Brownian dynamics of cylindrical capsule-like particles in a nanopore in an electrically biased solid-state membrane
Craig C.
Wells
 ,
Dmitriy V.
Melnikov
,
Dmitriy V.
Melnikov
 and
Maria E.
Gracheva
and
Maria E.
Gracheva
 *
*
Department of Physics, Clarkson University, Potsdam, NY 13699, USA. E-mail: gracheva@clarkson.edu
First published on 25th January 2022
Abstract
We use Brownian dynamics simulations to study the motion of cylindrical capsule-like particles (capsules) as they translocate through nanopores of various radii in an electrically biased silicon membrane. We find that for all pore sizes the electrostatic interaction between the particle and the pore results in the particle localization towards the pore 's center when the membrane and the particle have charges of the same sign (case 1) while in case of the opposite sign charges, the capsule prefers to stay near and along the nanopore wall (case 2). The preferential localization leads to all capsules rotating less while inside the pore compared to the bulk solution, with a larger net charge and/or particle length resulting in a smaller range of rotational movement. It also strongly affects the whole translocation process: in the first case, the translocation is due to the free diffusion along the pore axis and is weakly dependent on the particle charge and the nanopore radius while in the second case, the translocation time dramatically increases with the particle size and charge as the capsule gets “stuck” to the nanopore surface.
1 Introduction
The detection and characterization of biomolecules1–5 has been a growing field of interest with an increase in number of research studies focused on nanopore-based applications and the advancement of biosensing technologies.6–11 This rapid nanopore-based identification technology is frequently made possible by analyzing the ionic current blockade trace produced by a molecule translocating a nanopore embedded in a membrane.12–19 A system such as this works by having the nanoporous membrane separate two chambers filled with electrolyte solution, one where biomolecules initial reside in (the cis chamber), and the chamber they are encouraged to translocate into (the trans chamber) via the nanopore.Nanopores can also be utilized with particles whose shape and orientation affect the ionic current trace in a way that may be more difficult to interpret, such as for a non-globular molecule like a cylinder or capsule. In particular, cylinder-shaped particles have been the focus of several investigations.15,16,20–26 For example, one experimental study15 focused on unique features of the ionic current profile for a rod-shaped, silicon dioxide particle translocating a nanopore with irregularities to distinguish objects of different shapes. The results of the same investigation suggest that the rods will rotate while translocating the nanopore. Another study16 found that nanorods will tumble (a “tumble” refers to the capsule's major axis rotating over itself in the direction of the pore axis) while translocating a glass nanopipet, thus resulting in a current blockade dependent on the rod's angular orientation. In general, however, analysis of ionic current traces for these particles proved to be a challenge due to their unique shape and motion27.
One way to further our understanding of particle transloctaion dynamics is by employing computational modelling. This way, it is possible to investigate fine features of the molecule translocation process that would otherwise be difficult to observe experimentally. There are many ways to approach a nanopore system via modelling, from extremely detailed atomic level models28,29 to simpler models that yield broader statistical information on the system.30–33 Atomic level modelling can be expensive in computational resources and time, while modelling particles using Brownian dynamics (BD) and rigid, coarse-grained structures34 considerably reduces the computational cost and provides a good agreement with atomic level approaches.
In this work, the translocation dynamics of cylindrical capsule-like particles (hereby referred to as capsules) is studied using a BD model. Particles of various lengths and charges are studied to explore how each affects the particle's behavior in the pore. Additionally, different pore sizes and electric biases applied to the membrane are investigated to understand how the electric environment modifies the particle's movement.35,36
This work is organized as follows: The particle, system, and the computational model are described in Section 2. In Section 3, the results of our simulations are presented and discussed. Our findings are then summarized in Section 4.
