the following analyses. Each bottled water sample was associated with a negative control, run in parallel, in the same filtration ramp apparatus in order to standardize the final result.10 L of ultrapure water pre-filtered on a 0.45 μm MCE membrane was used as a negative control sample. At the end of each cycle, the filtration apparatus was cleaned using the procedures described above.
Counting and identification of microplastics
Particles were analyzed directly over the whole filter surface. Particle counting was performed with an optical microscope (Nikon Eclipse 80i Upright Microscope) equipped with two 5× and 20× optical objectives and a Nikon camera (Digital Sight series) using dark field illumination. Every detected particle was tracked by taking a photograph using ACT-2U acquisition software and analyzed by Raman spectroscopy to assess the polymer identity. Raman analysis was performed with a “Renishaw inVia instrument” with a Leica microscope with 50×/20×/5× objectives and a 785 nm diode laser. System calibration was performed on the 520.7 cm−1 peak of a silicon wafer (laser power of 100% and 1 s accumulation).Transparent, white, green or blue particles were recorded using a laser power of 5–10% (5–10 mW on sample), 10 s exposure time and three accumulations in the 500–1800 cm−1 region. Black particles were recorded using a laser power of 1–2% (1–2 mW on sample) to avoid particle overheating or burning. WiRE 3.4 software (Windows®-based Raman Environment) was used to process the Raman data. The obtained spectra were corrected by subtracting the polynomial baseline and matched with an online library (IRUG) and the literature.29 MPs were classified into five groups depending on their size: <10 μm, 10–20 μm, 20–50 μm, 50–100 μm and >100 μm by ImageJ software. In order to normalize the final count, MP concentration measured in the control sample was subtracted from the MP concentration measured in the corresponding sample according to the polymeric nature of the particles, as shown in the following formula:
| n. of MPs = n. of MPs (Sample) − n. of MPs (Control) |
Statistical analysis
All data were entered into a Microsoft Excel spreadsheet (2010) and statistically processed using MedCalc Software version 12.3 (MedCalc Software bvba, Ostend, Belgium). The arithmetic mean, standard deviation, maximum and minimum were calculated for each group of quantitative variables. Chi-squared tests and one-way ANOVA were performed for qualitative and quantitative variables, respectively, to verify the differences among groups.Results
MP concentration in the three different types of packaging
MP contamination was reported for all types of packaging. Microscopic observation showed that fragments made up 90.8% of the total MPs while the remaining part were fibres. In the controls, MPs were found with a mean of 0.94 ± 0.4 p L−1. Fig. 1 shows the distribution of the total number of MPs found in the whole set of samples analyzed for each packaging type. On average, glass bottles showed the highest particle concentration (p < 0.05) with a mean of 8.65 ± 5.39 p L−1, followed by PET bottles with 5.09 ± 3.28 p L−1. rPET bottles showed the lowest MP content with a mean of 3.31 ± 1.34 p L−1. The highest number of particles was found for glass bottles with a minimum of 28 particles and a maximum of 152 particles; for PET bottles the range was 18–114 particles; while rPET bottles showed a minimum of 20 and maximum of 48 particles.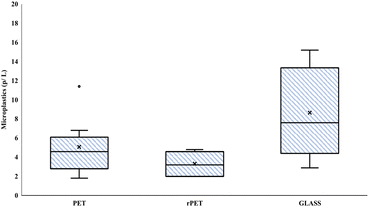 | ||
| Fig. 1 Box plot indicating the distribution in quartiles of microplastic concentration according to packaging. | ||
MP size
