Insights into D4h@metal-symmetry single-molecule magnetism: the case of a dysprosium-bis(boryloxide) complex†
Lewis R.
Thomas-Hargreaves
 a,
David
Hunger
b,
Michal
Kern
b,
Ashley J.
Wooles
a,
Joris
van Slageren
a,
David
Hunger
b,
Michal
Kern
b,
Ashley J.
Wooles
a,
Joris
van Slageren
 b,
Nicholas F.
Chilton
b,
Nicholas F.
Chilton
 *a and
Stephen T.
Liddle
*a and
Stephen T.
Liddle
 *a
*a
aDepartment of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: steve.liddle@manchester.ac.uk; nicholas.chilton@manchester.ac.uk
bInstitute of Physical Chemistry, University of Stuttgart, Pfaffenwaldring 55, Stuttgart, D-70569, Germany
First published on 11th December 2020
Abstract
We report the synthesis of the lanthanide-(bis)boryloxide complex [Dy{OB(NArCH)2}2(THF)4][BPh4] (2Dy, Ar = 2,6-Pri2C6H3), with idealised D4h@Dy(III) point-group symmetry. Complex 2Dy exhibits single-molecule magnetism (SMM), with one of the highest energy barriers (Ueff = 1565(298) K) of any six-coordinate lanthanide-SMM. Complex 2Dy validates electrostatic model predictions, informing the future design of lanthanide-SMMs.
With potential applications in quantum technologies and high-density data storage, the field of lanthanide (Ln) single-molecule magnetism (SMM) has expanded rapidly in recent years due to large energy barriers to reversal of magnetisation (Ueff) and blocking temperatures (TB).1 The principal factor controlling the performance of Ln-SMM is the crystal field (CF), and, following intuitive electrostatic principles, strong uniaxial CFs for Dy(III) stabilise the largest projections of the total angular momentum, mJ = ±15/2, which has an oblate spheroidal electron density distribution.2 Thus, there has been great interest in controlling the symmetry of the coordination sphere of Dy(III) complexes to engineer improved SMM performance.3 However, Ln-SMM is often subject to zero-field quantum tunnelling of the magnetization (QTM) that can bypass the anisotropy barrier, which is known to relate to hyperfine coupling and dipolar fields, but may also relate to spin-phonon coupling.4
As a result of the development of the electrostatic model,5 developing different geometries and thus metal point group symmetries has become a burgeoning area of investigation because some point groups prevent transverse CF terms by symmetry,6 meaning that the prevalence of QTM processes should be greatly diminished. Complexes with S8/D4d,7C5h/D5h,3d,8S12/D6d, D6h,9 and Cn (where n ≥ 7)10 have been found to be particularly effective. However, Ln-SMM complexes with D4h@Dy(III) symmetry at the metal remain rare,11 a vexatious situation considering the dominance of Oh and D4h complexes in coordination chemistry in general.
Around twenty (pseudo) Oh and D4h@Dy-SMM complexes are known, but few exhibit Ueff values >700 K: notable examples include [Dy(BIPM)2]− (721/813 K),12 [Dy{NC(NArCH)2}(Cl)2(THF)3] (803 K, Ar = 2,6-Pri2C6H3),13 [Dy(Cy3PO)2(I)3(CH3CN)] (1062 K),14 [Dy(DiMeQ)2(Cl)3(H2O)] (1100 K),15 and recently [Dy(OBut)2(Py-4-R)4]+ (1810–2075 K, R = Ph, N(CH2CH2)2CH2, N(CH2CH2)2).16 For D4h@Dy(III) SMMs, the latter three bis(alkoxide) complexes exhibit remarkable Ueff values, yet conversely D4h@Dy(III) [Dy(carbazolyl)2(L)4]+ (L = py, THF) exhibit Ueff barriers of 57–72 K.17 This is surprising because electrostatic models predict that [Dy(X)2(THF)4]+ (X = monoanionic ligand) species should exhibit Ueff values of ∼860–1500 K.5 The experimental D4h@Dy(III) Ueff variance of ∼2000 K suggests that much remains to be understood in electrostatic models, and so further data are required to validate or modify electrostatic potential theory.
