A photofunctional platform of bis-terpyridine ruthenium complex-linked coordination polymers with structural diversity†‡
Aichun
Wu
,
Yuanlin
Tang
,
Xinling
Li
,
Baohua
Zhang
 ,
Aiju
Zhou
,
Zhiwei
Qiao
,
Aiju
Zhou
,
Zhiwei
Qiao
 * and
Lianpeng
Tong
* and
Lianpeng
Tong
 *
*
School of Chemistry and Chemical Engineering, Guangzhou University, Higher Education Mega Center, No. 230 Wai Huan Xi Road, Guangzhou, 510006, P. R. China. E-mail: zqiao@gzhu.edu.cn; ltong@gzhu.edu.ch
First published on 3rd November 2022
Abstract
Solvothermal syntheses of the bis(4′-carboxyl-2,2′:6′,2′′-terpyridine) ruthenium complex [Ru(tpyCOO−)2] as a linker and different metal anions as nodes constructed a family of ten novel coordination polymers (CPs) with different topological types, ranging from one-dimensional (1D) chains and two-dimensional (2D) networks to three-dimensional (3D) frameworks. The structural diversity of CPs is achieved by the various coordinating geometries of metal nodes, including 3d transition (monometallic or bimetallic), lanthanide, and group 4 (zirconium and hafnium) metal anions. The Ru complex linker plays the role of a light-harvesting unit and renders multifarious photofunctions to the CP materials. While the ytterbium CP (Yb–Ru) exhibits near-infrared emission upon visible-light excitation, the 2D bimetallic Co/Ni CPs (Cox–Niy–Ru) and 3D interpenetrated copper CP (Cu–Ru) show high to modest efficiencies toward the photocatalytic hydrogen evolution reaction. We suggest the series of new CPs with versatile structural traits as a unique platform for the research of photocatalysis and other photonic functional applications.
Introduction
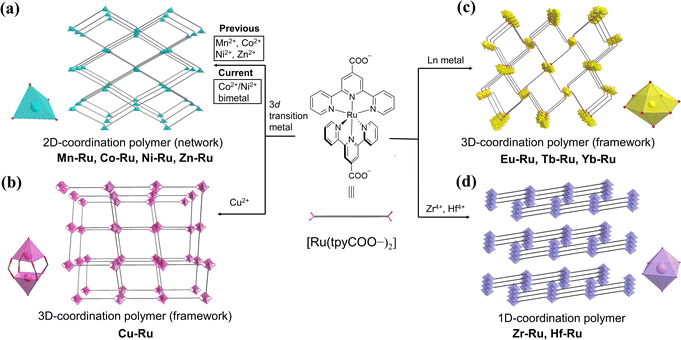
Coordination polymers (CPs), also known as metal–organic frameworks (MOFs), have emerged as a class of porous crystalline materials that attracted great interest across interdisciplinary research fields during the past decades.1–6 One unique merit of CP materials is that their topological structures can be elaborately designed and modified at the molecular level by the choice of the building components—organic linkers and inorganic nodes. The chemical and physical properties of CPs at the macroscopic level, otherwise, are determined by their geometrical morphology as well as the intrinsic features of their inorganic and organic building units. Incorporation of photo- and redox-active units in the construction of CPs may not only enhance the original functionalities of these building components but also generate new functionalities, due to the potential synergistic effect between the components within the specifical spatial environments of CPs.7–15 Recent studies have shown promising applications of photosensitizer-incorporated CPs in the areas of photosynthesis,16–21 photocatalysis,9,22–27 light-emitting materials,28–30 sensor materials,28,31–34 and light-harvesting complexes.35–37Polypyridyl ruthenium complexes are classic molecular photosensitizers with long-lived excited states and unique redox properties.38 The porous architectures of CPs built from polypyridyl Ru(II) photosensitizers have shown outstanding performance in photocatalysis, especially reactions related to solar fuel production.39–46 However, the tedious preparation of polypyridyl Ru(II) complexes and problematic crystallization of the frameworks, which might be attributed to the positive charge of the Ru(II) center and the obstacle of bulky polypyridyl ligands, have raised severe limitations over the research and development of functional CPs based on Ru(II) complex linkers.45,47–49 Systematic synthesis of polypyridyl Ru(II) complex-linked CPs with various metal nodes and versatile topological structures is rarely reported.
