C–H bond functionalization by dual catalysis: merging of high-valent cobalt and photoredox catalysis
Abstract
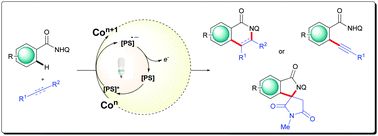
The merger of transition metal catalysis and photocatalysis has emerged as a versatile platform that opened the gateway to diverse low-energy pathways for several synthetic transformations. However, amidst the first-row transition metals, directed C–H bond functionalization mediated by high-valent cobalt catalysis has advanced with rising momentum owing to its unique reactivity and the ability to participate in both one- and two-electron transfer reactions. However, the use of expensive, privileged Cp* ligands or use of stoichiometric silver(I) or manganese(III) is unavoidable. Despite significant advances in their respective fields, the combination of these two “green” approaches to further the vested interest of the scientific research community towards the development of ecofriendly and sustainable protocols is noticeably limited. Thus, the methodology based on high-cobalt–photoredox dual-catalytic strategy has high dormant potential and is worthy to explore. Herein, we highlight the recent advances in the high-valent cobalt-catalyzed sustainable catalytic approach by harnessing light energy for oxidative C–H bond functionalization. With this, we hope to inspire the development of unexplored cobalt–photoredox-catalyzed reactions with improved efficiency and selectivity.


 Please wait while we load your content...
Please wait while we load your content...