Monoanionic O-donor ligands have proven particularly effective in Dy-SMM complexes. Our attention was drawn to the boryloxide ligand −OB(NArCH)2, which is isoelectronic to the imine −NC(NArCH)2 ligand used in [Dy{NC(NArCH)2}(Cl)2(THF)3].13 Though boryloxide ligands are poorer donors compared to the NH-imine, we surmised that a Dy-bis(boryloxide) complex could provide a high performance D4h@Dy(III) SMM complex to compare to electrostatic model predictions. Further motivation stemmed from the fact that N-substituted Ln–boryloxide complexes are rare, and have resulted from tris(pyrazoyl)borate decomposition rather than from targeted syntheses.18 Here, we present the realisation of our goal, which has resulted in a D4h@Dy(III) complex with one of the largest Ueff values to date. These results inform the electrostatic model and thus could assist the targeted design of improved lanthanide SMM.
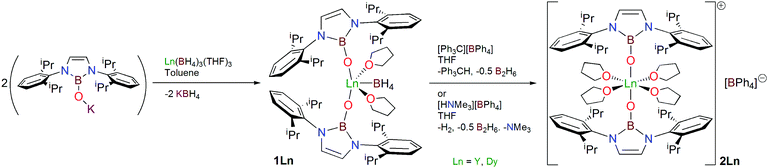
Treatment of [Dy(BH4)3(THF)3]19 with two equivalents of [KOB(NArCH)2]20 in toluene proceeds with elimination of KBH4 to give, after work-up, the dysprosium bis(boryloxide) complex [Dy{OB(NArCH)2}2(BH4)(THF)2] (1Dy), isolated as colourless crystals in 86% yield, Scheme 1.21 Following isolation, the coordinated BH4− group can be replaced with the non-coordinating BPh4− anion, using [Ph3C][BPh4] or [HNMe3][BPh4], in THF, to produce [Dy{OB(NArCH)2}2(THF)4][BPh4] (2Dy), Scheme 1. Both routes are synthetically effective, with isolated crystalline yields of 2Dy of 77 and 94%, respectively, though the latter route is more practicable not only for increased yield compared to the former but because gaseous by-products are formed, simplifying work-up. To give confidence in the formulations of 1Dy and 2Dy we also prepared the yttrium analogues 1Y and 2Y. Attempts to abstract the BH4− group from 1Ln in non-polar solvents did not give crystalline material, but subsequent addition of THF and recrystallisation gave 2Ln.
The 1H, 11B, and 13C NMR spectra of 1Y and 2Y are consistent with their structures, with the BH4− ligand in 1Y (11B δ −28.4 ppm) clearly being replaced by the BPh4− anion in 2Y (11B δ −6.5 ppm), though the characteristic boryl resonance is virtually unshifted from 1Y to 2Y (11B δ 21.5, 21.6 ppm, respectively).21 The IR spectra of 1Y and 1Dy exhibit absorptions that are characteristic of an ionic coordination mode of BH4−, and their absence in the corresponding spectra of 2Y and 2Dy is consistent with the exchange of BPh4− for BH4−.
