Metal–organic complex-based chemodynamic therapy agents for cancer therapy
Eunbin
Hwang
and
Hyo Sung
Jung
 *
*
Department of Biological Sciences, Hyupsung University, Hwasung-si, 18330, Korea. E-mail: hs0101j@uhs.ac.kr
First published on 3rd June 2020
Abstract
In recent years, many inorganic nanoparticle-based chemodynamic therapy (CDT) agents have been employed in cancer therapy; however, the relatively lower catalytic activity compared to that of other CDT agents and long-term toxicity owing to low biodegradability present significant challenges for their future clinical application. In light of this, metal–organic complex-based agents have been attracting attention as potential alternatives/complements to traditional CDT agents. During the past few years, many reports of agents with improved therapeutic potential have been published; however, no comprehensive review regarding metal–organic complex-based CDT agents has appeared to date. In this feature article, we present the different types and characteristics of metal–organic CDT agents and the potential future therapeutic applications associated with each of these. Representative agents that have been used in the field of CDT over the past 5 years are summarized, and recent advances aimed at improving the therapeutic efficacy in various tumors are highlighted. This framework allows us to discuss recent trends in the field of CDT. We also provide views as to where the field is moving and discuss how the potential of CDT agents can be broadened to include a range of clinical applications that go beyond standard CDT-based treatment strategies.
Introduction
Chemodynamic therapy (CDT) is an emerging therapeutic strategy that induces destruction of tumor cells or increases their susceptibility to other antitumor therapies, including chemotherapy, radiotherapy, photothermal therapy (PTT), and photodynamic therapy (PDT), by amplifying intracellular oxidative stress.1,2 It relies on intracellular Fenton or Fenton-like reactions to damage plasma membranes and DNA, decrease tumor vasculature, or promote an antitumor immune response, thereby conveying its antitumor activity via apoptosis or ferroptosis.3 One major CDT strategy, the so-called Fenton reaction, involves the process by which ferrous ions (Fe2+) react with endogenous hydrogen peroxide (H2O2) to generate hypertoxic hydroxyl radicals (˙OH) in tumor regions.4 Tumor cells often have high H2O2 levels (100 μM–1 mM) due to abnormal metabolic processes, rendering this approach viable.5 In addition to Fe2+, several metal ions, including Cu+, Mn2+, and Co2+, exhibit Fenton-like activities and thus can be utilized as CDT catalysts.6CDT is a highly specific and minimally invasive cancer treatment that does not damage surrounding healthy cells, as its effects remain localized to tumor regions containing CDT agents that are activated by specific tumor microenvironment (TME) conditions, including mild acidity, H2O2 overexpression, low levels of catalase, and hypoxia.7 PDT, one of the reactive oxygen species (ROS)-mediated minimally invasive treatment options, has limited clinical application due to reduced treatment efficiency within hypoxic tumors.8 Unlike PDT, CDT is not affected by this limitation, as ˙OH, which acts as a cytotoxin, originates from endogenous H2O2 and not oxygen. In addition, CDT does not require any external activation by light. It is thus suggested that CDT could emerge as a minimally invasive tumor treatment modality that could be more effective than not only PDT, but also traditional cancer treatments, such as radiotherapy and chemotherapy. In light of this, a considerable effort is being directed to the research of potential novel CDT agents, giving new hope to cancer patients.
Most studies have, thus far, focused on metal oxide nanoparticles, such as iron oxide nanoparticles, copper peroxide nanodots, and manganese dioxide nanoparticles, as CDT agents.2 Many of these nanoparticles have been widely used as Fenton catalyst sources for CDT; however, the relatively lower catalytic activity compared to that of other CDT agents and long-term toxicity owing to their non-biodegradability present significant challenges for their future clinical application.9 As potential complements of inorganic nanoparticles, metal–organic complexes have received significant attention in the past few years. These complexes are quite diverse, and their advantage lies in the point that, compared with metal oxide nanoparticles, metal–organic complexes exhibit higher catalytic activity because the low-coordinated or single metal atoms usually act as the active sites.10 These complexes also have excellent biodegradability and are ease to prepare and modify in many instances.10–14 In addition, they could be expected to function as efficient drug carriers in combination therapies.15,16
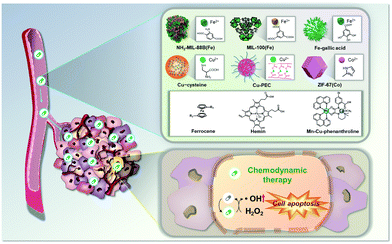
Common cancer targeting strategies involve passive targeting, active targeting, stimuli-responsive strategies, and combinational strategies;17 these have been applied to design CDT agents that depend on NH2-MIL-88B(Fe), MIL-100(Fe), Fe–gallic acid, Cu–cysteine, Cu–PEC, ZIF-67(Co), ferrocene, hemin, Mn–Cu-phenanthroline, etc. (Fig. 1). Enormous progress has been achieved using these different agents. However, to proceed to clinical trials, improvements, for example in their antitumor efficiency and more precise targeting, are likely to be necessary. In this regard, significant efforts have been devoted to the study of improved CDT systems, including selection of suitable Fenton reagents, regulation of the reaction conditions (increased amounts of H2O2, and decreased amounts of cellular antioxidant), and stimulation by an exogenous energy field (heat or light).1,2 In this feature article, these types, experimental details, and their therapeutic strategies are discussed in depth.