The analysis of the MPs showed that, overall, the most representative size classes were 50–100 μm (35.05%) and 20–50 μm (34.43%). Particles larger than 100 μm represented 25.98%, while the lowest percentage of particles was those smaller than 20 μm, with 3.92% for particles of 10–20 μm and 0.62% for particles <10 μm (Fig. 2). MPs showed a different size distribution (p = 0.003) according to packaging type (Table 1). Glass bottles showed the highest percentage of smaller particles compared to the other types of packaging: overall, 7.32% of them were <20 μm (0.49% < 10 μm, 6.83% of 10–20 μm) while the most representative size class was 20–50 μm (40.49%) followed by 50–100 μm (32.20%). Particles >100 μm made up 20% of the total. Single-use PET bottles showed 3.18% of smaller particles (0.53% < 10 μm, 2.65% of 10–20 μm). The size classes 20–50 μm and 50–100 μm were 33.86% and 34.92% of particles, respectively. Particles >100 μm represented 28.04% of the total. Improbably, rPET bottles were characterized by larger size particles. In particular, 1.10% of particles were <10 μm, while no particles of 10–20 μm were detected. Particles of 20–50 μm represented 21.98% of the total MPs, while particles of 50–100 μm were the most abundant (41.76%). Finally, particles >100 μm made up 35.16% of the total.| Particle size | PET (7 samples) | rPET (7 samples) | Glass (6 samples) | Total (20 samples) |
|---|---|---|---|---|
| <10 μm (%) | 0.53 | 1.10 | 0.49 | 0.62 |
| 10–20 μm (%) | 2.65 | 0.00 | 6.83 | 3.92 |
| 20–50 μm (%) | 33.86 | 21.98 | 40.49 | 34.43 |
| 50–100 μm (%) | 34.92 | 41.76 | 32.20 | 35.05 |
| >100 μm (%) | 28.04 | 35.16 | 20.00 | 25.98 |
Polymeric analysis of MPs
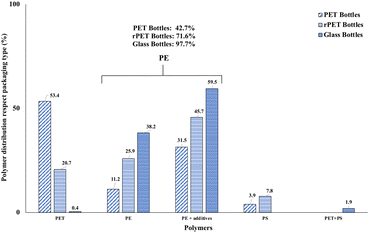
The polymer distribution according to packaging type is reported in Fig. 3. Total PE particles were split into “PE” and “PE + additives” in the obtained spectra. Overall, PE was the most abundant polymer (74.5%), comprising 26.9% of particles of common PE and 47.6% of PE + additives followed by PET (21.7%), PS (2.9%), and PET + PS (0.9%). However, differences among the types of packaging materials were found. PET represented the most abundant polymer (53.4%) in PET bottles followed by PE (42.7%) (11.2% of normal PE and 31.5% of PE + additives), while PS represented 3.9% of particles. rPET bottles showed a lower amount of PET particles (20.7%) and a higher percentage (71.6%) of PE (25.9% normal PE and 45.7% PE + additives) than PET bottles. Moreover, 7.8% of PS particles were found. Glass bottles showed the highest amount of PE particles with a total of 97.7% (38.2% of normal PE and 59.5% PE + additives). Whereas, very few PET (0.2%) and PET + PS (1.0%) particles were found in glass bottles. Consideration should be given to PE. Characteristic spectra of PE + additives were obtained on every measuring point from many samples. The direct analysis of the corresponding bottle cap returned a spectrum very similar to that obtained for fragments detected in water. Two spectra of fragments from water samples and a spectrum from a bottle cap are reported in Fig. 4.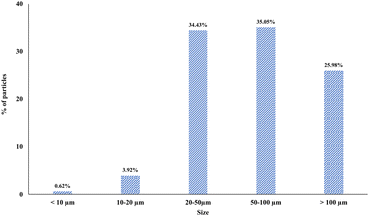 | ||
| Fig. 3 Polymer distribution by packaging type. PET (polyethylene terephthalate); PE (polyethylene); PS (polystyrene). | ||
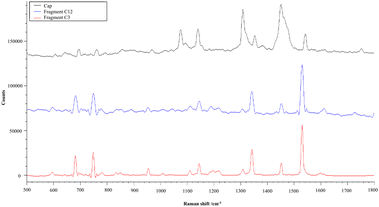 | ||
| Fig. 4 Spectrum of a bottle cap (black line) and spectra of two fragments found in water samples (blue and red lines). | ||
Discussion
In this study, one hundred and thirty water bottles made of three different materials were investigated. A total of 200 liters (70 for PET, 70 for rPET, 60 for glass) were analyzed for their microplastic content. Raman spectroscopy was applied for the research and analysis of MPs, as it was considered a more sensitive and specific technique capable of detecting particles down to 1 μm.25Overall, our results highlighted a low concentration of MPs in all samples, with a mean of 5.09 ± 3.28 p L−1 for PET bottles, 3.31 ± 1.34 p L−1 for rPET bottles and 8.65 ± 5.39 p L−1 for glass bottles, compared with other studies which used the same analytical methods28–30 (Table 2). In general, investigations which used different techniques, such as Fourier-transform infra-red spectroscopy (FTIR) and scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS), found a lower concentration of MPs in bottled water than Raman spectroscopy, mainly for smaller particles (Table 3).