2 Method
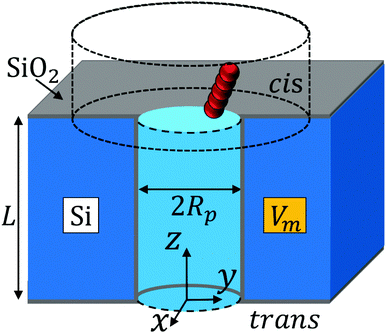
We consider a semiconductor membrane made of silicon (Si) with an 8 Å thick negatively charged surface layer of silicon dioxide (SiO2) with volume charge density of −4 × 1020e cm−3 (corresponding to a surface charge density of −0.32 e nm−2), where e is the elementary charge. An aqueous KCl electrolyte solution of bulk concentration CKCl = 0.1 M fills a cis and trans chambers separated by the L = 260 Å thick membrane and connected by a cylindrical nanopore of radius Rp = 40 Å to 60 Å as shown in Fig. 1. A capsule is initially placed in the cis chamber, requiring it to translocate the nanopore to move into the trans chamber. An electric bias of Vm = ±1 V is applied to Si, resulting in a positive or negative overall effective surface charge, respectively.37,38 The system's electric potential, ϕ(r), is numerically calculated using the Poisson–Nernst–Planck (PNP) approach, as described in our previous works.35,39 The dependence of the electric potential and electric field in and around the nanopore on the applied membrane bias and membrane surface charge are discussed in greater detail elsewhere.37,38In this work, we approximate a capsule of various length and charge through a coarse-grained method developed earlier36,38 and illustrated in Fig. 2. A capsule particle is comprised of N overlapping spheres (N ranges from 3 to 11) fixed in relative positions as a rigid body where each sphere has a radius Rb = 5 Å and a point charge qi = 0, −1e, −2e concentrated at each sphere's center. The ratio between the particle's length Lc and width 2Rb, 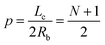 , can be used to define the perpendicular/parallel translational and the rotational drag coefficients relative to the capsule's major axis as:40,41
, can be used to define the perpendicular/parallel translational and the rotational drag coefficients relative to the capsule's major axis as:40,41
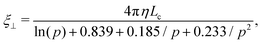 | (1) |
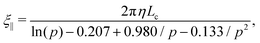 | (2) |
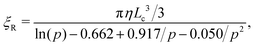 | (3) |
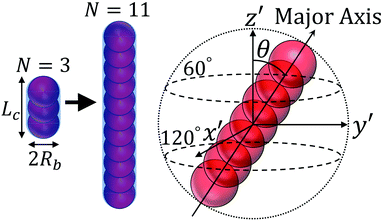 | ||
| Fig. 2 The simulated capsule and its center of mass reference frame (x′, y′, z′). Capsule orientation angle θ is the angle between the +z′-direction and the capsule's major axis. | ||
The movement of the capsule in the nanoporous system is simulated using BD approach, discussed fully in our previous studies36,42 and is similar to the method developed in ref. 41. The translational motion of the particle's center of mass position rcm(t)36 in the directions parallel and perpendicular to the capsule's major axis can be determined as
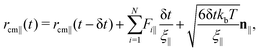 | (4a) |
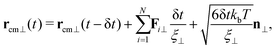 | (4b) |
Due to the capsule shape of the particle, rotational Brownian motion must be considered to accurately model its change in orientation. This change is effectively described by the angle δΩ by which particle's major axis turns and which in the center of mass (x′, y′, z′) coordinate system can be computed as36:
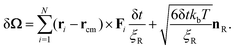 | (5) |
3 Results and discussion
3.1 Capsule's position and orientation inside the nanopore