The solid-state structure of 2Dy was determined by single crystal X-ray diffraction and is shown in Fig. 1. The compound crystallises as a separated ion pair where the cation features a pseudo-octahedral Dy ion22 with two axial trans boryloxide and four equatorial THF ligands. In the crystal examined, the shortest Dy⋯Dy interaction is 13.9151(6) Å. There is formally a C2 rotation axis passing through O2–Dy–O4 and a vertical mirror plane passing through the O2–O1–O4–O1A plane (hence there is disorder in the structure), but the complex is very close to D4h point symmetry at the metal: the O1–Dy–O1A angle is 175.9(3)° and the O1–Dy–O2/3/4 angles range from 86.2(7) to 94.5(7)°. The unique 2Dy Dy–O1 bond length is 2.136(5) Å, which is slightly longer than the Dy–OB distances of 2.089(10)/2.113(11) Å in 1Dy, which likely reflects the absence of a fourth equatorial donor in 1Dy compared to 2Dy. Unsurprisingly, in 2Dy the Dy–O2/3/4 bond lengths are considerably longer, ranging from 2.346(9) to 2.365(8) Å. Interestingly, the two boryloxide ligands tilt towards each other on one side of the complex (removing the putative C4 rotation axis), with B–O–Dy angles of 169.7(5)°, but despite the tilting the B–O distance of 1.367(10) Å is statistically indistinguishable to other complexes. Although in many regards the structures of 2Dy and 2Y are very similar, we note a key difference, which is in 2Dy the two boryloxide ligands adopt an eclipsed arrangement, whereas in 2Y they are arranged orthogonally and so 2Dy and 2Y are not isostructural with one another, which prevents meaningful magnetic doping dilution experiments in this case.
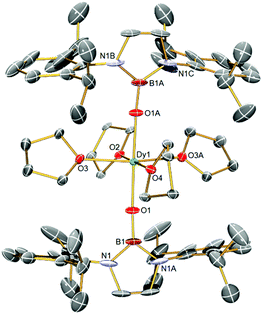 | ||
| Fig. 1 Solid state structure of the cation component of 2Dy at 150 K and displacement ellipsoids set to 40%. The BPh4− anion, hydrogen atoms, and THF disorder components are omitted for clarity. | ||
The χMT value of 14.71 cm3 mol−1 K for 2Dy at 298 K is indicative of a single Dy(III) ion (expected 14.2 cm3 mol−1 K) (Fig. S13, ESI†). This decreases only moderately until below 20 K where it falls precipitously to ca. 6 cm3 mol−1 K at 1.8 K. In zero direct current field, alternating current susceptibility data for 2Dy reveal peaks in the out-of-phase component up to 86 K (Fig. S15–S18, ESI†). Fitting these data to a generalised Debye model using the CC-FIT2 code23 gives relaxation rates and distribution parameters which we convert to estimated standard deviations (esds);24 note there is a high-frequency relaxation process that appears just above the frequency window of our instrument below 16 K, and so we fit the data using a two-process model but disregard the data for the poorly-defined fast-process. Fitting the temperature dependence of the magnetic relaxation rates with the expression τ − 1 = τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−Ueff/kT] + CTn + τQTM−1 gives Ueff = 1565(298) K, τ0 = 10−11.6(1.6) s, C = 10−3.05(0.45) s−1 K−n, n = 3.14(0.25), τQTM = 10−0.74(0.16) s, Fig. 2. This indicates a very large Orbach relaxation barrier, surpassed only by a handful of compounds3a–f and one D4h SMM (within esds);16 here the large uncertainty in the Ueff barrier is due to the limited temperature range over which the exponential relaxation process is observed coupled with the esds from the generalised Debye model. Magnetic hysteresis experiments performed from 1.8 to 15 K with a sweep-rate of ∼20 Oe s−1 reveal butterfly curves up to around 7 K, Fig. 3, however these are all closed at zero field, indicating efficient quantum tunnelling of the magnetisation.
exp[−Ueff/kT] + CTn + τQTM−1 gives Ueff = 1565(298) K, τ0 = 10−11.6(1.6) s, C = 10−3.05(0.45) s−1 K−n, n = 3.14(0.25), τQTM = 10−0.74(0.16) s, Fig. 2. This indicates a very large Orbach relaxation barrier, surpassed only by a handful of compounds3a–f and one D4h SMM (within esds);16 here the large uncertainty in the Ueff barrier is due to the limited temperature range over which the exponential relaxation process is observed coupled with the esds from the generalised Debye model. Magnetic hysteresis experiments performed from 1.8 to 15 K with a sweep-rate of ∼20 Oe s−1 reveal butterfly curves up to around 7 K, Fig. 3, however these are all closed at zero field, indicating efficient quantum tunnelling of the magnetisation.