| References | Packaging | Particle size | Abundance |
|---|---|---|---|
| Oßmann et al., 2018 (ref. 28) | PET | 1–10 μm | 2649 p L−1 |
| rPET | 4805.9 p L−1 | ||
| Glass | 5864.1 p L−1 | ||
| rPET | >10 μm | 83.1 p L−1 | |
| Glass | 434.1 p L−1 | ||
| Schymanski et al., 2018 (ref. 29) | PET | 5–1359 μm | 14 ± 14 p L−1 |
| rPET | 118 ± 88 p L−1 | ||
| Glass | 50 ± 52 p L−1 | ||
| Kankanige et al., 2020 (ref. 30) | PET | 6.5–50 μm | 125.8 p L−1 |
| Glass | 47.6 p L−1 | ||
| Our study | PET | >1 μm | 5.09 ± 3.28 p L−1 |
| rPET | 3.31 ± 1.34 p L−1 | ||
| Glass | 8.65 ± 5.39 p L−1 |
| Reference | Method | Packaging | Particle size | Abundance |
|---|---|---|---|---|
| Mason et al., 2018 (ref. 9) | FTIR | PET | 6.5–100 μm | 315 p L−1 |
| Glass | 195 p L−1 | |||
| PET | >100 μm | 10.4 p L−1 | ||
| Glass | 8.96 p L−1 | |||
| Weisser et al., 2021 (ref. 31) | FTIR | Glass | 11–500 μm | 317 ± 257 p L−1 |
| Almaiman et al., 2021 (ref. 32) | FTIR | PET | 25–500 μm | 0.99–4.2 p L−1 |
| Glass | — | |||
| Winkler et al., 2019 (ref. 33) | SEM/EDS | PET | ≥3 μm | 148 ± 253 p L−1 |
| Zuccarello et al., 2019 (ref. 34) | SEM/EDS | PET | 0.5–10 μm | 5.42 × 107 p L−1 |
| Kankanige et al., 2020 (ref. 30) | FTIR | PET | >50 μm | 14.7 p L−1 |
| Glass | 4 p L−1 | |||
| Li et al., 2023 (ref. 35) | LD-IR | PET | >10 μm | 65.62 ± 44.65 p L−1 |
| Glass | 87.94 ± 46.38 p L−1 |
In addition, our study showed that glass bottles contained more MPs than PET or rPET bottles. This finding is in line with those of Oßmann et al.28 and Schymanski et al.29 (Table 2), who, using Raman spectroscopy, also found more MPs in glass bottles than in plastic ones. Another recent survey,35 performed by laser direct infrared spectroscopy (LD-IR), confirmed this result, showing a greater content of MPs in glass-bottled water (87.94 ± 46.38 p L−1) than PET-bottled water (65.62 ± 44.65 p L−1) (Table 3). This suggested possible contamination during water bottling or a contribution from other plastic parts of the bottle (i.e., the bottle cap). In our study, the spectral analysis highlighted the meaningful role of bottle caps in MP contamination of glass bottles.
Our study also showed a lower MP mean concentration in rPET-bottled water than in PET, unlike other studies which used the same technique28,29 (Table 2). This aspect should be clarified in further studies.