 | ||
| Fig. 1 Schematic representation of the anticancer effect expected to be produced by metal–organic chemodynamic therapy agents. | ||
Design and working principle of metal–organic complex-based CDT agents
The Fenton reaction has a key role in amplifying oxidative stress for CDT of cancer, which is defined as the reaction of Fenton reagents and endogenous H2O2. In typical Fenton reactions, Fe2+ act as catalysts in converting H2O2 into hypertoxic ˙OH, represented as follows: Fe2+ + H2O2 → Fe3+ + ˙OH + OH−.18 Upon interaction with reducing metabolites, the resulting Fe3+ are easily reduced to Fe2+ and the reaction continues to generate the most toxic radical, the ˙OH, that cells can produce.19 In addition to Fe2+, several metal ions, such as Cu+, Mn2+, and Co2+, are also capable of catalyzing ˙OH production via a Fenton-like reaction.6Ideally, metal–organic CDT agents should meet the following criteria: (i) spatiotemporally restrict the agents to tumor cells to avoid potential damage to normal cells, (ii) suitably modify the reaction environment (for example, by increased amounts of H2O2, decreased amounts of cellular antioxidant, and stimulation with exogenous energy field) to attain a high antitumor efficacy, (iii) decompose quickly after treatment (to avoid adverse side effects), and (iv) be easily prepared and modified. Various metal–organic chemodynamic agents that comply with most of the criteria have been identified and investigated as potential CDT agents.
Fe–organic complex-based agents
Fe is used as a Fenton catalyst in typical CDT reactions. From the bio-applicability point of view, it has important benefits because Fe is an abundant and essential element for multiple life processes in the human body. Ferumoxytol (FMT), a clinically approved Fe supplement, leads to the inhibition of tumor growth through the Fenton reaction.20 However, it has limited outcomes in cancer treatment because of the lack of target specificity and low catalytic rate.7 Accordingly, appropriate strategies to improve the therapeutic efficacy are needed. Effective Fe delivery to target sites is a critical factor that determines the ability of CDT reactions to produce ˙OH. Also, efforts to amplify the CDT efficiency and cellular susceptibility to ˙OH will render this approach more effective. In recent years, improvements in tumor-targeted delivery and stimuli-responsive release have offered a solution to this issue, facilitating the design and preparation of many Fe–organic complex-based agents for CDT of cancer.Nanoscale metal–organic framework-based agents
Nanoscale metal–organic frameworks (NMOFs) for CDT consist of Fenton or Fenton-like metal ions coordinated to organic ligands to construct multi-dimensional structures.21 Compared to other agents, NMOFs have significant benefits in the application of CDT, including the availability of multiple catalytic sites and the ability to control their components. In addition, the agents can be used as effective drug delivery platforms owing to their intrinsic porous structures for loading of drugs, and thus can be combined with other treatment modalities. However, some factors, including aqueous solubility and biocompatibility, have limited the clinical utility of CDT.In 2017, a NH2-MIL-88B(Fe)-based reduced metal–organic framework with folic acid (FA) as a tumor targeting antenna (rMOF-FA) was developed and characterized by Ranji-Burachaloo et al. (Fig. 2).22 NH2-MIL-88B(Fe3+) is a typical Fe-based NMOF that is composed of Fe3+ and 2-aminoterephtalic acid (H2N-BDC) ligands, which further reduce to NH2-MIL-88B(Fe2+).23 rMOF-FA was formulated by conjugating FA to NH2-MIL-88B(Fe2+) via an amide coupling reaction. The rMOF-FA, designed to permit cancer targeting, was found to enter HeLa cells via folate receptor-mediated endocytosis. The arrival of rMOF-FA inside the cancer cells, followed by internalization into acidic endosomes, served to induce the controlled release of Fe2+. The generated Fe2+ then generated ˙OH via heterogeneous and homogeneous Fenton reactions. Intracellular ROS generation, presumed to be Fenton-catalyzed, and intracellular treatment efficiency were confirmed in cancer cells (HeLa cells) and noncancerous fibroblast cells (NIH-3T3 cells). Significantly more cytotoxicity was observed in HeLa cells than in NIH-3T3 cells (43 vs. 105 μg mL−1 IC50 for these two cell lines, respectively).