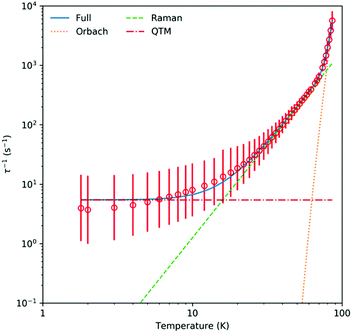 | ||
| Fig. 2 Temperature dependence of magnetic relaxation rates for 2Dy measured with alternating current susceptometry with zero direct current field. | ||
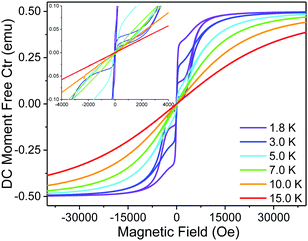 | ||
| Fig. 3 Magnetic hysteresis measurements performed 2Dy with a sweep rate of ∼20 Oe s−1. Inset shows a zoom around 0 field to show closing of hysteresis loops. | ||
Complete active space self-consistent field spin–orbit (CASSCF-SO) calculations using the crystallographic coordinates of the cation in 2Dy show, Fig. 4, that the ground Kramers doublet is 97% mJ = ±15/2 with effective g-values gx = gy = 0, gz = 19.86 (Table S2, ESI†). The first excited state at 763 K, whilst a mixture of 70% mJ = ±13/2 and 28% mJ = ∓13/2, retains very small transverse g-values (gx = 0.05, gy = 0.06, gz = 16.83), and the second excited state at 1309 K is dominated by 87% mJ = ±11/2, but here the transverse g-values grow (gx = 0.63, gy = 1.08, gz = 13.15); however, both first and second excited states share a common main magnetic axis with that of the ground state, deviating by 2.3° and 7.7°, respectively. However, the third excited state at 1536 K is very mixed (28% mJ = ±1/2, 26% mJ = ∓1/2, 18% mJ = ±9/2, 11% mJ = ∓9/2), has very large transverse g-values (gx = 3.53, gy = 6.79, gz = 11.18), and its main magnetic axis is roughly orthogonal to that of the ground state (84.1° deviation). Thus, ab initio calculations predict relaxation via the Orbach process to occur through the third excited state, which is in excellent agreement with the experimental Ueff.
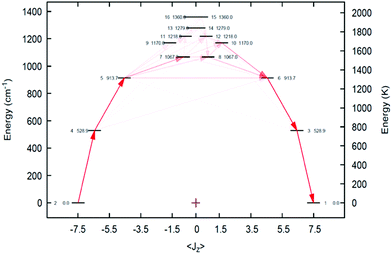 | ||
| Fig. 4 CASSCF-SO CF splitting of the cation in 2Dy. Arrows are likely Orbach transitions (av. Cartesian magnetic dipole operators); propensity is proportional to opacity. | ||
The Ueff and electronic structure of 2Dy can be compared to the theoretical models of [Dy(R)2(THF)4]+ (R = N(SiH3)2, CH(SiH3)2, C(SiH3)3), which were computed in a study examining the effects of linearity on Ueff and the effects of coordinated solvent and complex geometry.5b Pseudo-D4h symmetry was predicted to have a Ueff barrier of ∼1000 K for R = (H3Si)2N− and (H3Si)2(H)C−, and 1600 K when R = (H3Si)3C−. Considering the approximations made, these predictions are rather accurate.