We recently reported the syntheses of a series of two-dimensional (2D) CPs comprising bis(4′-carboxyl-2,2′:6′,2′′-terpyridine) ruthenium linker [Ru(tpyCOO−)2], which was readily prepared from commercially available compounds, and 3d transition-metal (Mn, Co, Ni, and Zn) nodes (Scheme 1a).50 These 2D coordination networks are capable of driving highly efficient photocatalytic hydrogen evolution from water without the assistance of any co-catalysts or co-sensitizers. The current work describes our effort of systematically assembling the [Ru(tpyCOO−)2] linker with various metal nodes and further outspreading a family of novel CPs. Besides the previous 2D monometallic coordination networks,50 we report here four categories of coordination polymers based on the same [Ru(tpyCOO−)2] linker: mixed Co2+/Ni2+ cations and [Ru(tpyCOO−)2] affording bimetallic 2D coordination networks Cox–Niy–Ru (Scheme 1a), Cu2+ nodes bridged by [Ru(tpyCOO−)2] affording an interpenetrated 3D coordination framework Cu–Ru (Scheme 1b), Ln nodes affording isostructural 3D coordination frameworks Ln–Ru (Ln = Eu, Tb, or Yb, Scheme 1c), and group 4 Zr4+ and Hf4+ nodes affording 1D coordination chains Zr–Ru and Hf–Ru (Scheme 1d). The structural diversity of these [Ru(tpyCOO−)2]-linked CPs explicitly demonstrates the viability of designing and tuning the crystalline structures of coordination polymers at the molecular level.
More importantly, integrating the light-harvesting [Ru(tpyCOO−)2] component with different metal nodes provides a unique CP platform of multifarious photofunctions.10,51–53 While the photoluminescence spectrum of Yb–Ru shows emission at 976 nm upon excitation of the [Ru(tpyCOO−)2] linker unit, the bimetallic Co2+/Ni2+ coordination networks (Cox–Niy–Ru) are efficient catalysts for the visible-light driven hydrogen evolution reaction. More interesting photofunctions are expected to be studied and exploited based on this CP platform via further constituent optimization or post-synthetic modification of the coordination frameworks.54,55
Results and discussion
Syntheses and structures
By following the previous synthetic conditions for Co–Ru and Ni–Ru, we prepared 2D bimetallic Co/Ni coordination polymers as micro crystals denoted as Cox–Niy–Ru, where the subscripts x and y represent the molar ratio of Co(NO3)2 and Ni(ClO4)2 salt precursors used in the syntheses (x/y = 1/1, 1/2, 1/4, and 1/6). The field-emission scanning electron microscopy (FE-SEM) images of Cox–Niy–Ru (Fig. S12,‡ taking Co1–Ni2–Ru as an example) display layered structures similar to the structural features of Co–Ru and Ni–Ru reported previously. The powder X-ray diffraction (PXRD) patterns of the bimetallic Cox–Niy–Ru bulk samples (Fig. S7‡) with different x/y ratios show identical peaks to the PXRD patterns of Co–Ru or Ni–Ru, indicating the same 2D morphology of Cox–Niy–Ru as that of Co–Ru or Ni–Ru.50 Inductively coupled plasma mass spectrometry (ICP-MS) confirms the contents of both Co and Ni ions in the Cox–Niy–Ru products (Table S8‡). The measured molar metal ratios (nCo/nNi) in the bimetallic CPs range from nCo/nNi = 15.2 (x/y = 1/1) to nCo/nNi = 1.7 (x/y = 1/6).