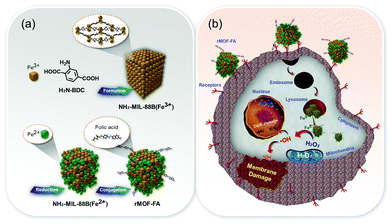 | ||
| Fig. 2 Schematic representation of (a) the synthesis and surface modification of rMOF-FA, and (b) the anti-cancer effect expected to be produced by rMOF-FA. Reprinted with permission from ref. 22. Copyright (2017) American Chemical Society. | ||
The same research group demonstrated polymeric formulation of rMOF-FA and optimized their CDT capacity as a modification of the hydrophilic polyethylene glycol (PEG) moieties to rMOF-FA.24 An optimized formulation (PEG monomer/nMOF composition, PEGMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rMOF = 2
rMOF = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio, P@rMOF-FA) exhibited improved stability in aqueous media. In vitro characterization revealed that P@rMOF-FA had a higher selectivity than rMOF-FA, and exhibited a greater CDT performance in HeLa cells than in NIH-3T3 cells (80% vs. 22% cell death, respectively, at 100 μg mL−1).
1 mass ratio, P@rMOF-FA) exhibited improved stability in aqueous media. In vitro characterization revealed that P@rMOF-FA had a higher selectivity than rMOF-FA, and exhibited a greater CDT performance in HeLa cells than in NIH-3T3 cells (80% vs. 22% cell death, respectively, at 100 μg mL−1).
A doxorubicin (DOX)-loaded MIL-100 NMOF system (DMH NP) was reported by Xue et al.15 MIL-100 is composed of Fe3+ and 2-aminoterephtalic acid (H2N-BDC) ligands and exhibits good CDT performance. Here, DOX was loaded into the MIL-100 NMOF before the system was further coated with hyaluronic acid (HA) moieties (Fig. 3). The surface modification with HA moieties significantly improved the aqueous solubility of MIL-100 NMOF. This also allowed for improved bio-applicability and chemotherapeutic outcomes that could complement the CDT. In this regard, DMH NP more effectively inhibited tumor growth in MCF-7 cells compared to the other variants (e.g., free DOX and the HA-free form of the MIL-100 NMOF (DM NP)) due to CD44 receptor-mediated targeting and the combined CDT and chemotherapy.
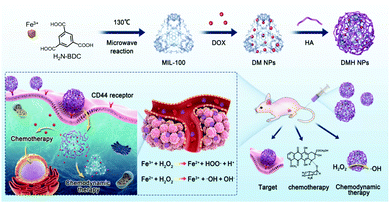 | ||
| Fig. 3 Schematic illustration of the synthesis and surface modification of DMH NPs and the anti-cancer effect of DMH NP for CDT/chemotherapy in cancer cells. Reproduced from ref. 15, with permission from The Royal Society of Chemistry. | ||
Ferrocene-based agents
Ferrocene (Fc) is an organometallic compound in which Fe is sandwiched between two cyclopentadienyl rings. It has high Fenton catalytic activity via good redox reversible characteristics, which originate from the electron donor–acceptor (D–A) conjugated structure in the Fc compound.25 Fc also exhibits unique properties; it is non-toxic, stable in biological media, and lipophilic (a property that aids in penetrating the cell membrane).Hagen et al. reported a functionalized aminoferrocene prodrug 1 for use in CDT.26 The aminoferrocene agent incorporates 4-[(carbonyloxy)methyl]phenylboronic acid pinacol ester residues via a carbamate linker to give prodrug 1 (Fig. 4). This was stable in normal fibroblasts but released the aminoferrocene and the quinone methide ROS scavenger via an H2O2-activated reaction in cancer cell lines (e.g., human promyelocytic leukemia (HL-60) and human glioblastoma–astrocytoma (U373)). The aminoferrocene then reacted with local H2O2 to produce toxic ˙OH. Simultaneously, the released quinone methide acted to deplete the local glutathione (GSH) levels, amplifying the Fenton reaction-based CDT efficiency. In this regard, prodrug 1 showed high toxicity in HL-60 cells, but negligible toxicity in normal fibroblasts (9 μM vs. 100 μM IC50 for these two cell lines, respectively).