Relaxation of the magnetisation occurs via the third excited state for 2Dy as well as the [Dy(OBut)2(Py-4-R)4]+ series, but the latter can have larger Ueff values; it is clear that shorter axial Dy–O distances (∼2.066(8)–2.122(5) Å) linearly expand the energy range of the excited mJ state manifold,16 but equatorial ligand coordination is still important. So, we compared the computed charges for the [Dy(OBut)2(Py-4-R)4]+ series and 2Dy; we find that the Dy, O, and N calculated LoProp charges for the former are 2.47 to 2.50, −1.09 to −1.12, and −0.39 to −0.40, and for 2Dy the Dy, OBoryl, and OTHF LoProp charges are 2.57, −1.02, and −0.56, respectively. These data suggest that the Dy atom in 2Dy is more positively charged as a result of less charge donation from the ligands overall, but the boryloxide ligands are weaker donors than the alkoxides and the THF ligands are stronger donors than the pyridine ligands resulting in a weaker axial and greater equatorial ligand field for 2Dy compared to the [Dy(OBut)2(Py-4-R)4]+ series, which is in-line with the observed Ueff data. Thus, whilst bond distances are a useful gross guide to likely magnetic performance, the potential donor strength of individual ligands is also a key factor within a range of effectiveness bracketed by the gross Dy–ligand distances.
To conclude, we have reported the synthesis of rare examples of Ln–boryloxide complexes utilising a borohydride elimination methodology. Complex 2Dy is a D4h@Dy(III) SMM with a large Ueff barrier likely surpassed by only one other D4h@Dy(III) SMM. These results validate the electrostatic model, but also clarify an appreciation that charges and thus donor strength of ligands is an important criterion for SMM performance within the framework of Dy–ligand distances.
We thank the EPSRC (EP/P002560/1, EP/P001386/1, EP/M027015/1), ERC (CoG612724, StG851504), Royal Society (University Research Fellowship to NFC), German DFG (SL104/10-1), and The Universities of Manchester and Stuttgart for support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) Z. Zhu, M. Guo, X.-L. Li and J. Tang, Coord. Chem. Rev., 2019, 378, 350 CrossRef CAS; (b) K. L. M. Harriman, D. Errulat and M. Murugesu, Trends Chem., 2019, 1, 425 CrossRef CAS; (c) A. K. Bar, P. Kalita, M. K. Singh, G. Rajaraman and V. Chandrasekhar, Coord. Chem. Rev., 2018, 367, 163 CrossRef CAS; (d) M. Feng and M.-L. Tong, Chem. – Eur. J., 2018, 24, 7574 CrossRef CAS PubMed; (e) S. G. McAdams, A.-M. Ariciu, A. K. Kostopoulos, J. P. S. Walsh and F. Tuna, Coord. Chem. Rev., 2017, 346, 216 CrossRef CAS; (f) S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655 RSC.
- J. Sievers, Z. Phys. B: Condens. Matter, 1982, 45, 289 CrossRef CAS.
- (a) Y.-S. Ding, T. Han, Y.-Q. Zhai, D. Reta, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Chem. – Eur. J., 2020, 26, 5893 CrossRef CAS PubMed; (b) Z. Zhu and J. Tang, Top. Organomet. Chem., 2019, 64, 191 CrossRef CAS; (c) K. R. McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492 RSC; (d) F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS PubMed; (e) C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439 CrossRef CAS PubMed; (f) Y.-S. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16071 CrossRef CAS PubMed; (g) Lanthanide Single Molecule Magnets, ed. J. Tang and P. Zhang, Springer-Verlag, Berlin, Heidelberg, 2015 Search PubMed; (h) N. F. Chilton, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and A. Soncini, Nat. Commun., 2013, 4, 2551J CrossRef PubMed; (i) D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC.