Ultrasonication in protic solvents allows exfoliation of the bimetallic Co/Ni CPs into thin nanosheets, which are shown by both the FE-SEM and transmission electron microscopy (TEM) images (Fig. 1 and S13–S15‡) of the ultrasonicated suspension. At the same time, the element mapping identified the presence of both Ni and Co metal elements in the Cox–Niy–Ru nanosheets (Fig. 1 and S15‡). The PXRD patterns of bimetallic CP nanosheets maintain all characteristic diffraction peaks of their networks prior to the exfoliation (Fig. S8,‡ taking Co1–Ni4–Ru as an example), indicating the preserved 2D coordination lattices in the nanosheets. These nanosheets are estimated to consist of 3–7 layers of 2D lattices with a thickness of 2–6 nm.50 The X-ray photoelectron spectroscopy (XPS) spectra of Co1–Ni4–Ru (Fig. S16 & S17‡) show binding energy peaks at 781.53 eV and 797.65 eV together with satellite peaks at 786.0 eV and 802.41 eV that are ascribed to the Co(II) ion.56,57 Further the XPS Ni 2p scan of Co1–Ni4–Ru (Fig. S18‡) illustrates peaks at 855.98 eV and 873.34 eV, suggesting the presence of the Ni(II) ion.58
Characterization and properties
The thermogravimetric analysis (TGA) demonstrates that all four categories of CPs begin to lose non-coordinating DMF and water molecules below 100 °C (Fig. S19–S22‡). A significant weight loss of these CPs occurs at higher temperatures ranging from 260 °C (for the bimetallic Co/Ni CPs) to 400 °C (for the Zr and Hf CPs), due to the degradation of the coordination frameworks. The N2 sorption measurement of Cu–Ru at 77 K reveals a type II isotherm (Fig. S23‡), corresponding to a Brunauer–Emmett–Teller (BET) surface area of 1.9 m2 g−1. The N2 sorption isotherm of Yb–Ru (Fig. S24‡), as an example of the Ln CPs, indicates a BET surface area of 7.6 m2 g−1. The weak N2 gas adsorption capability of these 3D CPs is ascribed to the presence of counterions that occupy the porous space within the crystal frameworks. In addition, the interpenetrated structure of Cu–Ru makes it almost imporous.The cyclic voltammogram (CV) of the bimetallic Co/Ni CP, taking Co1–Ni2–Ru as an example, displays redox waves at −1.6 and −2.0 V versus ferrocenium/ferrocene (Fc+/Fc, Fig. S26‡). Very similar redox features have been reported for the previously synthesized Co–Ru or Ni–Ru CPs and assigned to the redox events of [Ru(tpyCOO−)2] linkers.50 Such characteristic redox features at −1.6 and −2.0 V were also observed in the CVs of Cu–Ru and the lanthanide CPs (Fig. S27 and S28‡). Despite the different metal nodes and various structures of these CPs, they all retain the redox properties of the bis-terpyridine ruthenium complex.
The solid-state UV-vis absorption spectra of these CPs all exhibit broad absorbance bands at about 300 nm and 500 nm, besides shoulder absorbance bands of some CP samples at 550 nm (Fig. S30–S33‡). These longer-wave bands in the visible light region are attributed to the characteristic metal-to-ligand charge transfer (MLCT) of [Ru(tpyCOO−)2] linkers, while the short-wave bands at 300 nm originate from the π–π* excitation of tpy ligands. The solid-state emission bands of 3d transition-metal-based CPs, Cox–Niy–Ru and Cu–Ru, are centered at approximately 670–690 nm (Fig. S36‡),50 while the emission bands of lanthanide CPs are centered at about 670 nm (Fig. S37‡). The emission spectrum of [Ru(tpyCOOH)2](PF6)2 powder exhibits a broad band at λmax = 720 nm. These absorption and emission studies emphasize that the CPs preserve the photophysical properties of the Ru complex linker, whereas various coordinating metal nodes slightly alter the ground/excited states of the Ru linker unit.