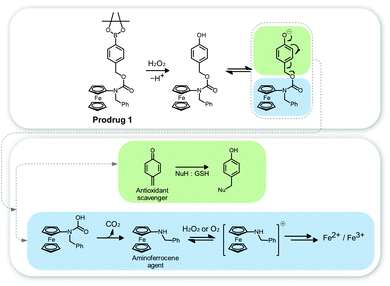 | ||
| Fig. 4 Chemical structure of prodrug 1 and its mode of action in the presence of H2O2. Reprinted with permission from ref. 26. Copyright (2012) American Chemical Society. | ||
Lei et al. demonstrated the feasibility of using a combination of CDT and PDT approaches to achieve an enhanced anticancer effect and to reduce the side effects of monotherapy (Fig. 5).27 In this study, a PDT sensitizer (TPP-NH2) was connected to a CDT catalyst (FC) for use as an anticancer conjugate (TNCF). The singlet oxygen (1O2) generated by the TPP-NH2 moiety under conditions of photoirradiation served to potentiate the FC-mediated CDT cytotoxicity in MCF-7 cells as a result of reduced GSH levels. Compared to the use of CDT alone, this conjugate system has significant efficiency and potency in attenuating tumor growth even at lower doses of the agents. The IC50 value of the TNCF (25 μM for MCF-7 cells) was considerably lower than that of the single CDT catalyst FC (>50 μM for MCF-7 cells).
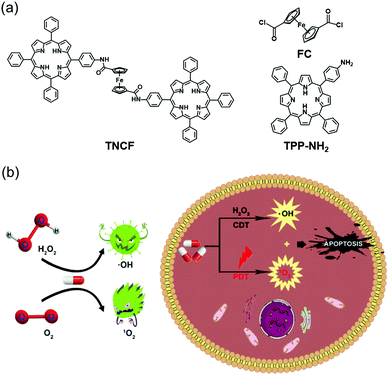 | ||
| Fig. 5 Schematic representation of (a) the chemical structures of FC, TPP-NH2, and TNCF, and (b) the proposed mechanism of TNCF for combined CDT/PDT in cancer cells. Reproduced from ref. 27 with permission from The Royal Society of Chemistry. | ||
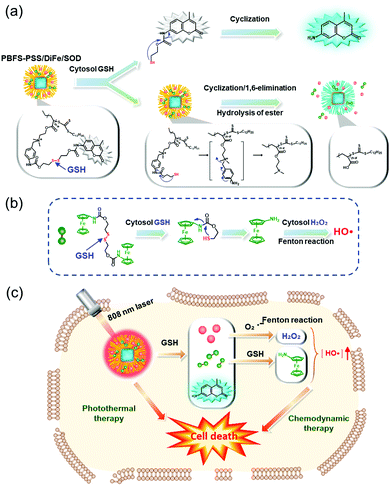
The Fc agent was used by Lei et al. to create the combined CDT/PTT nanoplatform system (PBFS–PSS/DiFe/SOD) (Fig. 6).28 In this system, a GSH-activatable cationic polymer (PSS), comprising coumarin fluorophores attached via disulfide triggering bonds, were connected to Prussian blue@fibrous SiO2 (PBFS) that further introduced a GSH-responsive Fc-based Fenton reagent (DiFe) and superoxide dismutase (SOD). The SOD in this system reacted with endogenous O2˙− to generate H2O2, facilitating rapid Fenton reactions. In MCF-7 tumor cells, this system exhibited excellent biocompatibility and the intracellular delivery process of PBFS–PSS/DiFe/SOD could be followed by using fluorescence microscopic imaging. They also showed that GSH activated Fenton reactions in tumor cells and simultaneously had good photothermal conversion efficiencies under laser irradiation (808 nm, 1.2 W cm−2, 5 min). Synergistic killing of PBFS–PSS/DiFe/SOD-treated MCF-7 cells was seen in cells treated with combined CDT/PTT, but not in cells that received individual CDT or PTT under the same particle concentrations. This study substantiated the use of dual CDT/PTT functional treatment.
Hemin-based agents
Hemin, an Fe-containing porphyrin complex, can be used as a biomimetic Fenton catalyst. Hemin has been widely explored as a versatile material in recent years owing to its unique photochemical properties and biomedical value.29–31 Hemin has also showed efficient PDT functionality.32 Consequently, effects of combined CDT and PDT are capable of contributing to an improved anticancer outcome. However, due to its hydrophobic nature, hemin tends to aggregate in aqueous media and thus its ability to generate ROS is limited.33 Inhibiting aggregation of hemin agents prevents the production of inactive dimers and their oxidative self-destruction in the photochemical system.34Wang et al. described a hemin-containing nanoplatform (PFO@CPPO@hemin–GOD) with both ROS-correlated chemiluminescence (CL) imaging performance and CDT capability (Fig. 7).35 This nanoplatform was prepared by coating 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(poly(ethylene glycol)) (DSPEPEG) and poly(styrene-co-maleic anhydride) (PSMA) over a formulation of bis(2,4,5-trichloro-6-(pentyloxycarbonyl)phenyl) oxalate (CPPO), hemin, and glucose oxidase (GOD), to provide a CL-monitoring CDT composite. Hemin loaded into the PFO@CPPO@hemin–GOD exhibited well-preserved catalytic activity for both CL imaging and CDT in biological media with no significant self-aggregation. In this platform, CPPO was rapidly decomposed by hemin-catalyzed ˙OH and thus served to provide CL activation. GOD in the system acted to supply enough H2O2 for the efficient Fenton reaction of hemin. By exploiting these properties, in vitro CL imaging, correlated to the CDT effect, was successfully performed with an excellent linear relationship between the production of ROS and the CL function. When administered intravenously to 4T1 tumor-bearing mice, the PFO@CPPO@hemin–GOD (GOD/PFO = 400 ng μg−1) exhibited efficient CL contrasts within tumor areas and effective CDT-mediated tumor attenuation.