With respect to MP size, unlike other studies28,34,35 which reported a higher content of smaller and potentially more dangerous particles, we found that the most representative particles in the analyzed water ranged from 20 to 100 μm, while smaller particles were more abundant in glass bottles than in the other packaging. The lower MP abundance together with the presence of larger fragments could reflect more recent contamination while smaller MPs generally appeared to be abundant in remote contamination. With regard to polymers, most particles were made of PE (74.5%) or PET (21.7%). Only a small percentage was represented by PS and PET + PS. Many PE particles, with particular reference to “PE + additives”, in water samples returned a spectrum that matches that obtained by the direct analysis of the bottle cap and similar to the spectrum found by Schymanski et al.29 (“unknown spectrum”). Moreover, additional analysis by ATR-FTIR on bottle caps revealed the presence of additives, probably slip additives such as those reported by Dulal et al.36 Regarding the presence of additives, as stated by Gall et al.,37 slip agents are very common additives that can be found in caps and represent one of the main classes of additives utilized as functional additives, after stabilizers. They are not considered harmful to humans since they are listed as authorized plastic additives and are not subject to specific migration limits according to EU regulation (10/2011)38 on plastic materials and articles intended to come into contact with food. For these reasons, we hypothesized that the MPs detected in water samples could originate from the bottle caps, especially in glass bottles which showed a higher abundance of “PE + additives” particles. The contribution of bottle caps in MP contamination of bottled water has been described by other authors.31,33 They found that the cap is the main contributor to the release of MPs into water and analysis of the MP content in the steps of bottled water production highlighted a significant increase in MP concentration after the filling and capping processes. However, a full understanding of the dynamics involved in the release of MPs from the cap requires further investigation, which should consider caps of different types and materials, which is beyond the scope of the present work. Finally, different methodological approaches are described in the literature11 that make data highly variable and hard to compare. The choice of a suitable analytical procedure is of primary importance for the reliability of results and is necessary for developing a monitoring plan for contaminants of emerging concern, which are receiving a great deal of attention due to their widespread environmental diffusion.39
Conclusions
This study found overall low MP contamination in bottled water packaged in three different materials, with a prevalence of larger particles, suggesting recent contamination, which likely occurs not at source but during the bottling and storage processes. Moreover, spectral analysis revealed that the bottle cap played an important role in MP contamination, especially for water in glass bottles.Raman spectroscopy was confirmed to be a sensible and tailored technique for MP detection. However, a standardized and shared analytical protocol would be useful to harmonize the results and make them comparable as well as to objectively assess human exposure to MPs through drinking water consumption, also in view of the monitoring plans for contaminants of emerging concern, which include MPs.40
Although there is increasing public awareness, it is important to highlight that the evidence of adverse human effects related to MPs has not been well determined, as reported by the WHO.41 In any case, monitoring of MPs in drinking water could help to quantify the phenomenon and limit human exposure.
Author contributions
Isabella Gambino: conceptualization, investigation, writing – original draft. Cosimino Malitesta: conceptualization, methodology. Francesco Bagordo: formal analysis, writing – review and editing. Tiziana Grassi: data curation, writing – original draft. Alessandra Panico: formal analysis, data curation, writing – original draft. Silvia Fraissinet: investigation, methodology. Antonella De Donno: editing. Giuseppe Egidio De Benedetto: conceptualization, resources, methodology, supervision.Conflicts of interest
There are no conflicts to declare.References
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- Plastic Europe, Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf, 2019.
- C. Arthur, J. Baker and H. Bamford, Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 2009 Search PubMed.
- M. Bergmann, L. Gutow and M. Klages, Marine anthropogenic litter, Springer Nature, 2015, p. 447 Search PubMed.
- A. A. Koelmans, N. H. Mohamed Nor, E. Hermsen, M. Kooi, S. M. Mintenig and J. De France, Microplastics in freshwaters and drinking water: Critical review and assessment of data quality, Water Res., 2019, 155, 410–422 CrossRef CAS PubMed.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor and A. E. Daugaard, et al., Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris, Environ. Sci. Technol., 2019, 53(3), 1039–1047 CrossRef CAS PubMed.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono and L. Brach, et al., Nanoplastic in the North Atlantic Subtropical Gyre, Environ. Sci. Technol., 2017, 51(23), 13689–13697 CrossRef CAS PubMed.
