Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An S-shaped double helicene showing both multi-resonance thermally activated delayed fluorescence and circularly polarized luminescence†
John Marques
dos Santos
 a,
Dianming
Sun
a,
Dianming
Sun
 a,
Juan Manuel
Moreno-Naranjo
b,
David
Hall
ac,
Francesco
Zinna
a,
Juan Manuel
Moreno-Naranjo
b,
David
Hall
ac,
Francesco
Zinna
 d,
Seán T. J.
Ryan
b,
Wenda
Shi
b,
Tomas
Matulaitis
d,
Seán T. J.
Ryan
b,
Wenda
Shi
b,
Tomas
Matulaitis
 a,
David B.
Cordes
a,
David B.
Cordes
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a,
David
Beljonne
a,
David
Beljonne
 c,
Stuart L.
Warriner
c,
Stuart L.
Warriner
 e,
Yoann
Olivier
e,
Yoann
Olivier
 *f,
Matthew J.
Fuchter
*f,
Matthew J.
Fuchter
 *b and
Eli
Zysman-Colman
*b and
Eli
Zysman-Colman
 *a
*a
aOrganic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk
bDepartment of Chemistry, Molecular Sciences Research Hub, Imperial College London, White City Campus, London, W12 0BZ, UK. E-mail: m.fuchter@imperial.ac.uk
cLaboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
dDipartimento di Chimica e Chimica Industriale, Università di Pisa, 56124 Pisa, Italy
eSchool of Chemistry, University of Leeds, Leeds, LS2 9JT, UK
fLaboratory for Computational Modeling of Functional Materials, Namur Institute of Structured Matter, Université de Namur, Rue de Bruxelles, 61, 5000 Namur, Belgium. E-mail: yoann.olivier@unamur.be
First published on 27th January 2022
Abstract
We present the first example of a multi-resonant thermally activated delayed fluorescent (MR-TADF) extended helicene, Hel-DiDiKTa. This S-shaped double helicene exhibits sky-blue emission, a singlet–triplet energy gap, ΔEST, of 0.15 eV and narrow emission at a peak maximum of 473 nm with a full-width at half-maximum of 44 nm in toluene. The MR-TADF character is confirmed by the small degree of positive solvatochromism and temperature-dependent increase in intensity of the delayed emission. The chiroptical properties of the separated enantiomers are similar to other large helicenes with comparable dissymmetry values, but with the added benefit of MR-TADF. (P)-Hel-DiDiKTa is stable towards enantiomerization, with a Gibbs free energy of activation for enantiomerization (ΔG‡e) of 31 ± 2 kcal mol−1 at 298 K, a value similar to other reported double helicenes. (P)-Hel-DiDiKTa is also thermally stable, with a 5% weight loss at 399 °C revealed by thermogravimetric analysis (TGA). Thus, this study further strengthens the burgeoning area of chiral TADF emitters for use in cutting-edge optoelectronic and photocatalytic molecules and materials.
Introduction
Helicenes are a class of fused polycyclic aromatic frameworks that possess a helically chiral framework,1,2 where overlapping rings render the enantiomers kinetically stable to racemization. Helicenes usually display strong circular dichroism (CD)3 and circularly polarized luminescence (CPL),4–8 which has led them to be investigated in a number of different applications.4,9 These include nonlinear optics,10 chemical sensors,11 asymmetric catalysis,12,13 circularly polarised luminescent materials14–16 and as ligands in Ir, Zn and Pt complexes,9 the latter of which have been employed in phosphorescent CP-OLEDs.17,18 They have also been exploited as fluorescent emitters,5,19 chiral additives for induced chiral fluorescence polymers20–23 and as components within donor–acceptor (D–A) TADF emitters24,25 in CP-OLEDs. Beyond chiroptical properties the high solubility of helicenes in organic solvents (compared to planar acenes), coupled with their excellent thermal stability has led to a wider array of optoelectronic applications, including as hole-transport materials in photovoltaic devices.26–28Thermally activated delayed fluorescence (TADF) materials have become increasingly attractive as sensors,29 in lasers,30 photodetectors,31 photocatalysis32 and bioimaging33 as well as both emitters and hosts in organic light-emitting diodes (OLEDs)34 due to their ability to harvest 100% of electrically generated excitons to generate light. This is accomplished by conversion of triplet excitons into singlets via reverse intersystem crossing (RISC)34–38 enabled by their small S1–T1 energy gap, ΔEST.39 The dominant molecular design relies on a strongly twisted donor–acceptor architecture that minimises the overlap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), resulting in a small ΔEST. This strategy also results in compounds with relatively large conformational flexibility and/or intramolecular/stretching vibrations, which in turn leads to broad emission characterized by a full width half maximum (FWHM) typically greater than 80 nm, resulting in poor color purity in resulting OLED devices.40 Hatakeyama and co-workers have advanced an alternative molecular design strategy to produce TADF materials with narrowband emission (FWHM < 40 nm), based on polycyclic aromatic hydrocarbons doped with electron-accepting B, and electron-donating N and O atoms.41,42 These so-called multi-resonant TADF (MR-TADF) emitters rely on p- and n-doping of polycyclic aromatic hydrocarbons to limit the overlap between the HOMO and the LUMO electron-density and thus produce a small ΔEST, while their rigid molecular structure results in negligible conformational reorganization in the excited state, and narrowband emission.40,42,43 There are now examples of blue,44–48 green48–52 and red43,53,54 MR-TADF materials used as emitters in high-efficiency OLEDs.55–57
The study and exploitation of CPL has accelerated over the past decade due to a recognition that employing CPL-active compounds can lead to an increased efficiency of OLEDs and other optoelectronic devices.17,58–62 Other technologies being investigated for the exploitation of CPL include quantum computing,63,64 3D information displays,23,65 spintronic devices,66 transistors,67 CPL lasers68 and biological probes.69,70 The CPL intensity of a chiral molecule can be quantified by the dissymmetry factor, gPL, which is represented by eqn (1):.7,71,72