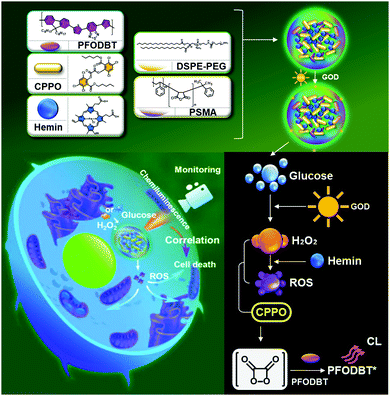 | ||
| Fig. 7 Schematic representation of the synthesis, modification, and proposed mechanism of PFO@CPPO@hemin–GOD for CL imaging monitoring CDT. Reprinted with permission from ref. 35. Copyright (2020) American Chemical Society. | ||
Xuan et al. used hemin in cooperation with adamantane-stabilized tetraoxane prodrug (T) decorated with a nucleotide DNA aptamer AS1411-6G (Ap-6G-H-2T) (Fig. 8).16 Here, Ap-6G-H-2T exhibited enhanced tumor uptake via AS1411-mediated nucleolin recognition. In this system, hemin mediated the Fenton reaction and simultaneously activated the production of active drugs containing toxic C-centered radicals via bioorthogonal reaction. Tumor cell targeting, presumed to be AS1411-mediated, and intracellular therapeutic behaviors were confirmed in HepG2 cells. Greater cytotoxicity was observed with Ap-6G-H-2T than with hemin-free Ap-6G-T under the same experimental conditions (IC50 = 4.79 μM vs. 6.88 μM for Ap-6G-H-2T and Ap-6G-2T, respectively). The benefits of Ap-6G-H-2T treatment were proposed to be due to a synergistic CDT effect.
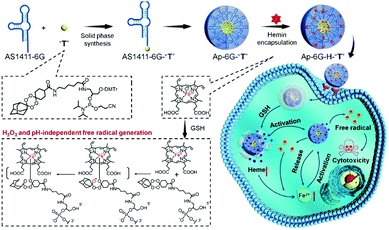 | ||
| Fig. 8 Schematic representation of the synthesis, modification, and proposed anti-cancer mechanism of ApPdC micelles in cancer cells. Reprinted with permission from ref. 16. Copyright (2020) American Chemical Society. | ||
Fe–polyphenol-based agents
Fe–polyphenol complexes display sustainable catalytic activity in catalyzing H2O2 to ˙OH, as polyphenol ligands (e.g., gallic acid (GA)) facilitate the conversion of Fe3+ to Fe2+.11 Consequently, they show biocompatible and superior Fenton catalytic stability over free Fe2+. Relative to other Fenton catalysts, including iron oxide nanoparticles, aminoferrocene, and hemin, under the same iron concentration, Fe–GA complexes exhibit the highest Fenton catalytic efficiencies in aqueous media.11 As a general rule, Fe–GA complexes also possess efficient PTT properties.12,36,37 As a result, both CDT and PTT effects are capable of contributing to effective anti-cancer activity.The Fe–GA complex was used by Dong et al. to create a biocompatible liposomal CDT agent (BSO/Fe–GA@liposome) (Fig. 9).11 The agent gave rise to significantly enhanced cytotoxicity, attributed to an elevation in oxidative stress caused by Fe–GA-mediated Fenton reaction and L-buthionine sulfoximine (BSO)-mediated GSH depletion. After labeling this agent with 99mTc4+ radioisotope, in vivo single photon emission computed tomography (SPECT) imaging was successfully performed, and significant tumor targeting and retention in the tumor site was seen 6 h post-injection. The potential utility of the agent was demonstrated in an in vivo 4T1 tumor mouse model, in which the anti-cancer activity was presumably due to the synergistic effect via a combination of CDT, chemotherapy, and radiotherapy.