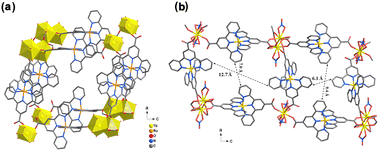
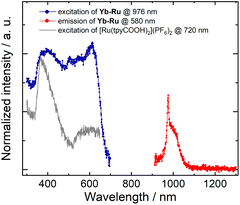
Since the Yb(III) ion emits luminescence in the near-infrared (NIR) range distinctively separated from the emission of the Ru linker in the visible-light range, the photochemical properties of Yb–Ru were further investigated. Upon excitation of the Ru linker of Yb–Ru at λex = 580 nm, a sharp NIR emission band at 978 nm assigned to the 2F5/2 → 2F7/2 transition of the Yb(III) ion59 was observed in the spectrum (Fig. 5), besides the characteristic Ru linker luminescent band at 670 nm (Fig. S37‡). Meanwhile, the excitation spectrum of Yb–Ru recorded upon the NIR luminescent band (λem = 976 nm) shows very similar featured bands to the excitation spectrum of the [Ru(tpyCOOH)2](PF6)2 complex (λem = 720 nm, Fig. 5). The excitation spectra of Yb–Ru collected upon the Yb(III) node emission (λem = 976 nm) and the Ru linker emission (λem = 670 nm) adopt similar profiles (Fig. S37‡). These spectroscopic results suggest that the Yb(III) node of Yb–Ru can be excited by the MLCT stated of the Ru linker, which plays the role of a sensitizer, tentatively via an energy transfer process.60–62 The time-resolved photoluminescence experiments (Fig. S38‡) reveal a reduced decay time of the excited [Ru(tpyCOOH)2]2+ complex upon coordination to the Yb(III) ions in Yb–Ru, consistent with the proposed energy transfer process throughout the CP framework.49,63
Photocatalytic hydrogen evolution
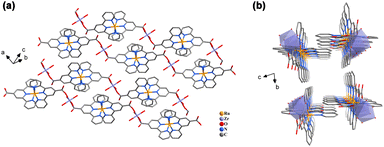
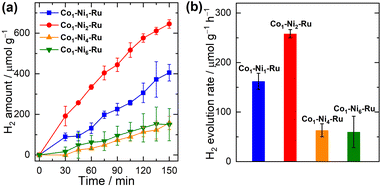
In the previous study, 2D Co–Ru and Ni–Ru nanosheets have been shown to effectively drive photocatalytic hydrogen evolution from an aqueous solution under the irradiation of visible light (λ > 420 nm).50 No co-catalyst or co-sensitizer was required for this photocatalytic hydrogen evolution process. The hydrogen evolution reaction is proposed to be initiated by quenching of the excited [Ru(tpyCOO−)2] linker. The resulting formally monovalent Ru(I) species transfer electrons to the Co or Ni nodes at the edge of CP nanosheets, where the reduction of protons occurs. The driving force for photocatalytic hydrogen evolution originates from the energy gap between the LUMO level of the [Ru(tpyCOO−)2] linker and the hydrogen evolution potential. Because the bimetallic Co/Ni CPs, the Cu–Ru, and the lanthanide CPs maintain the photochemical and electrochemical properties of the [Ru(tpyCOOH)2](PF6)2 complex, they were assessed for photocatalytic hydrogen evolution after being dispersed in aqueous solution. Sonicating Zr–Ru and Hf–Ru in aqueous solution leads to decomposition of these two CPs and thus their photocatalytic activity towards hydrogen evolution was not investigated.Under the irradiation of visible light (λ > 420 nm), the exfoliated Cox–Niy–Ru CP nanosheets effectively produced hydrogen from ascorbic acid solution without the presence of any co-catalysts or co-sensitizers (Fig. 6, S39 and S40‡). A pH range from 3 to 5 was tested and pH = 4 was found optimal for the photocatalytic performance of these CPs (Fig. S41‡). Among the four 2D bimetallic Co/Ni CPs, Co1–Ni2–Ru achieved the greatest initial reaction rate of 258 ± 8 μmol g−1 h−1, producing the most hydrogen amount of 645 ± 8 μmol g−1 in the 2.5 h reaction period (Fig. 6). More Ni content in the cases of Co1–Ni4–Ru and Co1–Ni6–Ru than Co1–Ni2–Ru significantly suppressed the photocatalytic hydrogen evolution activity, and the initial hydrogen evolution rates for Co1–Ni4–Ru and Co1–Ni6–Ru are merely 63 ± 13 and 60 ± 31 μmol g−1 h−1.