Using the above BD approach, we study how membrane potentials of Vm = ±1 and nanopores with radii of Rp = 40 Å, 44 Å, 48 Å, 52 Å, 56 Å, and 60 Å affect the dynamics of capsules constructed of N = 3, 5, 7, 9, and 11 beads where all beads have the same radius of Rb = 5 Å (capsule length Lc = 20 Å, 30 Å, 40 Å, 50 Å, and 60 Å) and the same bead charge of qi = 0, −1e or −2e. A single simulation starts with the particle placed at a random position and orientation inside the bounding box in the cis chamber and ends when the particle translocates into the trans chamber via the nanopore. This way, we are able to determine the capsule's dynamics in the nanopore (0 < z < L) including how frequently it rotates during the translocation process. About 1600 translocation simulations for each set were executed, ranging between approximately 800 to 6000 CPU hours of computational time for all simulations for each case (due to the total translocation time dependency on the simulation conditions). An example of a single simulation showing the capsule's center of mass trajectory while inside the nanopore is shown in Fig. 3. We can see that in this particular case the particle tends to move along the wall. This trace shows that the particle is most likely to be located near the membrane surface due to the electrostatic attraction between the negatively charged particle and nanopore with effectively positive charge.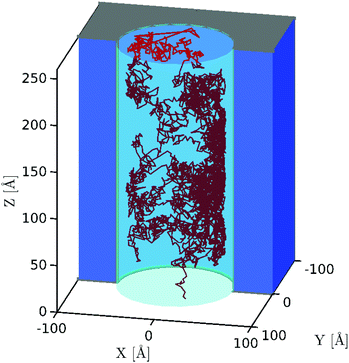 | ||
| Fig. 3 Capsule center of mass motion over the course of a single simulation (qi = −2e, Vm = 1 V, N = 5, Rp = 60 Å) while the particle is inside of the pore. | ||
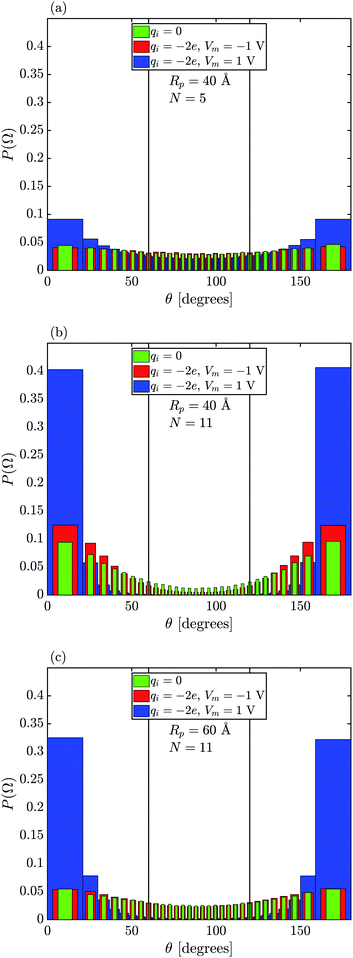
Fig. 4 shows how a capsule's orientation in the pore (defined through the angle θ between the particle major axis and nanopore axis, see Fig. 2) is influenced by particle size and charge, pore size, and membrane bias. Regardless of the particle charge and/or membrane bias, the capsule is discouraged from rotating away from the pore axis when the particle's length increases, as demonstrated by the increase of the probability P(Ω) of the capsule's angle θ at θ → 0°(180°) when comparing N = 5 (Fig. 4(a)) to N = 11 (Fig. 4(b and c)) because of the repulsion from the membrane surface preventing longer particles from exhibiting a horizontal orientation (in xy-plane). Also, for all cases considered in Fig. 4, the capsule's major axis is most often oriented along the pore axis when qi = −2e and Vm = 1 V (a similar but less prominent trend also emerges when qi = −1e and Vm = 1 V, not shown). This is due to the particle's attraction toward the membrane surface inside the pore, which in turn restricts its ability to rotate near the wall (and therefore its propensity to tumble, see Section 3.3).
When the capsule length is small compared to the pore radius (Lc < Rp), the particle's probability P(Ω) is weakly dependent on θ for Vm = −1 V regardless of its charge, also seen in Fig. 4(a and c). This is because in this case the particles are discouraged from being close to the wall (due to the LJ and repulsive electrostatic interactions for qi ≠ 0) and there is enough space inside the pore for the capsule to rotate. This is not so for more narrow pores, as seen in Fig. 4(b) when Rp = 40 Å and N = 11 (Lc = 60 Å). When the capsules are more restricted in their rotational movement, the neutral particle with qi = 0 is less likely to be oriented along the pore axis compared to the negatively charged particle subject to negative membrane bias because in this case the effective pore radius is smaller so that it becomes more difficult for the particle to rotate and the probability P(Ω) of finding the particle oriented along the pore axis (θ → 0° or 180°) increases.