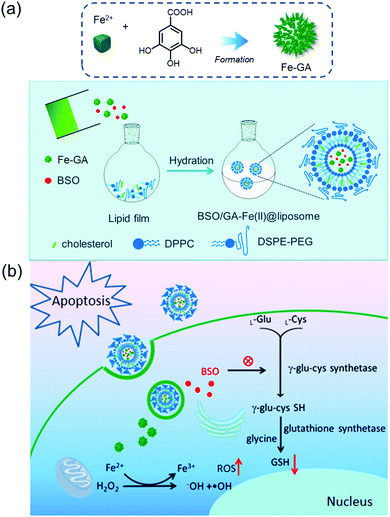 | ||
| Fig. 9 Schematic representation of (a) the synthesis, modification, and (b) the proposed mechanism of BSO/Fe–GA@liposome for combinational therapies of CDT, chemotherapy, and radiotherapy. Reprinted with permission from ref. 11. Copyright (2019) American Chemical Society. | ||
Zhang et al. studied another example of an Fe–GA complex-based system (Fe–GA/CaO2@PCM) that provides thermal-responsive enhanced CDT effect upon NIR laser irradiation (Fig. 10).12 Fe–GA/CaO2@PCM consists of an Fe–GA complex and ultra-small CaO2 encapsulated in organic phase-change materials (OPMs). Delivery of the system into cancer cells, followed by NIR laser irradiation (808 nm, 1.0 W cm−2, 20 min), released CaO2 from the Fe–GA complex. The free CaO2 then produced a large amount of Ca2+ and H2O2 in the TME; these H2O2 molecules acted to increase the ˙OH levels via Fenton reactions with the Fe–GA complex, providing a synergistic anticancer effect. This system as a theranostic agent was confirmed by in vivo photoacoustic (PA) imaging in HeLa cell-xenograft mice. The potential use of the system was verified through in vivo experiments in the same mice. The beneficial anti-cancer activity was proposed to be due to a combined CDT and PTT effect.
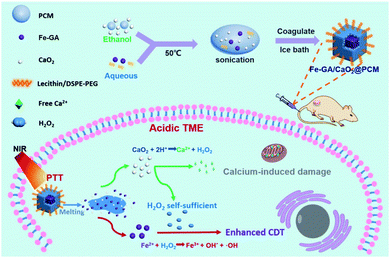 | ||
| Fig. 10 Schematic representation of the synthesis, modification, and proposed mechanism of Fe–GA/CaO2@PCM for combined CDT/PTT in cancer cells. Reproduced from ref. 12 with permission from The Royal Society of Chemistry. | ||
Cu–organic complex-based agents
The Cu+ ion-mediated Fenton-like reaction is kinetically and thermodynamically favorable in mild acidic TMEs. The highest Fenton-like reaction time for Cu+ was about 160-times faster than that of Fe2+.38,39 In this light, Cu–organic complexes are considered an attractive and potential alternative to traditional Fe-based agents.40–42 However, several factors, such as cellular antioxidants, can limit the CDT efficacy.43 In addition, residual Cu ions after treatment remain a potential concern.44,45 Their CDT activity can be improved by regulation of the CDT environment, including increased amounts of H2O2, or decreased amounts of cellular antioxidants.Peng et al. investigated the effects of Cu2+-diethyldithiocarbamate, Cu(DDC)2, in polymeric formulations and optimized the CDT/chemotherapy efficacy of the formulation in relation to their feeding ratios (Fig. 11).46 Highly stable polymeric formulations were built on a crosslinking of mPEG-block-poly(ester-carbonate) (PEC) filled with Cu2+ for effective loading of Cu(DDC)2, which was formed by bridging disulfiram (DSF) and Cu2+. In A547 tumor models, the optimized formulation (polymer/Cu(DDC)2 composition, PEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSF
DSF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1.5
Cu2+ = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed an efficiently combined Cu(DDC)2-based chemotherapy and Cu-catalyzed CDT effect, improving the anticancer outcome both in vitro and in vivo. The IC50 values were found to be 10.7 and 21.0 ng mL−1 for this polymeric formulation and Cu(DDC)2, respectively.
1) showed an efficiently combined Cu(DDC)2-based chemotherapy and Cu-catalyzed CDT effect, improving the anticancer outcome both in vitro and in vivo. The IC50 values were found to be 10.7 and 21.0 ng mL−1 for this polymeric formulation and Cu(DDC)2, respectively.