- (a) K. Irländer and J. Schnack, Phys. Rev. B, 2020, 102, 054407 CrossRef; (b) F. Ortu, D. Reta, Y.-S. Ding, C. A. P. Goodwin, M. P. Gregson, E. J. L. McInnes, R. E. P. Winpenny, Y.-Z. Zheng, S. T. Liddle, D. P. Mills and N. F. Chilton, Dalton Trans., 2019, 48, 8541 RSC; (c) E. Moreno-Pineda, G. Taran, W. Wernsdorfer and M. Ruben, Chem. Sci., 2019, 10, 5138 RSC; (d) Y.-S. Ding, K.-X. Yu, D. Reta, F. Ortu, R. E. P. Winpenny, Y.-Z. Zheng and N. F. Chilton, Nat. Commun., 2018, 9, 3134 CrossRef PubMed; (e) Y.-C. Chen, J.-L. Liu, W. Wernsdorfer, D. Liu, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, Angew. Chem., Int. Ed., 2017, 56, 4996 CrossRef CAS PubMed; (f) F. Pointillart, K. Bernot, S. Golhen, B. Le Guennic, T. Guizouarn, L. Ouahab and O. Cador, Angew. Chem., Int. Ed., 2015, 54, 1504 CrossRef CAS; (g) N. Ishikawa, M. Sugita and W. Wernsdorfer, J. Am. Chem. Soc., 2005, 127, 3650 CrossRef CAS PubMed.
- (a) L. Ungur and L. F. Chibotaru, Inorg. Chem., 2016, 55, 10043 CrossRef CAS PubMed; (b) N. F. Chilton, Inorg. Chem., 2015, 54, 2097 CrossRef CAS PubMed; (c) M. A. Aldamen, J. M. Clemente-Juan, E. Coronado, C. Marti-Gastaldo and A. Gaita-AriÇo, J. Am. Chem. Soc., 2008, 130, 8874 CrossRef CAS; (d) N. F. Chilton, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and A. Soncini, Nat. Commun., 2013, 4, 2551 CrossRef PubMed; (e) Y. Bi, Y.-N. Guo, L. Zhao, Y. Guo, S.-Y. Lin, S.-D. Jiang, J. Tang, B.-W. Wang and S. Gao, Chem. – Eur. J., 2011, 17, 12476–12481 CrossRef CAS PubMed; (f) S. D. Jiang, B. W. Wang, H. L. Sun, Z. M. Wang and S. Gao, J. Am. Chem. Soc., 2011, 133, 4730 CrossRef CAS PubMed.
- (a) J.-L. Liu, Y.-C. Chen and M.-L. Tong, Chem. Sci., 2018, 47, 2431 CAS; (b) L. Sorace, C. Benelli and D. Gatteschi,, Chem. Soc. Rev., 2011, 40, 3092 RSC; (c) C. Görller-Walrand and K. Binnemans, in Handbook on the Physics and Chemistry of Rare Earths, Elsevier, 1996, vol. 23 Search PubMed.
- Recent S8/D4d examples: (a) K. Katoh, S. Yamashita, N. Yasuda, Y. Kitagawa, B. K. Breedlove, Y. Nakazawa and M. Yamashita, Angew. Chem., Int. Ed., 2018, 57, 9262 CrossRef CAS PubMed; (b) J. Wu, J. Jung, P. Zhang, H. Zhang, J. Tang and B. L. Guennic, Chem. Sci., 2016, 7, 3632 RSC.
- Recent C5h/D5h examples: (a) A. B. Canaj, M. K. Singh, C. Wilson, G. Rajaraman and M. Murrie, Chem. Commun., 2018, 54, 8273 RSC; (b) Y.-C. Chen, J.-L. Liu, Y. Lan, Z.-Q. Zhong, A. Mansikkamaki, L. Ungur, Q.-W. Li, J.-H. Jia, L. F. Chibotaru, J.-B. Han, W. Wernsdorfer, X.-M. Chen and M.-L. Tong, Chem. – Eur. J., 2017, 23, 5708 CrossRef CAS PubMed; (c) Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829 CrossRef CAS PubMed.