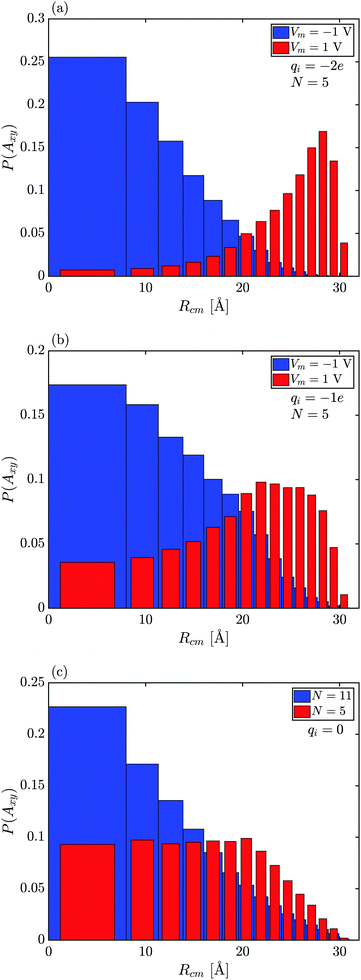
To understand where capsule's are positioned in the nanopore, histograms for the probability of the capsule's radial center of mass location in the pore (Rcm) for annuli of equal area in the xy-plane, P(Axy), are computed (Fig. 5). The radial position of the particle while in the pore for qi = −2e, Vm = 1 V, N = 5, Rp = 40 Å, Fig. 5(a), reveals that the capsule's center of mass has the highest probability of being located toward the membrane surface. When qi = − 2e for Vm = −1 V (Fig. 5(a)), the P(Axy) is large near the center of pore, as the capsule is repelled from the membrane surface. This trend is less obvious for qi = −1e (Fig. 5(b)), where the particle's peak probability is still located close to the membrane wall but the maximum P(Axy) is halved compared to the qi = −2e case, and the particle spends significantly more time near the center of the pore. This is because the attractive electric interaction in this case is not strong enough to overcome the stochastic effects and repulsive particle–membrane LJ interaction, and therefore, a particle will have a non-negligible probability of being found towards the center of the pore.
For a small neutral particle that can more freely rotate inside the nanopore (N = 5 in Fig. 5(c)), P(Axy) remains nearly constant until the particle is close enough to the membrane wall, and P(Axy) → 0. In Fig. 5(c), it is also seen for a long neutral particle (N = 11) that P(Axy) is larger for smaller Rcm, as a longer particle will have a tendency to orient itself along the pore axis (see Fig. 4(b)) with the capsule's center of mass near the pore center.
3.2 Translocation dynamics
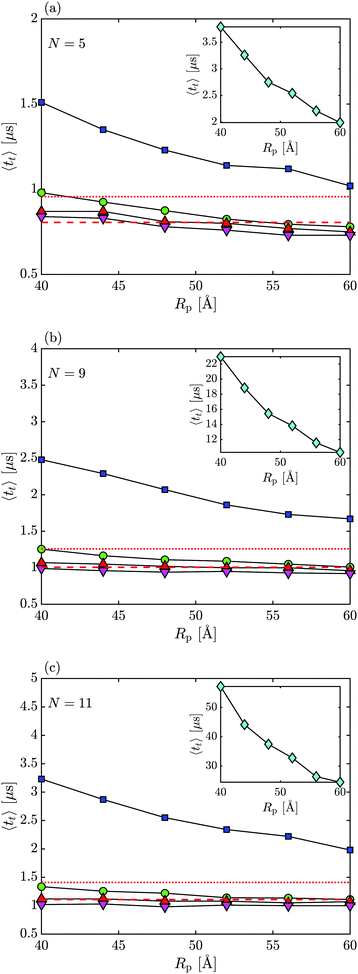
Next, we consider how capsule's translocation time, tt, depends on its length and charge. We define translocation time as the time it takes for a capsule to move from the cis chamber into the trans chamber via the nanopore in one successful attempt (without returning back to the cis chamber). The average translocation times 〈tt〉 vs. pore radius Rp for select capsule sizes (N = 5, N = 9, and N = 11) are shown in Fig. 6. For all cases observed, smaller capsule sizes and increased pore radius result in a shorter 〈tt〉. This is due to the shorter capsules having a smaller drag coefficient, reduced LJ particle–wall interactions, and a reduced electrostatic interaction with the wall compared to larger capsules in a more confined space of nanopores with smaller radius.When the capsule and membrane are both negatively charged (or the capsule is neutral), translocation times are the lowest with similar values (∼1 μs) which do not increase significantly with the bead's number N. This result is due to capsules being repelled from the pore walls, producing quick translocations but with many failed translocation attempts. The fact that 〈tt〉 for negatively charged particles and Vm < 0 is very close to the values for the neutral particle suggests that the particles are driven by free diffusion along the pore's axis. In this case, 〈tt〉 ≈ L2ξ‖/6kbT (shown by a horizontal dashed line in Fig. 6) for most capsule sizes independent of the pore radius, and consistent with results for the mean first passage times45.