Based on our previous investigation,50 we propose that the photocatalytic hydrogen evolution by Cox–Niy–Ru nanosheets undergoes a reductive quenching pathway, where the excited Ru linker is first reduced by the sacrificial donor and then transfers one electron to the Co/Ni node. The driving force for the photocatalytic reaction is estimated to be about 0.7 eV at pH = 4.0, according to the redox potentials of Co/Ni CPs (Fig. S43‡). The critical step of the catalytic cycle is believed as proton association with the reduced and unsaturated Ni or Co node. Even though the previous findings concluded the more catalytically efficient Ni node at the edge of nanosheets than the edge Co node, a too large portion of Ni ions present in the bimetallic Co/Ni CPs might create a significant number of defective sites and thus hinder the electron transfer within the bimetallic CP nanosheets. In addition, these defective sites are detrimental to the completeness of CP crystalline and reduce the amount of active catalytic nodes at the edge of nanosheets.
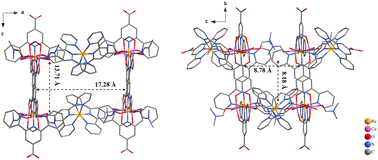
The Cu–Ru coordination polymer, after being dispersed by ultrasonic treatment, is also able to catalyze visible-light driven hydrogen evolution in the pH = 4 ascorbic acid solution with a modest reaction rate of 151 ± 19 μmol g−1 h−1 (Fig. S42‡). In contrast, the lanthanide CP Eu–Ru, Tb–Ru or Yb–Ru does not display photocatalytic activity under the same light-driven hydrogen evolution conditions described above. The type of metal nodes rather than the topologies of CPs is ascribed to the photocatalytic disparity.
Conclusions
In the present work, we synthesized a family of ten coordination polymers composed of Ru(II) linker [Ru(tpyCOO−)2] and different metal cations, including the mixed bimetallic Co/Ni ion nodes. Single-crystal X-ray diffraction analysis revealed topological diversity of these porous coordination polymers depending on the type of metal ions. The series of CPs provide a promising platform for the development of multifarious functionalities based on the photochemical properties of the polypyridyl Ru linker. The obtained CPs were screened as catalysts for the photocatalytic hydrogen evolution reaction. Both the 2D bimetallic Co/Ni CP (Cox–Niy–Ru) and 3D copper CP (Cu–Ru) showed notable catalytic performance without the addition of co-catalysts or co-sensitizers. Moreover, the 3D yttrium CP (Yb–Ru) exhibited characteristic near-infrared emission upon excitation of the ruthenium complex linker. These results elaborately demonstrate how various structures and functions of CPs derive from the interplay of their organic and inorganic components. Design and syntheses of CPs based on novel bis-terpyridine ruthenium complex derivatives are now in progress, aiming at further development of multifunctional materials.Author contributions
L. Tong and Z. Qiao conceived the concept and designed the experiments. L. Tong supervised this study. A. Wu, Y. Tang, and X. Li prepared the samples, conducted the characterization, and assessed the catalytic activity. B. Zhang carried out the photochemical investigation and processed the data. A. Zhou resolved the single crystal X-ray diffraction data and determined the CP structures. All authors discussed the results, contributed to the manuscript writing, and approved its final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by Guangzhou University under the funding number of 202201020230, and the State Key Laboratory of Fine Chemicals, Dalian University of Technology (KF2014). We also gratefully thank the National Natural Science Foundation of China (21978058 and 21676094), the Pearl River Talent Recruitment Program (2019QN01L255) and the R & D Program of the Joint Institute of GZHU and ICoST (GI202102) for financial support.Notes and references
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh and J. Reedijk, CrystEngComm, 2012, 14, 3001–3004 RSC
.
-
S. R. Batten, S. M. Neville and D. R. Turner, Coordination Polymers: Design, Analysis and Application, The Royal Society of Chemistry, 2009 Search PubMed
.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC
.
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. Paik Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CrossRef CAS
.
- U. Ryu, S. Jee, P. C. Rao, J. Shin, C. Ko, M. Yoon, K. S. Park and K. M. Choi, Coord. Chem. Rev., 2021, 426, 213544 CrossRef CAS PubMed
.
- A. Kirchon, L. Feng, H. F. Drake, E. A. Joseph and H. C. Zhou, Chem. Soc. Rev., 2018, 47, 8611–8638 RSC
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed
.
- Y. B. Huang, J. Liang, X. S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC
.
- J. D. Xiao and H. L. Jiang, Acc. Chem. Res., 2019, 52, 356–366 CrossRef CAS
.
- Y. Cui, B. Li, H. He, W. Zhou, B. Chen and G. Qian, Acc. Chem. Res., 2016, 49, 483–493 CrossRef CAS PubMed
.
- S. Fu, S. Yao, S. Guo, G. C. Guo, W. Yuan, T. B. Lu and Z. M. Zhang, J. Am. Chem. Soc., 2021, 143, 20792–20801 CrossRef CAS PubMed
.
- S. N. Sun, L. Z. Dong, J. R. Li, J. W. Shi, J. Liu, Y. R. Wang, Q. Huang and Y. Q. Lan, Angew. Chem., Int. Ed., 2022, 61, e202207282 CAS
.
- S. J. Yao, N. Li, J. Liu, L. Z. Dong, J. J. Liu, Z. F. Xin, D. S. Li, S. L. Li and Y. Q. Lan, Inorg. Chem., 2022, 61, 2167–2173 CrossRef CAS
.
- J. Zhang, Y. Wang, H. Wang, D. Zhong and T. Lu, Chin. Chem. Lett., 2022, 33, 2065–2068 CrossRef CAS
.
- T. Lu, T. Li, D. Shi, J. Sun, H. Pang, L. Xu, J. Yang and Y. Tang, SmartMat, 2021, 2, 591–602 CrossRef CAS
.
- Z.-Y. Gu, J. Park, A. Raiff, Z. Wei and H.-C. Zhou, ChemCatChem, 2014, 6, 67–75 CrossRef CAS
.
- Y. Guo, Y. Wang, Y. Shen, Z. Cai, Z. Li, J. Liu, J. Chen, C. Xiao, H. Liu, W. Lin and C. Wang, J. Am. Chem. Soc., 2020, 142, 21493–21501 CrossRef CAS PubMed
.
- S. Li, Y. Gao, N. Li, L. Ge, X. Bu and P. Feng, Energy Environ. Sci., 2021, 14, 1897–1927 RSC
.
- X. Y. Zhang, C. F. Xie, S. Q. Wang, X. M. Cheng, Y. Zhang, Y. Zhao, Y. Lu and W. Y. Sun, Inorg. Chem., 2022, 61, 1590–1596 CrossRef CAS PubMed
.
- N.-Y. Huang, J.-Q. Shen, X.-W. Zhang, P.-Q. Liao, J.-P. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2022, 144, 8676–8682 CrossRef CAS PubMed
.