- A. A. Souza Machado, W. Kloas, C. Zarfl, S. Hempel and M. C. Rillig, Microplastics as an emerging threat to terrestrial ecosystems, Global Change Biol., 2018, 24(4), 1405–1416 CrossRef PubMed.
- S. A. Mason, V. G. Welch and J. Neratko, Synthetic Polymer Contamination in Bottled Water, Front. Chem., 2018, 6, 407 CrossRef PubMed.
- S. M. Mintenig, M. G. J. Löder, S. Primpke and G. Gerdts, Low numbers of microplastics detected in drinking water from ground water sources, Sci. Total Environ., 2019, 648, 631–635 CrossRef CAS PubMed.
- I. Gambino, F. Bagordo, T. Grassi, A. Panico and A. De Donno, Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge, Int. J. Environ. Res. Public Health, 2022, 19(9), 5283 CrossRef CAS PubMed.
- M. F. Diaz-Basantes, J. A. Conesa and A. Fullana, Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants, Sustainability, 2020, 12(14), 5514 CrossRef.
- M. E. Iñiguez, J. A. Conesa and A. Fullana, Microplastics in Spanish Table Salt, Sci. Rep., 2017, 7(1), 8620 CrossRef PubMed.
- S. Fraissinet, A. Pennetta, S. Rossi, G. E. De Benedetto and C. Malitesta, Optimization of a new multi-reagent procedure for quantitative mussel digestion in microplastic analysis, Mar. Pollut. Bull., 2021, 173, 112931 CrossRef CAS PubMed.
- L. Van Cauwenberghe and C. R. Janssen, Microplastics in bivalves cultured for human consumption, Environ. Pollut., 2014, 193, 65–70 CrossRef CAS PubMed.
- N. P. Ivleva, A. C. Wiesheu and R. Niessner, Microplastic in Aquatic Ecosystems, Angew. Chem., Int. Ed., 2017, 56(7), 1720–1739 CrossRef CAS PubMed.
- D. Zhu, Q. F. Bi, Q. Xiang, Q. L. Chen, P. Christie and X. Ke, et al., Trophic predator-prey relationships promote transport of microplastics compared with the single Hypoaspis aculeifer and Folsomia candida, Environ. Pollut., 2018, 235, 150–154 CrossRef CAS PubMed.
- S. N. Athey, S. D. Albotra, C. A. Gordon, B. Monteleone, P. Seaton and A. L. Andrady, et al., Trophic transfer of microplastics in an estuarine food chain and the effects of a sorbed legacy pollutant, Limnol. Oceanogr. Lett., 2020, 5(1), 154–162 CrossRef.
- L. W. D. van Raamsdonk, M. van der Zande, A. A. Koelmans, R. L. A. P. Hoogenboom, R. J. B. Peters and M. J. Groot, et al., Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain, Foods, 2020, 9(1), 72 CrossRef CAS PubMed.
- C. Yong, S. Valiyaveettil and B. Tang, Toxicity of Microplastics and Nanoplastics in Mammalian Systems, Int. J. Environ. Res. Public Health, 2020, 17(5), 1509 CrossRef CAS PubMed.
- E. Danopoulos, M. Twiddy, R. West and J. M. Rotchell, A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells, J. Hazard. Mater., 2022, 427, 127861 CrossRef CAS PubMed.
- M. Kosuth, S. A. Mason and E. V. Wattenberg, Anthropogenic contamination of tap water, beer, and sea salt. Zhou Z, curatore, PLoS One, 2018, 13(4), e0194970 CrossRef PubMed.
- I. Gambino, F. Bagordo, B. Coluccia, T. Grassi, G. D. Filippis and P. Piscitelli, et al., PET-Bottled Water Consumption in View of a Circular Economy: The Case Study of Salento (South Italy), Sustainability, 2020, 12(19), 7988 CrossRef.