On the other hand, when the applied membrane bias is positive, Vm > 0, the particle's motion is inhibited so that the translocation times are longer, and there are fewer failed attempts to translocate from the cis into the trans chamber since the particle is attracted to the pore surface, making it less likely for the capsule to escape from either end of the pore once inside. As can be observed from Fig. 6, in this case the average translocation time changes linearly with the particle's net particle charge, 〈tt〉 ∝ Nqi when qi = −1e and 〈tt〉 ∝ exp(Nqi) for qi = −2e resulting in a dramatic increase in translocation time values (∼40 μs for qi = −2e vs. ∼2.5 μs for qi = −1e, N = 11, for example). The escalation of the average translocation time values can be attributed to the capsule's electrostatic interaction with the membrane being the dominating factor in its motion through the pore (corresponding to the biased diffusion case45), especially for longer capsules and smaller pores since in this case the capsule is forced to be oriented along the pore axis more often which encourages strong capsule–pore interactions (the particles effectively “sticks” to the wall along the entire length of the particle). Note also a strong dependence of the translocation time on the pore radius: As Rp decreases, the translocation time increases which is consistent with the particle–pore interaction becoming stronger with decreasing curvature of the pore.
3.3 Tumbling dynamics
Next, we focus on how the membrane bias, the pore radius, and size/charge of the capsule affect the particle's tumbling dynamics as it translocates through the nanopore. Using the orientation angle θ between the capsule's major axis and the pore's axis (see Fig. 2 and Section 3.1), a single “tumble” is defined by the capsule's major axis rotating from an upper threshold (θup = 60°) to a lower threshold (θdown = 120°) orientation or vice versa. These values of θup(down) were chosen since for a freely moving particle (subjected only to a stochastic force), the expectation value, 〈θ〉, for 0° < θ < 90° is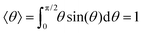 rad ≈60° (
rad ≈60° (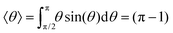 rads ≈ 120° when 90° < θ < 180°).
rads ≈ 120° when 90° < θ < 180°).
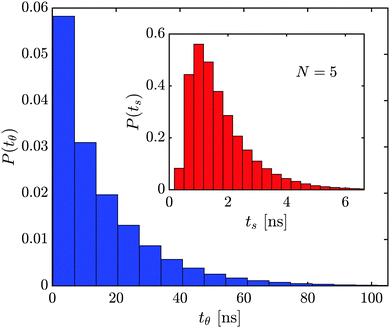
The average time required for the capsule to rotate from θup to θdown successfully in a single attempt, 〈ts〉, is primarily determined by the free rotational diffusion when 〈ts〉 ≈ ξR(θup − θdown)2/6kbT. Since longer capsules have a larger value of ξR, 〈ts〉 increases from 0.8 ns for N = 3 to 7.0 ns for N = 11 (see inset in Fig. 7 for N = 5, histograms for other N are similar). Despite capsules with the same N but different charges and in different electrostatic environments having similar 〈ts〉 values, the frequency of tumbling inside the nanopore exhibited by these particles can differ greatly. Therefore, an average time for a particle to tumble that includes the particle's failed tumbling attempts, referred to as 〈tθ〉, is much greater than ts, as seen in Fig. 7, and is considerably more dependent on the system's parameters.