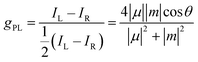 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = ± 1) to each other. Unfortunately, chiral molecules that display high photoluminescence quantum yields (ΦPL) typically show small gPL and vice versa.15,16 For instance, lanthanoid complexes typically show high gPL (0.1–1)73–75 but in order to reach high brightness values76 they need suitable antenna ligands to overcome the Laporte-forbidden nature of their transitions. In contrast, organic molecules can attain high absorption and emission intensities but typically show relatively low gPL (≤10−2) due to their significantly larger electric transition dipole moments compared to their magnetic transition dipole moments, coupled with their relatively smaller molecular size.7,8,77,78
θ = ± 1) to each other. Unfortunately, chiral molecules that display high photoluminescence quantum yields (ΦPL) typically show small gPL and vice versa.15,16 For instance, lanthanoid complexes typically show high gPL (0.1–1)73–75 but in order to reach high brightness values76 they need suitable antenna ligands to overcome the Laporte-forbidden nature of their transitions. In contrast, organic molecules can attain high absorption and emission intensities but typically show relatively low gPL (≤10−2) due to their significantly larger electric transition dipole moments compared to their magnetic transition dipole moments, coupled with their relatively smaller molecular size.7,8,77,78
Presently, there exists a small number of organic compounds displaying CP-TADF, with most of the reports emerging over the past few years. A number of these have been used in CP-TADF OLEDs,18,60,62,79,80 showing maximum external quantum efficiencies (EQEmax) surpassing 30%80 but with gEL typically ≤10−3 (Fig. 1a). The overwhelming majority of compounds showing CP-TADF are based on a donor–acceptor design that incorporates a remote stereogenic unit (asymmetric centre or axis) to induce chirality,18,60,61,81–83 and so do not show narrow emission spectra. Materials showing concomitantly narrow emission, TADF and CPL are of significant interest, and a class of compounds that can meet these requirements are chiral MR-TADF compounds. To date, there exists only a handful of reports of chiral MR-TADF compounds. Compounds OBN-2CN-BN and OBN-4CN-BN,84 were based loosely on Hatakeyama's DABNA-141 design and contain a peripheral chiral (R/S)-octahydro-binaphthol group that is not directly implicated in the emissive short-range charge transfer excited state.84 A similar strategy was used for QAO-PhCz,85 by introducing a 9-phenyl-9H-carbazole appended group to the MR-framework to lock the helical structure and obtain chirality. There are only two reports where the MR-TADF core structure shows intrinsic chirality and both are boron/nitrogen-based helicenes: compounds BN586 and OBN-Cz (aka 1a).87
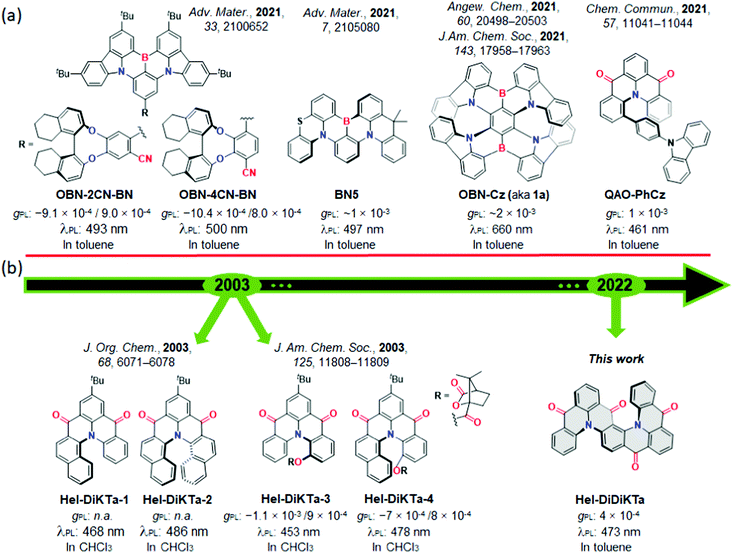 | ||
| Fig. 1 (a) Structure of prior examples of MR-TADF materials able to display CPL.84–87 (b) Structure of prior examples of triangulene helicenes and Hel-DiDiKTa.14,88 | ||
The first CPL data reported for helicenoids belong to two bridged triphenylamine diketone derivatives, reported by Venkataraman et al., (renamed here as Hel-DiKTa-3 and Hel-DiKTa-4, Fig. 1b) with gPL: –1.1 × 10−3/9 × 10−4 at 453 nm for (M)-Hel-DiKTa-3 and (P)-Hel-DiKTa-3 and −7 × 10−4/8 × 10−4 at 478 nm for (M)-Hel-DiKTa-4 and (P)-Hel-DiKTa-4, respectively.14 Given our previous work on diketone-based MR-TADF emitters (namely DiKTa and DDiKTa, structures shown in Fig. S1, ESI†),44,49 we envisaged that developing a helical analogue of DiKTa would be an elegant strategy to obtain a CPL-active molecule without compromising MR-TADF. Our preliminary quantum calculations for Hel-DiKTa-288 (Fig. 1a, renamed here for clarity), however, revealed that its ΔEST is too large (0.51 eV) for it to show MR-TADF due to triplet stabilization on the naphthalene group (vide infra).
Here we report an intrinsically helically chiral MR-TADF molecule that is CPL-active and is based on an extended helicene structure, Hel-DiDiKTa (Fig. 1b); an S-shaped double [4]helicene. Despite a large current interest in multiple helicenes, the chiroptical properties of these molecules remain understudied.89 We report in-depth structural, photophysical and chiroptical data for Hel-DiDiKTa and believe our study paves the way for further chiral MR-TADF molecules, based on extended or multiple helicene frameworks.
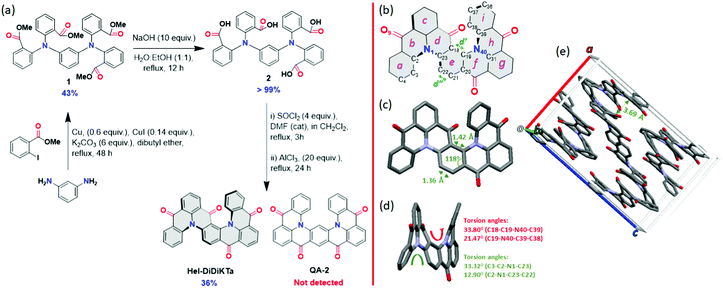
The synthesis of Hel-DiDiKTa is shown in Scheme 1a. A Cu-catalysed Ullman coupling between m-phenylenediamine and methyl 2-iodobenzoate afforded intermediate 1 in moderate yield (43%). Saponification proceeded quantitatively to yield 2, which was then subjected to a four-fold intramolecular Friedel–Crafts acylation of the in situ-prepared acyl chloride derivative in the presence of the Lewis acid AlCl3 to afford the title compound, (rac)-Hel-DiDiKTa, in 36% yield as a racemate; the C-shaped compound QA-2, which was recently reported by Yasuda et al.45 using a different synthetic route (and crystallised as a non-chiral meso isomer), was not detected. We performed Density Functional Theory (DFT) and coupled cluster calculations to probe which of the two products, (rac)-Hel-DiDiKTa or QA-2, is more stable in the ground state. QA-2 was calculated to be more stable than Hel-DiDiKTa by between 18 and 29 kJ mol−1, irrespective of the methodology applied (Fig. S19 and Table S7, ESI†), thus (rac)-Hel-DiDiKTa represents the kinetic product. The identity and purity of Hel-DiDiKTa was established by a combination of NMR spectroscopy, HRMS, melting point determination, HPLC measurements and elemental analysis. Additionally, single crystals of sufficient quality were grown by slow evaporation of a CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc solution (7
EtOAc solution (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio), enabling determination of the single crystal structure.
3 ratio), enabling determination of the single crystal structure.