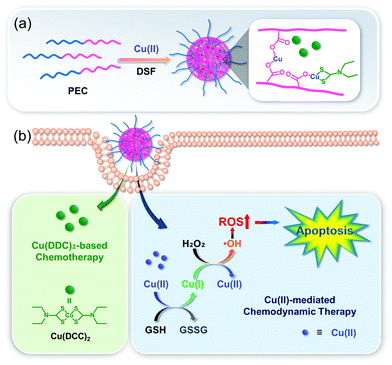 | ||
| Fig. 11 Schematic illustration of (a) the synthesis and modification and (b) the proposed mechanism of the Cu(DDC)2-loaded nanoformulation for combined chemotherapy/CDT. Reprinted with permission from ref. 46. Copyright (2019) American Chemical Society. | ||
Ma et al. prepared self-assembled Cu–cysteine nanomaterials (Cu–Cys NPs) for TME-responsive CDT (Fig. 12).43 The Cu–Cys NPs easily penetrated the tumor cells and released Cu+ from the nanoparticles through a sequence of demetallization and reduction by local GSH. The released Cu+ reacted with local H2O2 in the TME to produce ˙OH. Simultaneously, the ions acted to deplete the local GSH levels through oxidation of GSH to GSSG, significantly improving the Cu-based CDT efficacy. The Cu–Cys NPs exhibited high toxicity against cancer cells (HeLa, MCF-7, and MCF-7R), and less toxicity against normal cells (hADSCs, hbMSCs, and HK-2 cells), under identical experimental conditions. The in vivo application of Cu–Cys NPs was demonstrated in DOX-resistant MCF-7 tumor-bearing orthotopic xenograft mice, whereby Cu–Cys NPs significantly attenuated the drug-resistant breast cancer without a significant effect to the overall body weight of the mice.
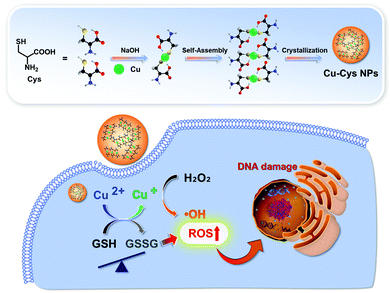 | ||
| Fig. 12 Schematic illustration of the synthesis, modification, and proposed mechanism of Cu–Cys NPs for enhanced CDT. Reprinted with permission from ref. 43. Copyright (2019) American Chemical Society. | ||
Mn–organic complex-based agents
Mn is an essential metal in the human biological system and can be applied to the composition of CDT agents for the production of ˙OH to kill tumor cells. To exhibit an efficient Mn-based CDT effect, the presence of bicarbonate (HCO3−) is indispensable.47 Because enough HCO3− for the CDT effect can be sufficiently provided from HCO3−/CO2, as one of the main physiological buffer systems, it is possible for Mn-based CDT to have excellent antitumor effects. Mn-based systems also have interesting paramagnetic properties, so they can be used in T1-MRI-guided CDT therapy. According to a recent report, a significant effect of improving CDT functionality was confirmed in a bimetallic complex in which Mn ions were combined with other Fenton metal ions, such as Cu, as described below.Cao et al. first reported a Mn–Cu bimetallic complex 1 for enhancing antitumor CDT efficacy (Fig. 13).48 Complex 1 produced ˙OH through a Fenton-like function induced by an Mn complex subunit. Simultaneously, an elevation in oxidative stress in tumor cells was induced by Cu-mediated GSH depletion. Consequently, it showed a significant dose-dependent cytotoxic effect via a combined CDT and GSH depletion effect in four cancer cells (HepG2, MCF7, A549, and 4T1), and the IC50 value of complex 1 (2.29 μM in A549 cells) was dramatically lower than that of the anti-cancer chemotherapy drug, cisplatin (28.5 μM in A549 cells).
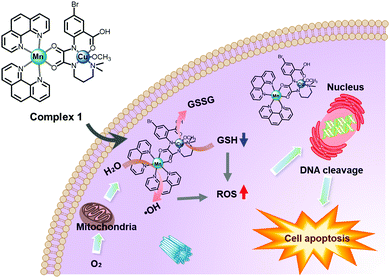 | ||
| Fig. 13 Schematic illustration of the proposed mechanism of complex 1 for CDT in cancer cells. Reproduced from ref. 48 with permission from The Royal Society of Chemistry. | ||
Currently, CDT agents based on Mn-organic complexes are very rare, except for inorganic-based agents.49 In the near future, the use of Mn in CDT is expected to lead to the development of important new materials in the field of cancer theranostics.