- Recent D6h examples: (a) J. Li, S. Gomez-Coca, B. S. Dolinar, L. Yang, F. Yu, M. Kong, Y.-Q. Zhang, Y. Song and K. R. Dunbar, Inorg. Chem., 2019, 58, 2610 CrossRef CAS; (b) A. B. Canaj, S. Dey, E. R. Marti, C. Wilson, G. Rajaraman and M. Murrie, Angew. Chem., Int. Ed., 2019, 58, 14146 CrossRef CAS PubMed; (c) Z. H. Li, Y.-Q. Zhai, W.-P. Chen, Y. S. Ding and Y.-Z. Zheng, Chem. – Eur. J., 2019, 25, 16219 CrossRef CAS.
- Recent Cn (where n ≥ 7) examples: (a) D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Buchnera and A. A. Popov, Chem. Commun., 2017, 53, 7901 RSC; (b) F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Buchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef CAS PubMed.
- A list of six-coordinate Dy(III) SMMs is located in the ESI†.
- M. Gregson, N. F. Chilton, A.-M. Ariciu, F. Tuna, I. F. Crowe, W. Lewis, A. J. Blake, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and S. T. Liddle, Chem. Sci., 2016, 7, 155 RSC.
- B.-C. Liu, N. Ge, Y.-Q. Zhai, T. Zhang, Y.-S. Ding and Y.-Z. Zheng, Chem. Commun., 2019, 55, 9355 RSC.
- M. Li, H. Wu, Z. Xia, L. Ungur, D. Liu, L. F. Chibotaru, H. Ke, S. Chen and S. Gao, Inorg. Chem., 2020, 59, 7158 CrossRef CAS PubMed.
- M. J. Giansiracusa, S. Al-Badran, A. K. Kostopoulos, G. F. S. Whitehead, D. Collison, F. Tuna, R. E. P. Winpenny and N. F. Chilton, Dalton Trans., 2019, 48, 10795 RSC.
- X.-L. Ding, Y.-Q. Zhai, T. Han, W.-P. Chen, Y.-S. Ding and Y.-Z. Zheng, Chem. – Eur. J., 2020 DOI:10.1002/chem.202003931.
- J. Long, A. N. Selikhov, E. Mamontova, K. A. Lyssenko, Y. Guari, J. Larionova and A. A. Trifonov, Dalton Trans., 2020, 49, 4039 RSC.
- (a) F. Ortu, H. Zhu, M.-E. Boulon and D. P. Mills, Inorganics, 2015, 3, 534 CrossRef CAS; (b) A. Domingos, M. R. J. Elsegood, A. C. Hillier, G. Lin, S. Y. Liu, I. Lopes, N. Marques, G. H. Maunder, R. McDonald, A. Sella, J. W. Steed and J. Takats, Inorg. Chem., 2002, 41, 6761 CrossRef CAS PubMed; (c) D.-L. Deng, Y.-H. Zhang, C.-Y. Dai, H. Zeng, C.-Q. Ye and R. Hage, Inorg. Chim. Acta, 2000, 310, 51 CrossRef CAS.
- S. M. Cendrowski-Guillaume, G. Le Gland, M. Nierlich and M. Ephritikhine, Organometallics, 2000, 19, 5654 CrossRef CAS.
- Y. K. Loh, M. Á. Fuentes, D. C. H. Do and S. Aldridge, Angew. Chem., Int. Ed., 2019, 58, 4847 CrossRef CAS PubMed.
- See the ESI† for full details.
- Continuous shape measurement of first coordination sphere in 2Dy gives 0.802 for Oh with all other six-coordinate polyhedra >10. See: (a) S. Alvarez, D. Avnir, M. Llunell and M. Pinsky, New J. Chem., 2002, 26, 996 RSC; (b) J. Cirera, M. Llunell, P. Alemany, D. Avnir and S. Alvarez, J. Am. Chem. Soc., 2004, 126, 1755 CrossRef PubMed.
- http://www.nfchilton.com/cc-fit, accessed 1st November 2020.
- D. Reta and N. F. Chilton, Phys. Chem. Chem. Phys., 2019, 21, 23567 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational details. CCDC 2043760–2043763. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc07446b |
| This journal is © The Royal Society of Chemistry 2021 |