Crystallographic data of Hel-DiDiKTa obtained by single crystal X-ray diffraction revealed that the compound crystalizes as a racemate. The helical pitch through each of the two [4]helicenes varies between 12.90(15) and 33.80(15)° (torsion angles C3-C2-N1-C23, C2-N1-C23-C22, C18-C19-N40-C39 and C19-N40-C39-C38) and is similar to that found in DiKTa (27.2°) (Scheme 1d). Typically, in helicenes, the benzene ring at the centre of the helical structure is distorted, with two very different C–C bond lengths; a longer C–C bond facing the inner helix and opposite it a much shorter one facing the outer helix (distances d′ and d′′ in Scheme 1b). This also leads to internal bond angles that are considerably different from that of an ideal planar benzene ring. This is not usually the case for S-shaped double helicenes,90–93 and as such, in Hel-DiDiKTa, the central benzene e-ring (Scheme 1b) has internal bond angles between 118.73(10) and 122.54(10)° and C–C bond distances between 1.3641(17) and 1.4207(15) Å (Scheme 1c). There is only one π−π stacking interaction between adjacent molecules (packing diagram displayed in Scheme 1e), the c-ring interacting with the g-ring [centroid···centroid distance 3.6875(6) Å], resulting in π-stacked chains running along the b-axis.
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) recorded in degassed dichloromethane (Fig. 2a) revealed an irreversible oxidation wave at Eox = 1.81 V vs. SCE and a reversible reduction wave at Ered = −1.16 V vs. SCE. The corresponding HOMO and LUMO energy levels are −6.15 and −3.18 eV, respectively. The LUMO is only slightly stabilized compared to that of the parent compounds DiKTa (−3.11 eV) while the HOMO is considerably more stabilized than that of DiKTa (−5.93 eV).44 The electrochemical data are summarized in Table S6 (ESI†).
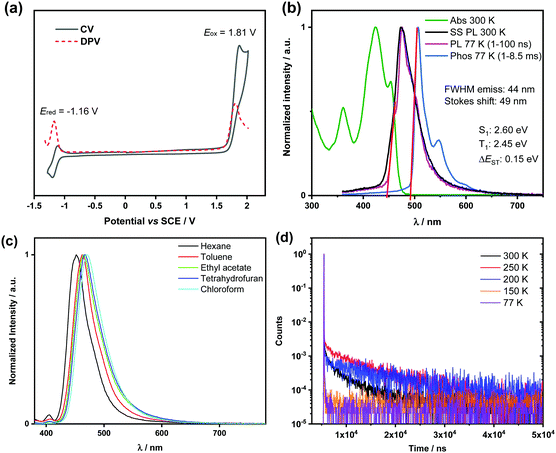 | ||
| Fig. 2 Optoelectronic characterization of (rac)-Hel-DiDiKTa: (a) cyclic and differential pulse voltammograms in degassed CH2Cl2 with 0.1 m [nBu4N]PF6 as the supporting electrolyte and Fc/Fc+ as the internal reference (Fc/Fc+ = 0.46 V vs. SCE).94 (b) Absorption (green line) and steady-state (SS) PL spectra obtained in toluene at 300 K (black line) and 77 K (pink line), and phosphorescence (Phos.) spectrum in toluene glass at 77 K (blue line) (delay: 1 ms; gate time: 8.5 ms, λexc = 343 nm). (c) Solvatochromic PL study (λexc = 350 nm). (d) Temperature-dependent lifetime for 1 wt% (rac)-Hel-DiDiKTa in mCP. | ||
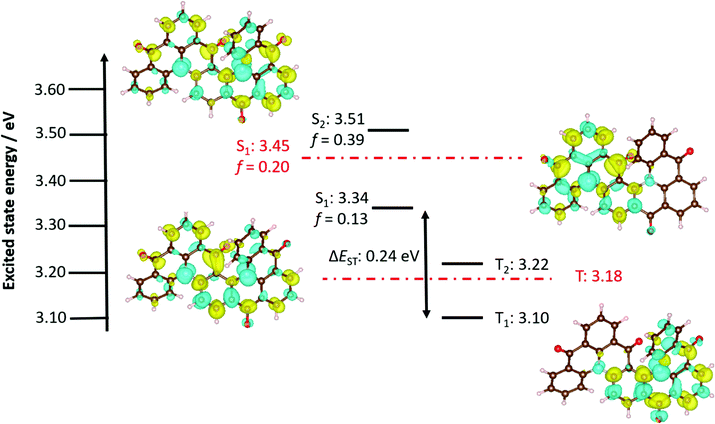
The UV-vis absorption spectrum of (rac)-Hel-DiDiKTa in toluene (Fig. 2b and Fig. S8a, ESI†) is significantly different than that of DiKTa. There is a high-energy, high-intensity band at 360 nm (ε = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 M−1 cm−1) assigned by Spin-Component Scaling second-order approximate Coupled-Cluster (SCS-CC2) calculations as a π–π* transition centered on one half of the emitter (Fig. S23, ESI†). There is a higher-intensity, low-energy band at 425 nm (ε = 21
917 M−1 cm−1) assigned by Spin-Component Scaling second-order approximate Coupled-Cluster (SCS-CC2) calculations as a π–π* transition centered on one half of the emitter (Fig. S23, ESI†). There is a higher-intensity, low-energy band at 425 nm (ε = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 M−1 cm−1) assigned as a short-range charge-transfer (SRCT) transition, which, according to the calculations, is associated with a transition to the S2 state at 3.51 eV. The band at 453 nm is associated with a transition to the S1 state, and has a lower ε of 14
140 M−1 cm−1) assigned as a short-range charge-transfer (SRCT) transition, which, according to the calculations, is associated with a transition to the S2 state at 3.51 eV. The band at 453 nm is associated with a transition to the S1 state, and has a lower ε of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 M−1 cm−1 than the band at 425 nm; a trend in line with the lower calculated oscillator strength, f, of 0.13 for S1 compared to 0.39 for S2. This assignment is further corroborated by the energy difference between these states, calculated to be 0.17 eV (Fig. 3) and measured to be 0.18 eV (Fig. 2b). S1 is also SRCT in nature, with S1 centered more around the middle of the molecule and S2 associated with electronic density across the entire molecule, hence the oscillator strength changes (Fig. 3). This low-energy S1 band is red-shifted compared to that of DiKTa (433 nm), with lower molar absorptivity (ε of DiKTa ∼21
292 M−1 cm−1 than the band at 425 nm; a trend in line with the lower calculated oscillator strength, f, of 0.13 for S1 compared to 0.39 for S2. This assignment is further corroborated by the energy difference between these states, calculated to be 0.17 eV (Fig. 3) and measured to be 0.18 eV (Fig. 2b). S1 is also SRCT in nature, with S1 centered more around the middle of the molecule and S2 associated with electronic density across the entire molecule, hence the oscillator strength changes (Fig. 3). This low-energy S1 band is red-shifted compared to that of DiKTa (433 nm), with lower molar absorptivity (ε of DiKTa ∼21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1)44 in line with the change in energy of the S1 state and the lower oscillator strength, which are 3.45 eV and 0.20 for DiKTa, and 3.34 eV and 0.13 for Hel-DiDiKTa (Fig. 3).
000 M−1 cm−1)44 in line with the change in energy of the S1 state and the lower oscillator strength, which are 3.45 eV and 0.20 for DiKTa, and 3.34 eV and 0.13 for Hel-DiDiKTa (Fig. 3).
The room temperature steady-state (SS) photoluminescence (PL) spectrum of (rac)-Hel-DiDiKTa, recorded in toluene, peaks at 473 nm, which is slightly red-shifted compared to that of DiKTa (λPL = 453 nm). The PL spectrum is narrow (FWHM = 44 nm) and shows a small Stokes shift of 49 nm, both of which reflect the conformationally rigid structure and the small degree of geometrical relaxation in the excited state (Fig. 2b). This FWHM value is considerably smaller than many of the helicene compounds reported in the literature (often 50 – 80 nm).1,16,19,81,91–93,95 The PL spectrum at 77 K is nearly identical to that at room temperature. Furthermore, a very small degree of positive solvatochromism is observed (Fig. 2c and Table S1, ESI†), behavior that is a hallmark of MR-TADF compounds.44,96 Unlike para-disposed nitrogen and ketone groups that leads to a significant bathochromic shift in the emission compared to their meta-functionalized analogue, as evidenced in the case of the emitters DMQA and QA-1 (c.f., Fig. S1, ESI†) recently reported by Yasuda et al.,45 the emission energy of (rac)-Hel-DiDiKTa changes very little compared to that of DiKTa. This is despite of the increased conjugation length of Hel-DiDiKTa and is due to the meta-disposition of the amine and ketone groups. The S1 and T1, determined from the peaks of the prompt fluorescence and phosphorescence spectra in toluene at 77 K, respectively, are 2.60 and 2.45 eV, leading to a moderate ΔEST of 0.15 eV. These values are in reasonable accord with the predicted by SCS-CC2 calculations (ΔEST = 0.24 eV) and with those of other reported ketone-containing MR-TADF compounds, including DiKTa (0.20 eV).44,45,49 The ΦPL in toluene is very low at 1% and is due to significant non-radiative decay that is not uncommon in helicene compounds. No delayed emission was detected by time-resolved PL measurements in toluene (Fig. S13, ESI†). Recently, Wu et al. highlighted that many MR-TADF emitters, including N/ketone-based emitters, may not show TADF behavior in solvents while TADF can be observed in a suitable host due to exciplex-like host-emitter interactions.97 The SS PL spectrum is only slightly affected by the presence of air (Fig. S9, ESI†). The ΦPL improved to 4.1% as a 1 wt% doped film in 1,3-bis(N-carbazolyl)benzene (mCP) and to 6.2% in 1 wt% doped film in PMMA. We note that the ΦPL remained essentially constant at ∼5% regardless of the doping concentration in mCP (Table S2, ESI†). These results are similar to the ΦPL values reported for unsubstituted helicenes.16
In mCP (1 wt% of emitter) the SS spectrum peaks at 477 nm (Fig. S10a, ESI†), the FWHM value is 50 nm, and, in contrast to what is seen in toluene solution (Fig. S9, ESI†), both the PL intensity and lifetime of (rac)-Hel-DiDiKTa decrease moderately in the presence of O2 (Fig. S10a and b, ESI†). Temperature-dependent time-resolved PL measurements revealed an increase in the contribution of the delayed component of the emission decay with increasing temperature (Fig. 2d and Table S4, ESI†), which corroborates the TADF character of Hel-DiDiKTa. The S1, T1 and ΔEST values, determined from the peaks of the prompt fluorescence and phosphorescence spectra at 77 K, in mCP are 2.59, 2.44 and 0.15 eV, respectively, while in PMMA, these are essentially the same at 2.58, 2.45 and 0.13 eV, respectively. The temperature-dependent steady-state PL emission of (rac)-Hel-DiDiKTa was measured in 1% mCP, and the data are shown in Fig. S12 (ESI†). Analysis of the evolution of the SS PL of (rac)-Hel-DiDiKTa with decreasing temperature reveals a competition between phosphorescence and delayed fluorescence, with the phosphorescence intensity increasing considerably at lower temperatures (150 K and 77 K, Fig. S12, ESI†), which indicates that non-radiative decay is suppressed at lower temperatures and the phosphorescence becomes more competitive (Table S4, ESI†). We also observed from the temperature dependent transient decay profile of (rac)-Hel-DiDiKTa (1 wt% in mCP, Fig. 2d) that the delayed fluorescence intensity increases upon going from 300 K to 250 K, indicating decreased non-radiative decay due to suppression of molecular vibrations at 250 K. The delayed fluorescence intensity then decreases considerably upon reaching 77 K, which we ascribe to the quenching of the TADF (Table 1). The photophysical data is summarized in Table 1.
| In toluene | In film | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ abs /nm | ε /M−1 cm−1 | λ PL /nm | Φ PL in N2 (air)c /% | FWHMd /nm (eV) | S 1 /eV | T 1 /eV | ΔESTf/eV | λ PL /nm | Φ PL in N2 (air) /% | FWHMd/nm (eV) | S1 /eV | T1 /eV | ΔEST /eV |
| a UV-vis absorption band of interest. b PL in toluene degassing with N2. c Photoluminescence quantum yield in toluene relative to quinine sulfate in 1 N H2SO4 (ΦPL = 54.6%). d Full width at half maximum. e Obtained using an integrating sphere under N2. f Energy gap between S1 and T1 calculated from the difference of the peaks of the fluorescence and phosphorescence spectra in toluene glass at 77 K. g 1 wt% (rac)-Hel-DiDiKTa doped in mCP. h 1 wt% (rac)-Hel-DiDiKTa doped in PMMA. | |||||||||||||
| 360/425/453 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917/21 140/14 917/21 140/14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 292 |
473 | 1.34 (1.27) | 44 (0.25) | 2.60 | 2.45 | 0.15 | 478g | 4.1 (4.0)g | 52 (0.29)g | 2.59g | 2.44g | 0.15g |
| 480h | 6.2 (5.4)h | 58 (0.33)h | 2.58h | 2.45h | 0.13h | ||||||||
Density Functional Theory (DFT) calculations were first performed to optimize the geometry of the ground state, taking the crystal structure geometry as the starting point. Compared to DiKTa (−6.20 eV), the calculated HOMO energy is slightly stabilized to −6.26 eV for Hel-DiDiKTa (Table S8, ESI†). A larger stabilization of the LUMO energy exists at −2.53 eV (for DiKTa, ELUMO = −2.23 eV). The relative HOMO/LUMO levels corroborate the experimental values of −5.93 eV/−3.11 eV for DiKTa and −6.15 eV/−3.18 eV for Hel-DiDiKTa. Previously, we have highlighted that DFT is not appropriate as it does not accurately predict the excited state energies of MR-TADF emitters.96 We thus used Spin-Component Scaling second-order approximate Coupled-Cluster (SCS-CC2) calculations, which we have shown previously to provide an excellent set of predictions of ΔEST and S1 energies.44,49,98,99 SCS-CC2 calculations predict the S1 state to be 3.34 eV and the T1 state to be 3.10 eV, resulting in a ΔEST of 0.24 eV, which is slightly smaller than that calculated for DiKTa (ΔEST = 0.27 eV). The difference density plots are shown in Fig. 3 and reveal how the electronic density evolves from the ground state to the excited states. The pattern of the difference density plots for S1 and S2 is indicative of SRCT states involving the entirety of the helicene structure, while the plots for T1 and T2 likewise show the alternating increase and decrease in electron density pattern, but this is localized on different fragments of the helicene corresponding to the DiKTa core structure. The differences in orbital type between the singlet and triplet excited states ensure that RISC can occur directly between these states;100 the presence of an intermediate triplet excited state should also facilitate RISC.101–103 The SRCT character of the S1 and T1 states explains the moderate ΔEST. We also performed SCS-CC2 calculations on the previously reported compound Hel-DiKTa-2. This compound possesses a very large ΔEST of 0.51 eV (Table S8, ESI†) due to triplet stabilisation by the naphthalene group104 and so is unlikely to show TADF (Fig. S21, ESI†).
(rac)-Hel-DiDiKTa was separated into its P- and M-enantiomers using peak recycling chiral HPLC (cHPLC; Fig. S14, ESI†), demonstrating high enantiomeric excess (>99% and >97%, respectively; Fig. S15, ESI†). The circular dichroism (CD) spectra for the separated enantiomers were recorded in toluene (Fig. 4a) and showed mirror-image CD signals (Fig. S17, ESI† right), which exhibited multiple bisignate cotton effects throughout the ground state absorption spectral range (300–480 nm). Time-dependent DFT calculation (PBE0/6-31g(d,p)) was employed to predict the CD spectra and assign the absolute configuration (P and M) of Hel-DiDiKTa (Fig. S24, ESI†). The TD-DFT predicted CD spectra shown in Fig. S24 (ESI†) align with the CD spectra obtained experimentally. A maximum |gabs| of 2.8 × 10−3 was observed at 320 nm (Fig. S17, ESI†). These results are of a similar order of magnitude to other chiral small molecule TADF systems.2,14 Circularly polarized photoluminescence (CPPL) spectra (Fig. S17, ESI†) display mirror image monosignate bands peaking at around 465 nm, confirming the transference of chirality to the excited state, with a maximum |gPL| of 4 × 10−4 for both enantiomers (Fig. S18, ESI†). These values are also similar to other helicenes and chiral small molecule TADF systems reported in the literature,76 and align with those reported for helicenoids with two bridged triphenylamine diketone derivatives (10−4−10−3, see Fig. 1).14 As expected for helicenes7 where the ground and emitting excited state geometries are generally similar, the sign and magnitude of gPL align with those of gabs calculated at around 455 nm. Upon optimization of the molecular structure at the lowest-energy singlet excited state (S1) at the PBE0/6-31g(d,p) level of theory, we calculated a gPL value of 6.94 × 10−4 (Table S9, ESI†), which aligns closely with the measured gPL. The main limiting factor for the magnitude of gPL, based on our calculations, is the nearly perpendicular alignment of the |μ| and |m| vectors [cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.116918 (θ = 83.28573)]. Although the magnitude of |m| is low at 0.348671679 × 10−20 esu cm−1, compared to |μ| (234.811511 × 10−20 erg G−1), it is within the range typically observed for helicenes.3,15,16,95 This result suggests that our molecular design is promising to generate strong CPL MR-TADF emitters if the alignment between |μ| and |m| can be solved.3,15,16,95 While these molecules do not have suitable ΦPL to warrant exploration in OLEDs, they provide valuable insight into CP-TADF material design, particularly integrating MR-TADF into helicenes.
θ = 0.116918 (θ = 83.28573)]. Although the magnitude of |m| is low at 0.348671679 × 10−20 esu cm−1, compared to |μ| (234.811511 × 10−20 erg G−1), it is within the range typically observed for helicenes.3,15,16,95 This result suggests that our molecular design is promising to generate strong CPL MR-TADF emitters if the alignment between |μ| and |m| can be solved.3,15,16,95 While these molecules do not have suitable ΦPL to warrant exploration in OLEDs, they provide valuable insight into CP-TADF material design, particularly integrating MR-TADF into helicenes.
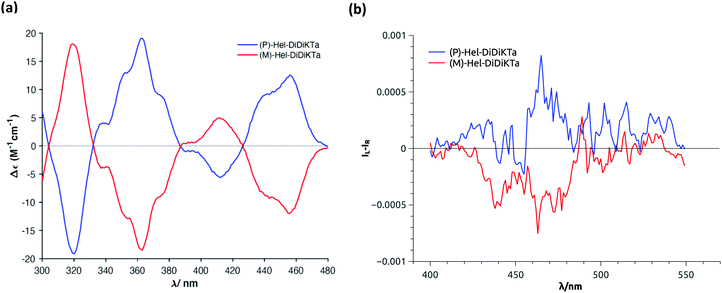 | ||
| Fig. 4 (a) Circular dichroism and (b) CPL spectra for (P)-Hel-DiDiKTa (blue) and (M)-Hel-DiDiKTa (red) in toluene. | ||
After the resolution of both enantiomers, the enantiomerisation process was studied. The CD signal decay from (P)-Hel-DiDiKTa in p-xylene at different temperatures (T = 75 °C, 80 °C, 85 °C and 90 °C) was monitored over time (see Fig. S25–S28, ESI†). An inversion barrier characterised by ΔH‡e = 39 ± 1 (kcal mol−1) and ΔS‡e = 26 ± 1 (cal K−1 mol−1) was found (see Table S10, ESI†). This results in a Gibbs free energy of activation for the enantiomerisation process (ΔG‡e) of 31±2 (kcal mol−1) at 298 K. (P)-Hel-DiDiKTa possesses a similar inversion barrier (298 K) to other double helicenes found in the literature (Table S10, ESI†).105,106(P)-Hel-DiDiKTa also appears to have a similar enantiomerisation barrier to other bridged phenylamines previously reported (Table S10, ESI†).107,108 Moreover, when compared to [5] and [6]-helicene (f and g in Table S10, ESI†), (P)-Hel-DiDiKTa possesses a similar barrier as [6]helicene.109 The half-life obtained for the enantiomerisation is ca. 310 years at room temperature.
Not only is (P)-Hel-DiDiKTa stable to enantiomerization but the compound is also stable towards thermal decomposition. Thermogravimetric analysis (TGA) showed that no decomposition was observed in the temperature range of the enantiomerization study and the decomposition temperature was found to be 399 °C, corresponding to 5% weight loss (Fig. S30, ESI†).
In summary, we report a novel S-shaped triphenylamine diketone double [4]helicene that exhibits MR-TADF. Unlike the previously reported triphenylamine diketone helicene (Hel-DiKTa-2), Hel-DiDiKTa was predicted by SCS-CC2 calculations to show a ΔEST appropriate for TADF, which was confirmed experimentally (0.15 eV in 1 wt% doped mCP film). As expected from a MR-TADF emitter, the emission peak at 473 nm is narrow, with a FWHM of 44 nm in toluene, a value considerably smaller than often observed for helicenoids. In 1 wt% mCP the emission peak at 477 nm shows a FWHM value of 50 nm. The TADF behavior of Hel-DiDiKTa was confirmed by temperature-dependent time-resolved photoluminescence measurements, and the chiroptical behavior of the enantiomers was determined, with a maximum |gabs| of 2.8 × 10−3 and a |gPL| of 4 × 10−4. While the low ΦPL precluded the use of this compound as an emitter in OLEDs, we believe that this report will serve as a foundation to develop intrinsically chiral, CPL-active MR-TADF molecules for OLEDs. Further work is undergoing in our lab to improve the optoelectronic and chiroptical properties of chiral MR-TADF compounds. Finally, the enantiomerization studies and the thermogravimetric analysis demonstrate the high inherent stability of (P)-Hel-DiDiKTa.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The St Andrews team would also like to thank the Leverhulme Trust (RPG-2016-047) and EPSRC (EP/P010482/1) for financial support. EZ-C is a Royal Society Leverhulme Trust Senior Research Fellow (SRF\R1\201089). We are also grateful for financial support from the University of St Andrews Restarting Research Funding Scheme (SARRF) which is funded through the Scottish Funding Council grant reference SFC/AN/08/020. D. S. acknowledges support from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska Curie Individual Fellowship grant agreement No. 838009 (TSFP). Computational resources have been provided by the Consortium des Équipements de Calcul In-tensif (CÉCI), funded by the Fonds de la Recherche Scientifiques de Belgique (F. R. S.-FNRS) under Grant no. 2.5020.11. The Imperial team would like to thank the EPSRC for funding (EP/R00188X/1). F. Z. gratefully acknowledges financial support from the University of Pisa (PRA 2020_21). This project also received funding from the European Commission Research Executive Agency (Grant Agreement Number: 859752 HEL4CHIR-OLED H2020-MSCA-ITN-2019). Y. O. acknowledges funding by the Fonds de la Recherche Scientifique-FNRS under Grant no F.4534.21 (MIS-IMAGINE). D. B. is a FNRS Research Director.References
- F. Saal, F. Zhang, M. Holzapfel, M. Stolte, E. Michail, M. Moos, A. Schmiedel, A. M. Krause, C. Lambert, F. Würthner and P. Ravat, J. Am. Chem. Soc., 2020, 142, 21298–21303 CrossRef CAS PubMed.
- K. Dhbaibi, L. Favereau and J. Crassous, Chem. Rev., 2019, 119, 8846–8953 CrossRef CAS PubMed.
- Y. Nakai, T. Mori and Y. Inoue, J. Phys. Chem. A, 2012, 112, 7372–7385 CrossRef PubMed.
- Y. Shen and C. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed.
- K. Dhbaibi, L. Abella, S. Meunier-Della-Gatta, T. Roisnel, N. Vanthuyne, B. Jamoussi, G. Pieters, B. Racine, E. Quesnel, J. Autschbach, J. Crassous and L. Favereau, Chem. Sci., 2021, 12, 5522–5533 RSC.
- C. Shen, G. Zhang, Y. Ding, N. Yang, F. Gan, J. Crassous and H. Qiu, Nat. Commun., 2021, 12, 2786 CrossRef CAS PubMed.
- H. Tanaka, Y. Inoue and T. Mori, ChemPhotoChem, 2018, 2, 386–402 CrossRef CAS.
- J. L. Greenfield, J. Wade, J. R. Brandt, X. Shi, T. J. Penfold and J. Fuchter, Chem. Sci., 2021, 12, 1–15 RSC.
- W. L. Zhao, M. Li, H. Y. Lu and C. F. Chen, Chem. Commun., 2019, 55, 13793–13803 RSC.
- T. Verbiest, S. Van Elshocht, M. Kauranen, L. Hellemans, J. Snauwaert, C. Nuckolls, T. J. Katz and A. Persoons, Science, 1998, 282, 913–916 CrossRef CAS PubMed.
- O. Kel, A. Fürstenberg, N. Mehanna, C. Nicolas, B. Laleu, M. Hammarson, B. Albinsson, J. Lacour and E. Vauthey, Chem. – A Eur. J., 2013, 19, 7173–7180 CrossRef CAS.
- S. D. Dreher, T. J. Katz, K. Lam and A. L. Rheingold, J. Org. Chem., 2000, 65, 815–822 CrossRef CAS.
- K. Yavari, P. Aillard, Y. Zhang, F. Nuter, P. Retailleau, A. Voituriez and A. Marinetti, Angew. Chem., Int. Ed., 2014, 53, 861–865 CrossRef CAS PubMed.
- J. E. Field, G. Muller, J. P. Riehl and D. Venkataraman, J. Am. Chem. Soc., 2003, 125, 11808–11809 CrossRef CAS PubMed.
- H. Kubo, D. Shimizu, T. Hirose and K. Matsuda, Org. Lett., 2020, 22, 9276–9281 CrossRef CAS PubMed.
- H. Kubo, T. Hirose, T. Nakashima, T. Kawai, J. Hasegawa and K. Matsuda, J. Phys. Chem. Lett., 2021, 12, 686–695 CrossRef CAS.
- J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- D. Zhang, M. Li and C. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 RSC.
- S. Sahasithiwat, T. Mophuang, L. Menbangpung, S. Kamtonwong and T. Sooksimuang, Synth. Met., 2010, 160, 1148–1152 CrossRef CAS.
- L. Wan, X. Shi, J. Wade, A. J. Campbell and M. J. Fuchter, Adv. Opt. Mater., 2021, 9, 2100066 CrossRef CAS.
- L. Wan, J. Wade, F. Salerno, O. Arteaga, B. Laidlaw, X. Wang, T. Penfold, M. J. Fuchter and A. J. Campbell, ACS Nano, 2019, 13, 8099–8105 CrossRef CAS PubMed.
- J. Wade, J. N. Hilfiker, J. R. Brandt, L. Liirò-Peluso, L. Wan, X. Shi, F. Salerno, S. T. J. Ryan, S. Schöche, O. Arteaga, T. Jávorfi, G. Siligardi, C. Wang, D. B. Amabilino, P. H. Beton, A. J. Campbell and M. J. Fuchter, Nat. Commun., 2020, 11, 1–11 Search PubMed.
- Y. Yang, R. C. Da Costa, D. M. Smilgies, A. J. Campbell and M. J. Fuchter, Adv. Mater., 2013, 25, 2624–2628 CrossRef CAS PubMed.
- A. Klimash, P. Pander, W. T. Klooster, S. J. Coles, P. Data, F. B. Dias and P. J. Skabara, J. Mater. Chem. C, 2018, 6, 10557–10568 RSC.
- M. Li, Y. Wang, D. Zhang, D. Zhang, Z. Hu, L. Duan and C. Chen, Sci. China Mater., 2021, 64, 899–908 CrossRef CAS.
- Y. Wei, A. Zheng, X. Xie, J. Zhang, L. He and P. Wang, ACS Mater. Lett., 2021, 3, 947–955 CrossRef CAS.
- Y. S. Lin, S. Y. Abate, C. I. Wang, Y. S. Wen, C. I. Chen, C. P. Hsu, C. C. Chueh, Y. T. Tao and S. S. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 20051–20059 CrossRef CAS.
- J. Wang, Y. Wang, X. Xie, Y. Ren, B. Zhang, L. He, J. Zhang, L. D. Wang and P. Wang, ACS Energy Lett., 2021, 6, 1764–1772 CrossRef CAS.
- N. R. Paisley, C. M. Tonge and Z. M. Hudson, Front. Chem., 2020, 8, 229 CrossRef CAS PubMed.
- C. Adachi and A. S. D. Sandanayaka, CCS Chem., 2020, 2, 1203–1216 CrossRef CAS.
- X. Wang, D. Zhou, J. Huang and J. Yu, Appl. Phys. Lett., 2015, 107, 043303 CrossRef.
- M. A. Bryden and E. Zysman-Colman, Chem. Soc. Rev., 2021, 50, 7587–7680 RSC.
- V. N. Nguyen, A. Kumar, M. H. Lee and J. Yoon, Coord. Chem. Rev., 2020, 425, 213545 CrossRef CAS.
- M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29, 1–54 Search PubMed.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS.
- G. Méhes, H. Nomura, Q. Zhang, T. Nakagawa and C. Adachi, Angew. Chem., Int. Ed., 2012, 51, 11311–11315 CrossRef.
- T. Nakagawa, S. Y. Ku, K. T. Wong and C. Adachi, Chem. Commun., 2012, 48, 9580–9582 RSC.
- S. Hirata, Y. Sakai, K. Masui, H. Tanaka, S. Y. Lee, H. Nomura, N. Nakamura, M. Yasumatsu, H. Nakanotani, Q. Zhang, K. Shizu, H. Miyazaki and C. Adachi, Nat. Mater., 2015, 14, 330–336 CrossRef CAS PubMed.
- C. A. Parker and C. G. Hatchard, Trans. Faraday Soc., 1961, 57, 1894–1904 RSC.
- H. W. Chen, J. H. Lee, B. Y. Lin, S. Chen and S. T. Wu, Light: Sci. Appl., 2018, 7, 17168 CrossRef CAS.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Nat. Photonics, 2019, 13, 678–682 CrossRef CAS.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS.
- D. Hall, S. M. Suresh, P. L. dos Santos, E. Duda, S. Bagnich, A. Pershin, P. Rajamalli, D. B. Cordes, A. M. Z. Slawin, D. Beljonne, A. Köhler, I. D. W. Samuel, Y. Olivier and E. Zysman-Colman, Adv. Opt. Mater., 2020, 8, 1901627 CrossRef CAS.
- H. Min, I. S. Park and T. Yasuda, Angew. Chem., Int. Ed., 2021, 60, 7643–7648 CrossRef CAS.
- F. Chen, L. Zhao, X. Wang, Q. Yang, W. Li, H. Tian, S. Shao, L. Wang, X. Jing and F. Wang, Sci. China: Chem., 2021, 64, 547–551 CrossRef CAS.
- J. Wei, C. Zhang, D. Zhang, Y. Zhang, Z. Liu, Z. Li, G. Yu and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 12269–12273 CrossRef CAS PubMed.
- S. Oda, W. Kumano, T. Hama, R. Kawasumi, K. Yoshiura and T. Hatakeyama, Angew. Chem., Int. Ed., 2021, 60, 2882–2886 CrossRef CAS PubMed.
- D. Sun, S. M. Suresh, D. Hall, M. Zhang, C. Si, D. B. Cordes, A. M. Z. Slawin, Y. Olivier, X. Zhang and E. Zysman-Colman, Mater. Chem. Front., 2020, 4, 2018–2022 RSC.
- N. Ikeda, S. Oda, R. Matsumoto, M. Yoshioka, D. Fukushima, K. Yoshiura, N. Yasuda and T. Hatakeyama, Adv. Mater., 2020, 32, 2004072 CrossRef CAS PubMed.
- S. Zou, C. Peng, S. Yang, Y. Qu, Y. Yu, X. Chen, Z. Jiang and L. Liao, Org. Lett., 2021, 23, 958–962 CrossRef CAS.
- Y. Qi, W. Ning, Y. Zou, X. Cao, S. Gong and C. Yang, Adv. Funct. Mater., 2021, 2102017, 1–7 Search PubMed.
- M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- F. Huang, K. Wang, Y. Z. Shi, X. C. Fan, X. Zhang, J. Yu, C. S. Lee and X. H. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 36089–36097 CrossRef CAS PubMed.
- S. Madayanad Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Adv. Funct. Mater., 2020, 30, 1908677 CrossRef CAS.
- Y. Zhang, J. Wei, D. Zhang, C. Yin, G. Li, Z. Liu, X. Jia, J. Qiao and L. Duan, Angew. Chem., Int. Ed., 2022, 61, e202113206 CAS.
- P. Jiang, J. Miao, X. Cao, H. Xia, K. Pan, T. Hua, X. Lv, Z. Huang, Y. Zou and C. Yang, Adv. Mater., 2021, 2106954 Search PubMed.
- T. Imagawa, S. Hirata, K. Totani, T. Watanabe and M. Vacha, Chem. Commun., 2015, 51, 13268–13271 RSC.
- M. Li, S. Li, D. Zhang, M. Cai, L. Duan, M. Fung and C. Chen, Angew. Chem., Int. Ed., 2018, 57, 2889–2893 CrossRef CAS PubMed.
- L. Zhou, G. Xie, F. Ni and C. Yang, Appl. Phys. Lett., 2020, 117, 130502 CrossRef CAS.
- J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 0045 CrossRef CAS.
- X. Li, Y. Xie and Z. Li, Adv. Photonics Res., 2021, 2, 2000136 CrossRef.
- T. Y. Li, Y. M. Jing, X. Liu, Y. Zhao, L. Shi, Z. Tang, Y. X. Zheng and J. L. Zuo, Sci. Rep., 2015, 5, 14912 CrossRef CAS PubMed.
- J. F. Sherson, H. Krauter, R. K. Olsson, B. Julsgaard, K. Hammerer, I. Cirac and E. S. Polzik, Nature, 2006, 443, 557–560 CrossRef CAS PubMed.
- Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light Sci. Appl., 2021, 10, 1–18 CrossRef PubMed.
- R. Farshchi, M. Ramsteiner, J. Herfort, A. Tahraoui and H. T. Grahn, Appl. Phys. Lett., 2011, 98, 162508 CrossRef.
- Y. Yang, R. Correa, M. J. Fuchter and A. J. Campbell, Nat. Photonics, 2013, 7, 634–638 CrossRef CAS.
- J. Jiménez, L. Cerdán, F. Moreno, B. L. Maroto, I. García-Moreno, J. L. Lunkley, G. Muller and S. De La Moya, J. Phys. Chem. C, 2017, 121, 5287–5292 CrossRef PubMed.
- R. Tempelaar, A. Stradomska, J. Knoester and F. C. Spano, J. Phys. Chem. B, 2011, 115, 10592–10603 CrossRef CAS PubMed.
- T. Wu, P. Bouř and V. Andrushchenko, Sci. Rep., 2019, 9, 1068 CrossRef PubMed.
- P. M. L. Blok and H. P. J. M. Dekkers, Chem. Phys. Lett., 1989, 161, 188–194 CrossRef CAS.
- J. A. Schellman, Chem. Rev., 1975, 75, 323–331 CrossRef CAS.
- R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673–7686 RSC.
- F. Zinna and L. D. I. Bari, Chirality, 2015, 27, 1–13 CrossRef CAS PubMed.
- J. L. Lunkley, D. Shirotani, K. Yamanari, S. Kaizaki and G. Muller, J. Am. Chem. Soc., 2008, 130, 13814–13815 CrossRef CAS PubMed.
- L. Arrico, L. Di Bari and F. Zinna, Chem. – A Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed.
- E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. De La Moya, Chem. – A Eur. J., 2015, 21, 13488–13500 CrossRef PubMed.
- N. Chen and B. Yan, Molecules, 2018, 23, 3376 CrossRef PubMed.
- Z. L. Tu, Z. P. Yan, X. Liang, L. Chen, Z. G. Wu, Y. Wang, Y. X. Zheng, J. L. Zuo and Y. Pan, Adv. Sci., 2020, 7, 1–6 Search PubMed.
- Z. Wu, H. Han, Z. Yan, X. Luo, Y. Wang and Y. Zheng, Adv. Mater., 2019, 31, 1900524 CrossRef PubMed.
- Y. Li, A. Yagi and K. Itami, J. Am. Chem. Soc., 2020, 142, 3246–3253 CrossRef CAS PubMed.
- L. Frédéric, A. Desmarchelier, R. Plais, L. Lavnevich, G. Muller, C. Villafuerte, G. Clavier, E. Quesnel, B. Racine, S. Meunier-della-gatta, J. Dognon, P. Thuéry, J. Crassous, L. Favereau and G. Pieters, Adv. Funct. Mater. Mater., 2020, 30, 2004838 CrossRef PubMed.
- Y.-P. Zhang, X. Liang, X.-F. Luo, S.-Q. Song, S. Li, Y. Wang, Z.-P. Mao, W.-Y. Xu, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Angew. Chem., Int. Ed., 2021, 60, 8435–8440 CrossRef CAS PubMed.
- Y. Xu, Q. Wang, X. Cai, C. Li and Y. Wang, Adv. Mater., 2021, 2100652 CrossRef CAS PubMed.
- S.-Y. Yang, S.-N. Zou, F.-C. Kong, X.-J. Liao, Y.-K. Qu, Z.-Q. Feng, Y.-X. Zheng, Z.-Q. Jiang and L.-S. Liao, Chem. Commun., 2021, 57, 11041–11044 RSC.
- X. Wu, J. Huang, B. Su, S. Wang, L. Yuan, W. Zheng, H. Zhang, Y. Zheng, W. Zhu and P. Chou, Adv. Mater., 2021, 2105080 Search PubMed.
- J.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A. C.-H. Sue, X.-Y. Cao and X.-Y. Wang, J. Am. Chem. Soc., 2021, 143, 17958–17963 CrossRef CAS PubMed.
- J. E. Field, T. J. Hill and D. Venkataraman, J. Org. Chem., 2003, 68, 6071–6078 CrossRef CAS PubMed.
- T. Mori, Chem. Rev., 2021, 121, 2373–2412 CrossRef CAS PubMed.
- N. J. Schuster, L. A. Joyce, D. W. Paley, F. Ng, M. L. Steigerwald and C. Nuckolls, J. Am. Chem. Soc., 2020, 142, 7066–7074 CrossRef CAS PubMed.
- Q. Jiang, Y. Han, Y. Zou, H. Phan, L. Yuan, T. Herng, J. Ding and C. Chi, Chem. – A Eur. J., 2020, 26, 5613–15622 Search PubMed.
- S. Kinoshita, R. Yamano, Y. Shibata, Y. Tanaka, K. Hanada, T. Matsumoto, K. Miyamoto, A. Muranaka, M. Uchiyama and K. Tanaka, Angew. Chem., Int. Ed., 2020, 59, 11020–11027 CrossRef CAS PubMed.
- G. Zhang, J. Tan, L. Zhou, C. Liu, J. Liu, Y. Zou, A. Narita and Y. Hu, Org. Lett., 2021, 23, 6183–6188 CrossRef CAS PubMed.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef CAS PubMed.
- H. Tanaka, M. Ikenosako, Y. Kato, M. Fujiki, Y. Inoue and T. Mori, Commun. Chem., 2018, 1, 38 CrossRef.
- A. Pershin, D. Hall, V. Lemaur, J. C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Nat. Commun., 2019, 10, 597 CrossRef CAS PubMed.
- X. Wu, B. K. Su, D. G. Chen, D. Liu, C. C. Wu, Z. X. Huang, T. C. Lin, C. H. Wu, M. Zhu, E. Y. Li, W. Y. Hung, W. Zhu and P. T. Chou, Nat. Photonics, 2021, 15, 780–786 CrossRef CAS.
- J. A. Knçller, G. Meng, X. Wang, D. Hall, A. Pershin, D. Beljonne, Y. Olivier, S. Laschat, E. Zysman-colman and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 3156–3160 CrossRef PubMed.
- S. M. Suresh, E. Duda, D. Hall, Z. Yao, S. Bagnich, D. Beljonne, M. Buck, Y. Olivier, A. Ko, A. M. Z. Slawin, H. Ba and E. Zysman-colman, J. Am. Chem. Soc., 2020, 142, 6588–6599 CrossRef CAS PubMed.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- Y. Wada, H. Nakagawa, S. Matsumoto, Y. Wakisaka and H. Kaji, Nat. Photonics, 2020, 14, 643–649 CrossRef CAS.
- C. Y. Chan, M. Tanaka, Y. T. Lee, Y. W. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Nat. Photonics, 2021, 15, 203–207 CrossRef CAS.
- J. U. Kim, I. S. Park, C. Y. Chan, M. Tanaka, Y. Tsuchiya, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1–8 Search PubMed.
- Y. Y. Pan, J. Huang, Z. M. Wang, D. W. Yu, B. Yang and Y. G. Ma, RSC Adv., 2017, 7, 26697–26703 RSC.
- Q. Jiang, Y. Han, Y. Zou, H. Phan, L. Yuan, T. S. Herng, J. Ding and C. Chi, Chem. – A Eur. J., 2020, 26, 15613–15622 CrossRef CAS PubMed.
- T. Katayama, S. Nakatsuka, H. Hirai, N. Yasuda, J. Kumar, T. Kawai and T. Hatakeyama, J. Am. Chem. Soc., 2016, 138, 5210–5213 CrossRef CAS PubMed.
- B. D. Gliemann, A. G. Petrovic, E. M. Zolnhofer, P. O. Dral, F. Hampel, G. Breitenbruch, P. Schulze, V. Raghavan, K. Meyer, P. L. Polavarapu, N. Berova and M. Kivala, Chem. – Asian J., 2017, 12, 31–35 CrossRef CAS PubMed.
- H. Nishimura, K. Tanaka, Y. Morisaki, Y. Chujo, A. Wakamiya and Y. Murata, J. Org. Chem., 2017, 82, 5242–5249 CrossRef CAS PubMed.
- R. H. Martin and M. J. Marchant, Tetrahedron Lett., 1972, 13, 3707–3708 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: A summary of prior examples of carbonyl-containing MR-TADF emitters, experimental details, synthesis procedures and characterization data, NMR spectra, HRMS, HPLC, X-ray crystallographic data, supplemental photophysical data, electrochemical data, chiral HPLC, CD spectra, CPPL data, computational simulations, enantiomerisation kinetics data and thermogravimetric analysis. CCDC 2105660. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2tc00198e. The research data supporting this publication can be accessed at https://doi.org/10.17630/25585cf8-5f19-4b26-b95e-569787e10399. |
| This journal is © The Royal Society of Chemistry 2022 |