- J. Zhang, D. Zhong and T. Lu, Acta Phys.-Chim. Sin., 2021, 37, 2008068 Search PubMed
.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Angew. Chem., Int. Ed., 2016, 55, 5414–5445 CrossRef CAS PubMed
.
- H. Wang, Q.-L. Zhu, R. Zou and Q. Xu, Chem, 2017, 2, 52–80 CAS
.
- X. Feng, Y. Pi, Y. Song, C. Brzezinski, Z. Xu, Z. Li and W. Lin, J. Am. Chem. Soc., 2020, 142, 690–695 CrossRef CAS
.
- H. An, H. Luo, T. Xu, S. Chang, Y. Chen, Q. Zhu, Y. Huang, H. Tan and Y. G. Li, Inorg. Chem., 2022, 61, 10442–10453 CrossRef CAS PubMed
.
- Y. N. Gong, J. W. Liu, J. H. Mei, X. L. Lin, J. H. Deng, X. Li, D. C. Zhong and T. B. Lu, Inorg. Chem., 2021, 60, 14924–14931 CrossRef CAS PubMed
.
- J. H. Zhang, Y. N. Gong, H. J. Wang, Y. C. Wang, W. Yang, J. H. Mei, D. C. Zhong and T. B. Lu, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2118278119 CrossRef CAS PubMed
.
- H. Q. Yin and X. B. Yin, Acc. Chem. Res., 2020, 53, 485–495 CrossRef CAS PubMed
.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS
.
- T. N. Nguyen, F. M. Ebrahim and K. C. Stylianou, Coord. Chem. Rev., 2018, 377, 259–306 CrossRef CAS
.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC
.
- T. Rasheed and F. Nabeel, Coord. Chem. Rev., 2019, 401, 213065 CrossRef CAS
.
- E. A. Dolgopolova, A. M. Rice, C. R. Martin and N. B. Shustova, Chem. Soc. Rev., 2018, 47, 4710–4728 RSC
.
- Z. Jia, Z. Han, K. Wang, T. Zhou, H. Min, T. Sun, Y. Liao, L. Wang, P. Cheng and W. Shi, Inorg. Chem., 2022, 61, 14313–14321 CrossRef CAS PubMed
.
- C. W. Kung, S. Goswami, I. Hod, T. C. Wang, J. Duan, O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2020, 53, 1187–1195 CrossRef CAS PubMed
.
- D. Ren, H.-L. Xia, K. Zhou, S. Wu, X.-Y. Liu, X. Wang and J. Li, Angew. Chem., Int. Ed., 2021, 60, 25048–25054 CrossRef CAS
.
- C. Fiankor, J. Nyakuchena, R. S. H. Khoo, X. Zhang, Y. Hu, S. Yang, J. Huang and J. Zhang, J. Am. Chem. Soc., 2021, 143, 20411–20418 CrossRef CAS PubMed
.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS
.
- C. Wang, Z. Xie, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445–13454 CrossRef CAS PubMed
.
- T. C. Zhuo, Y. Song, G. L. Zhuang, L. P. Chang, S. Yao, W. Zhang, Y. Wang, P. Wang, W. Lin, T. B. Lu and Z. M. Zhang, J. Am. Chem. Soc., 2021, 143, 6114–6122 CrossRef CAS PubMed
.
- G. Lan, Z. Li, S. S. Veroneau, Y. Y. Zhu, Z. Xu, C. Wang and W. Lin, J. Am. Chem. Soc., 2018, 140, 12369–12373 CrossRef CAS
.
- D. Kim, D. R. Whang and S. Y. Park, J. Am. Chem. Soc., 2016, 138, 8698–8701 CrossRef CAS PubMed
.
- Z. H. Yan, M. H. Du, J. Liu, S. Jin, C. Wang, G. L. Zhuang, X. J. Kong, L. S. Long and L. S. Zheng, Nat. Commun., 2018, 9, 3353 CrossRef PubMed
.