- J. Tosun, U. Scherer, S. Schaub and H. Horn, Making Europe go from bottles to the tap: Political and societal attempts to induce behavioral change, Wiley Interdiscip. Rev.: Water, 2020, 7(3), 1435 CrossRef.
- C. Schwaferts, R. Niessner, M. Elsner and N. P. Ivleva, Methods for the analysis of submicrometer- and nanoplastic particles in the environment, TrAC, Trends Anal. Chem., 2019, 112, 52–65 CrossRef CAS.
- Y. Q. Zhang, M. Lykaki, M. Markiewicz, M. T. Alrajoula, C. Kraas and S. Stolte, Environmental contamination by microplastics originating from textiles: Emission, transport, fate and toxicity, J. Hazard. Mater., 2022, 430, 128453 CrossRef CAS PubMed.
- World Health Organization, Microplastics in drinking-water, World Health Organization, Geneva, 2019, Available at: https://apps.who.int/iris/handle/10665/326499 Search PubMed.
- B. E. Oßmann, G. Sarau, H. Holtmannspötter, M. Pischetsrieder, S. H. Christiansen and W. Dicke, Small-sized microplastics and pigmented particles in bottled mineral water, Water Res., 2018, 141, 307–316 CrossRef PubMed.
- D. Schymanski, C. Goldbeck, H. U. Humpf and P. Fürst, Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water, Water Res., 2018, 129, 154–162 CrossRef CAS PubMed.
- D. Kankanige and S. Babel, Smaller-sized micro-plastics (MPs) contamination in single-use PET-bottled water in Thailand, Sci. Total Environ., 2020, 717, 137232 CrossRef CAS PubMed.
- J. Weisser, I. Beer, B. Hufnagl, T. Hofmann, H. Lohninger and N. P. Ivleva, et al., From the Well to the Bottle: Identifying Sources of Microplastics in Mineral Water, Water, 2021, 13(6), 841 CrossRef CAS.
- L. Almaiman, A. Aljomah, M. Bineid, F. M. Aljeldah, F. Aldawsari and B. Liebmann, et al., The occurrence and dietary intake related to the presence of microplastics in drinking water in Saudi Arabia, Environ. Monit. Assess., 2021, 193(7), 390 CrossRef CAS PubMed.
- A. Winkler, N. Santo, M. A. Ortenzi, E. Bolzoni, R. Bacchetta and P. Tremolada, Does mechanical stress cause microplastic release from plastic water bottles?, Water Res., 2019, 166, 115082 CrossRef CAS PubMed.
- P. Zuccarello, M. Ferrante, A. Cristaldi, C. Copat, A. Grasso and D. Sangregorio, et al., Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study, Water Res., 2019, 157, 365–371 CrossRef CAS PubMed.
- H. Li, L. Zhu, M. Ma, H. Wu, L. An and Z. Yang, Occurrence of microplastics in commercially sold bottled water, Sci. Total Environ., 2023, 867, 161553 CrossRef CAS PubMed.
- N. Dulal, R. Shanks, T. Gengenbach, H. Gill, D. Chalmers and B. Adhikari, et al., Slip-additive migration, surface morphology, and performance on injection moulded high-density polyethylene closures, J. Colloid Interface Sci., 2017, 505, 537–545 CrossRef CAS PubMed.
- M. Gall, A. Schweighuber, W. Buchberger and R. W. Lang, Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity, Sustainability, 2020, 12(24), 10378 CrossRef.
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food.
- M. T. Montagna, O. De Giglio, C. Calia, C. Pousis, F. Triggiano and S. Murgolo, et al., Microbiological and Chemical Assessment of Wastewater Discharged by Infiltration Trenches in Fractured and Karstified Limestone (SCA.Re.S. Project 2019–2020), Pathogens, 2020, 9(12), 1010 CrossRef CAS PubMed.
- Directive (Eu) 2020/2184 of the European Parliament and of the Council.
- WHO, Dietary and inhalation exposure to nano- and microplastic particles and potential implications for human health, World Health Organization, Geneva, 2022, ISBN 978-92-4-005460-8 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |