Unraveling the growth mechanism of W18O49 nanowires on W surfaces†
Abstract
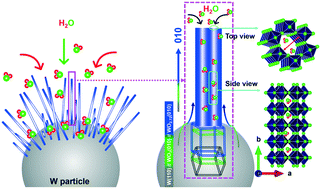
Understanding the growth mechanism is crucial for controlling the morphology and further designing efficient nanostructured materials. Non-stoichiometric tungsten oxides, in particular, W18O49 nanostructures have shown their prominence for various functional applications. This work presents the growth mechanism of W18O49 nanowires on W surfaces under H2O(v) atmosphere. The phase evolutions are unfolded in view of the detailed thermodynamic considerations. The reaction between W and H2O(v) produces WO3 along with H2 as a by-product. H2 acts as a reductant for WO3 and facilitates the W18O49 phase formation. The complete crystallographic relation of the phase transformation could be represented as W(110)//WO3(010)//W18O49(010). The oxygen vacancy-induced planar defects are responsible for the 1d [010] growth of W18O49 NWs. The hexagonal tunnels along the NW length create a passage for H2O(v) to repeat the reaction and increase the NW length.
- This article is part of the themed collection: Nanomaterials

 Please wait while we load your content...
Please wait while we load your content...