- M. Elcheikh Mahmoud, H. Audi, A. Assoud, T. H. Ghaddar and M. Hmadeh, J. Am. Chem. Soc., 2019, 141, 7115–7121 CrossRef CAS PubMed
.
- T. Toyao, M. Saito, S. Dohshi, K. Mochizuki, M. Iwata, H. Higashimura, Y. Horiuchi and M. Matsuoka, Chem. Commun., 2014, 50, 6779–6781 RSC
.
- S. Zhang, L. Li, S. Zhao, Z. Sun and J. Luo, Inorg. Chem., 2015, 54, 8375–8379 CrossRef CAS PubMed
.
- A. Kobayashi, Y. Suzuki, T. Ohba, T. Ogawa, T. Matsumoto, S. Noro, H. C. Chang and M. Kato, Inorg. Chem., 2015, 54, 2522–2535 CrossRef CAS PubMed
.
- D. Luo, T. Zuo, J. Zheng, Z.-H. Long, X.-Z. Wang, Y.-L. Huang, X.-P. Zhou and D. Li, Mater. Chem. Front., 2021, 5, 2777–2782 RSC
.
- L. Martins, L. K. Macreadie, D. Sensharma, S. Vaesen, X. Zhang, J. J. Gough, M. O'Doherty, N. Y. Zhu, M. Ruther, J. E. O'Brien, A. L. Bradley and W. Schmitt, Chem. Commun., 2019, 55, 5013–5016 RSC
.
- D. Huo, F. Lin, S. Chen, Y. Ni, R. Wang, H. Chen, L. Duan, Y. Ji, A. Zhou and L. Tong, Inorg. Chem., 2020, 59, 2379–2386 CrossRef CAS
.
- J. Hao, X. Xu, H. Fei, L. Li and B. Yan, Adv. Mater., 2018, 30, e1705634 CrossRef PubMed
.
- H. Zhang, J. Nai, L. Yu and X. W. Lou, Joule, 2017, 1, 77–107 CrossRef CAS
.
- X. Guo, X. Wan, Q. Liu, Y. Li, W. Li and J. Shui, eScience, 2022, 2, 304–310 CrossRef
.
- P. Deria, J. E. Mondloch, O. Karagiaridi, W. Bury, J. T. Hupp and O. K. Farha, Chem. Soc. Rev., 2014, 43, 5896–5912 RSC
.
- S. M. Cohen, J. Am. Chem. Soc., 2017, 139, 2855–2863 CrossRef CAS
.
- W. Li, W. Fang, C. Wu, K. N. Dinh, H. Ren, L. Zhao, C. Liu and Q. Yan, J. Mater. Chem. A, 2020, 8, 3658–3666 RSC
.
- Q. Wang, J. Lu, Y. Jiang, S. Yang, Y. Yang and Z. Wang, Chem. Eng. J., 2022, 443, 136483 CrossRef CAS
.
- S. Rezaee and S. Shahrokhian, Appl. Catal., B, 2019, 244, 802–813 CrossRef CAS
.
- S. V. Eliseeva and J. C. Bunzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC
.
- L.-J. Xu, G.-T. Xu and Z.-N. Chen, Coord. Chem. Rev., 2014, 273–274, 47–62 CrossRef CAS
.
- D. Guo, C. Y. Duan, F. Lu, Y. Hasegawa, Q. J. Meng and S. Yanagida, Chem. Commun., 2004, 1486–1487, 10.1039/b403519d
.
- M. D. Ward, Coord. Chem. Rev., 2007, 251, 1663–1677 CrossRef CAS
.
- M. C. So, G. P. Wiederrecht, J. E. Mondloch, J. T. Hupp and O. K. Farha, Chem. Commun., 2015, 51, 3501–3510 RSC
.
Footnotes |
| † This paper is dedicated to Prof. Licheng Sun on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2009804, 2024242, 2024244, 2024245, 2178964 and 2178965. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ta07219j |
| This journal is © The Royal Society of Chemistry 2022 |