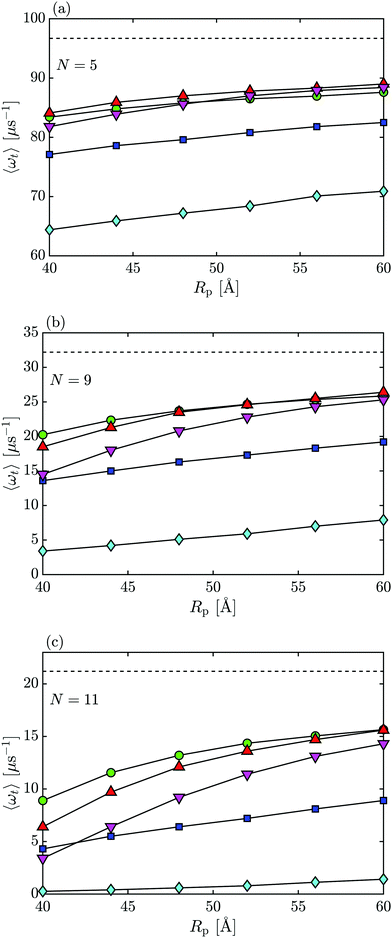
To establish how the capsule's tumbling dynamics is influenced by nanopore system properties, we investigate the average tumbling rate, i.e., the number of tumbles per μs, 〈ωt〉 = 1/〈tθ〉, for each set of parameters. For all cases studied, the 〈ωt〉 of the capsule in the bulk is higher compared to 〈ωt〉 inside of the nanopore (see Fig. 8 where the dashed line is for the bulk). We can also see in Fig. 8 that the shorter the capsule (smaller N), the higher the 〈ωt〉, due to the smaller rotational drag and less number of attempts needed to complete a tumble. Additionally, a more narrow pore exhibit smaller 〈ωt〉, revealing how a more confined space reduces the ability for the capsule to tumble.
For most cases studied, particularly those with larger N, the tumbling rate 〈ωt〉 for the neutral particle was higher than for capsules with qi = −1e, Vm = 1 V, which in turn had higher 〈ωt〉's than for qi = −2e, Vm = 1 V cases. The cases with qi = −2e exhibit a particular sharp drop in tumbling rates from ∼65 μs−1 for N = 5 to ∼0.25 μs−1 for N = 11. As discussed earlier, the capsule's “sticking” to the membrane wall discourages rotations (see Fig. 4 and 5), which is why long capsules with charge qi = −2e tumble the least.
On the other hand, the effects that N, Rp, and qi have on the 〈ωt〉 for a negatively biased membrane are not as straightforward. For small N (N = 5, Fig. 8(a)), the rate of tumbling for negatively charged particles in a Vm = −1 V pore is about the same (it is even slightly higher for N = 3, not shown) as 〈ωt〉 for a neutral capsule. A trend begins to emerge when the capsule becomes longer (N = 9, Fig. 8(b)) and pore radius decreases (Rp = 40 Å) in which case, qi = −2e particles have a smaller 〈ωt〉 than capsules with qi = −1e as well as the neutral particle case. As N increases further, this difference in tumbling rates extends to larger pore radii, see Fig. 8(c) for N = 11.
When Vm = −1 V, the particle is repelled from the membrane surface via the electrostatic repulsion as well as the particle–membrane LJ interactions. For smaller capsules, the repulsion has little effect on the tumbling rate because it can easily rotate completely regardless of its position in the nanopore. When N increases, the particle's ability to freely rotate becomes inhibited as the effective pore radius for the negatively charged particles subject to a negative membrane bias is much smaller than for the neutral particle. As a result, it becomes more difficult for the capsule to have angle θ to change by a large amount from 〈θup〉 to 〈θdown〉 to ensure a full rotation and hence, the tumbling rate decreases.
By combining the data presented in Fig. 6 and 7, we also note that despite the fact the lowest tumbling rates occur for the opposite effective charges on the particle and the membrane (when qi < 0, and Vm = 1 V), the total average number of rotations during a translocation time is larger in this case than when Vm = −1 V (∼10 vs. 4 for N = 11, qi = −2e, Vm = 1 V and Vm = −1 V). This is due to an increase in time it takes the capsule to go through the pore with the particle spending most of the time being “stuck” next to the membrane surface and only occasionally managing to rotate.
4 Concluding remarks
In this work, we studied the interactions that affect cylindrical capsule-like particles orientation and motion through a solid-state nanopore. The movement of rigid, rod-like structures comprised of overlapping spherical beads is modeled using a BD method alongside a PNP approach. We investigated how the applied membrane bias, pore radius, and particle size/charge relate to capsule location and orientation while translocating the nanopore. The purpose of considering these parameters and the resulting particle dynamics were to reveal how they contribute to the particle's propensity to tumble inside the nanopore.We find that the parameter that influences the tumbling frequency the most is the particle's size, with shorter capsules rotating more frequently than longer capsules. However, other factors such as pore width also play a role, resulting in smaller tumbling rates due to the space confinement. We also find that neutral capsules tend to not favor any particular orientations if they are short but will mostly align itself along the pore axis if they are long compared to the nanopore radius.
The particle–membrane LJ interactions influence charged capsules in a similar way to neutral capsules, but the electric force changes the particle dynamics in those cases. For negative particles subjected to a positive membrane bias, the attraction toward the wall results in the capsule typically orienting itself along the pore axis. These trends are emphasized for longer and more negatively charged particles resulting in the decrease of the particle's rate of tumbling while greatly increasing the time it takes for it to translocate the nanopore.
The dynamics of negatively charged particles subjected to a negative membrane bias is not as intuitive. For short capsules/wide pores, such particles have the largest tumbling frequencies, close to bulk values (∼10% lower). When the capsule is longer (or the pore is more narrow), the rate of tumbling decreases. Furthermore, the capsules with a greater negative charge tumble less as the electrostatic interaction focuses particle motion along the pore's axis precluding its frequent rotations. This focusing also affects the translocation time, resulting in it being governed by free diffusion independent of the capsule's length and charge.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSF through a CAREER award No. 1352218 (M. G.). We would like to acknowledge the use of Orion cluster at Clarkson University.References
- M. Firnkes, D. Pedone, J. Knezevic, M. Doblinger and U. Rant, Nano Lett., 2010, 10, 2162 CrossRef CAS PubMed.
- L.-Q. Gu and J. W. Shim, Analyst, 2010, 135, 441 RSC.
- A. de la Escosura-Muñiz and A. Merkoçi, ACS Nano, 2012, 6, 7556 CrossRef PubMed.
- D. H. Stoloff and M. Wanunu, Curr. Opin. Biotechnol., 2013, 24, 699 CrossRef CAS PubMed.
- Y. Feng, Y. Zhang, C. Ying, D. Wang and C. Du, Genom., Proteom. Bioinform., 2015, 13, 4 CrossRef CAS PubMed.
- D. Fologea, B. Ledden, D. S. McNabb and J. Li, Appl. Phys. Lett., 2007, 91, 053901 CrossRef PubMed.
- L. Movileanu, Trends Biotechnol., 2009, 27, 333 CrossRef CAS PubMed.
- P.-H. Lee, V. Helms and T. Geyer, J. Chem. Phys., 2012, 137, 145105 CrossRef PubMed.
- M. Muthukumar, J. Chem. Phys., 2014, 141, 081104 CrossRef CAS PubMed.
- J. Larkin, R. Y. Henley, M. Muthukumar, J. K. Rosenstein and M. Wanunu, Biophys. J., 2014, 106, 696 CrossRef CAS PubMed.
- H. Arjmandi-Tash, L. A. Belyaeva and G. F. Schneider, Chem. Soc. Rev., 2016, 45, 476 RSC.
- J. J. Kasianowicz, E. Brandin, D. Branton and D. W. Deamer, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 13770 CrossRef CAS PubMed.
- R. M. Smeets, U. F. Keyser, D. Krapf, M.-Y. Wu, N. H. Dekker and C. Dekker, Nano Lett, 2006, 6, 89 CrossRef CAS PubMed.
- M. Wanunu, J. Sutin, B. McNally, A. Chow and A. Meller, Biophys. J., 2008, 95, 4716 CrossRef CAS PubMed.
- Y. Qiu, P. Hinkle, C. Yang, H. E. Bakker, M. Schiel, H. Wang, D. Melnikov, M. Gracheva, M. E. Toimil-Molares and A. Imhof, et al. , ACS Nano, 2015, 9, 4390 CrossRef CAS PubMed.
- Y. Zhang, M. A. Edwards, S. R. German and H. S. White, J. Phys. Chem. C, 2016, 120, 20781 CrossRef CAS.
- S. Howorka and Z. Siwy, Chem. Soc. Rev., 2009, 38, 2360 RSC.
- D. S. Talaga and J. Li, J. Am. Chem. Soc., 2009, 131, 9287 CrossRef CAS PubMed.
- C. Plesa, S. W. Kowalczyk, R. Zinsmeester, A. Y. Grosberg, Y. Rabin and C. Dekker, Nano Lett, 2013, 13, 658 CrossRef CAS PubMed.
- Z. Hu, M. D. Fischbein, C. Querner and M. Drndić, Nano Lett, 2006, 6, 2585 CrossRef CAS PubMed.
- M. Ali, R. Neumann and W. Ensinger, ACS Nano, 2010, 4, 7267 CrossRef CAS PubMed.
- Y. Ai and S. Qian, Phys. Chem. Chem. Phys., 2011, 13, 4060 RSC.
- H. Wu, Y. Chen, Q. Zhou, R. Wang, B. Xia, D. Ma, K. Luo and Q. Liu, Anal. Chem., 2016, 88, 2502 CrossRef CAS PubMed.
- A. J. McMullen, J. X. Tang and D. Stein, ACS Nano, 2017, 11, 11669 CrossRef CAS PubMed.
- J. Sha, W. Si, B. Xu, S. Zhang, K. Li, K. Lin, H. Shi and Y. Chen, Anal. Chem., 2018, 90, 13826 CrossRef CAS PubMed.
- R. Maugi, P. Hauer, J. Bowen, E. Ashman, E. Hunsicker and M. Platt, Nanoscale, 2020, 12, 262 RSC.
- K. E. Venta, M. B. Zanjani, X. Ye, G. Danda, C. B. Murray, J. R. Lukes and M. Drndic, Nano Lett, 2014, 14, 5358 CrossRef CAS PubMed.
- S. K. Kannam, S. C. Kim, P. R. Rogers, N. Gunn, J. Wagner, S. Harrer and M. T. Downton, Nanotechnology, 2014, 25, 155502 CrossRef PubMed.
- W. Si and A. Aksimentiev, ACS Nano, 2017, 11, 7091 CrossRef CAS PubMed.
- A. Ammenti, F. Cecconi, U. Marini Bettolo Marconi and A. Vulpiani, J. Phys. Chem. B, 2009, 113, 10348 CrossRef CAS PubMed.
- F. Cecconi, M. Bacci and M. Chinappi, Protein Pept. Lett., 2014, 21, 227 CrossRef CAS PubMed.
- I. Jou and M. Muthukumar, Biophys. J., 2017, 113, 1664 CrossRef CAS PubMed.
- Z. Liu, X. Shi and H. Wu, Nanotechnology, 2019, 30, 165701 CrossRef CAS PubMed.
- X. Sun, T. Lin and J. D. Gezelter, J. Chem. Phys., 2008, 128, 234107 CrossRef PubMed.
- A. Nadtochiy, D. Melnikov and M. Gracheva, ACS Nano, 2013, 7, 7053 CrossRef CAS PubMed.
- I. A. Jou, D. V. Melnikov and M. E. Gracheva, Nanotechnology, 2016, 27, 205201 CrossRef PubMed.
- M. E. Gracheva, J. Vidal and J.-P. Leburton, Nano Lett, 2007, 7, 1717 CrossRef CAS PubMed.
- C. C. Wells, D. V. Melnikov and M. E. Gracheva, J. Chem. Phys., 2019, 150, 115103 CrossRef PubMed.
- A. Nikolaev and M. E. Gracheva, Nanotechnology, 2011, 22, 165202 CrossRef PubMed.
- M. M. Tirado, C. L. Martnez and J. G. de la Torre, J. Chem. Phys., 1984, 81, 2047 CrossRef.
- H. Löwen, Physical Review E, 1994, 50, 1232 CrossRef PubMed.
- C. C. Wells, D. V. Melnikov, J. T. Cirillo and M. E. Gracheva, Physical Review E, 2020, 102, 063104 CrossRef CAS PubMed.
- D. L. Ermak and J. McCammon, J. Chem. Phys., 1978, 69, 1352 CrossRef CAS.
- N. Watari, M. Doi and R. G. Larson, Physical Review E, 2008, 78, 011801 CrossRef PubMed.
- S. Redner, A guide to first-passage processes, Cambridge University Press, 2001 Search PubMed.
| This journal is © the Owner Societies 2022 |