Co–organic complex-based agents
Co can perform redox reactions in biological systems and often acts as a Fenton-like agent for converting endogenous H2O2 into cytotoxic ˙OH in the presence of bicarbonate (HCO3−), inducing an anticancer effect.50 As excess free Co ions may mediate severe biosystemic toxicity in normal cells, the use of Co should be tightly controlled to allow for precise targeting only at the tumor site. However, the use of Co–organic complex-based agents remains attractive, and we have summarized the efforts to advance Co–organic complexes for utilization in CDT.ZIF-67 is a typical Co-based MOF composed of Co2+ and 2-methylimidazole ligands. It has unique features, such as a tunable porous structure, multiple catalytic centers, and highly stable composition.51 In 2019, ZIF-67 was used by Gao et al. to fabricate the catalytic framework, CaO2@DOX@ZIF-67 (Fig. 14).52 This framework, designed to achieve pH-responsive tumor targeting, was found to provide a good therapeutic effect in accordance with a combined chemotherapy/CDT function. In weakly acidic TMEs, it decomposed into Co2+ and DOX via a pH responsive reaction, which induced CDT and chemotherapy processes, respectively. The CaO2 included in this framework reacts with H2O to facilitate the generation of O2 and H2O2, thereby achieving an improved therapeutic result for hypoxic tumors. In MCF-7 tumor models, the framework showed excellent therapeutic performance both in vitro and in vivo, with no obvious effect of significant long-term toxicity.
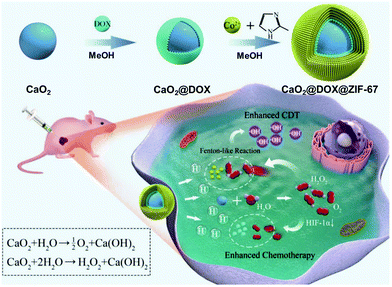 | ||
| Fig. 14 Schematic illustration of the synthesis, modification, and proposed mechanism of CaO2@DOX@ZIF-67 for combined chemotherapy/CDT in cancer cells. Reproduced with permission from ref. 52. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In another report, Sang et al. demonstrated the cancer treatment potential of ZIF-67-consisting multifunctional material, PZIF67-AT, for intensive CDT (Fig. 15).53 In this case, 3-amino-1,2,4-triazole (3-AT) as a catalase inhibitor, was modified on a ZIF-67 framework that was further coated with a PEG moiety. The material has SOD like activity, which reacted with endogenous O2˙− to facilitate the generation of H2O2 in the tumor site. At the same time, the material decomposed into 3-AT under the weakly acidic conditions, which protected against the elimination of H2O2 by the enzyme catalase. Simultaneously, an elevation in oxidative stress in tumor cells was mediated in part by ZIF-67-catalyzed GSH oxidation. Similar and significant cytotoxicity in three cancer cell lines (HeLa, A549, and 4T1) was observed, whereas reduced tumor volume in H22 xenograft tumor models was confirmed, with no other adverse effects. These benefits were believed to be due to a combination of SOD-like activity, catalase inhibition, and GSH depletion.
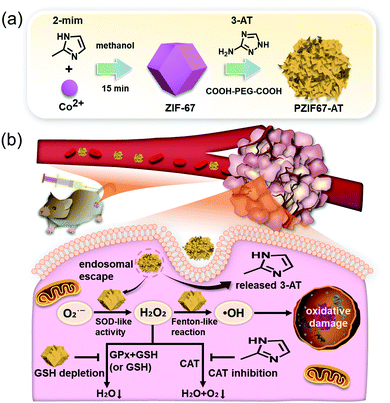 | ||
| Fig. 15 Schematic illustration of the synthesis, modification, and proposed mechanism of PZIF67-AT for intensive CDT in cancer cells. Reprinted with permission from ref. 53. Copyright (2020) American Chemical Society. | ||
Concluding remarks
Considerable progress in the field of metal–organic complex-based CDT agents has been made in recent years. A number of metal–organic complexes, including NH2-MIL-88B(Fe), MIL-100(Fe), Fe–gallic acid, Cu–cysteine, Cu–PEC, ZIF-67(Co), ferrocene, hemin, and Mn–Cu-phenanthroline, have been identified, which exhibit good chemodynamic effects. Compared to the more widely studied inorganic nanoparticles for CDT applications, metal–organic complex-based CDT agents offer considerable benefits, such as high catalytic activity, improved safety, and a tighter control over the overall structure. It is thus anticipated that the agents will provide new insights into the development of novel tumor therapies with potential clinical application.The appealing CDT strategy involves the Fenton process, by which Fenton- (Fe2+) or Fenton like-metal ions (Cu+, Mn2+, and Co2+) released from metal–organic complexes react with endogenous H2O2 to convert it to highly cytotoxic ˙OH in tumor sites, producing an anticancer effect. Among the agents discussed in this feature article, most CDT agents are based on Fe-containing complexes, such as Fe–organic frameworks, ferrocenes, hemins, and Fe–polyphenols. However, in order to proceed to clinical application, appropriate strategies to improve the catalytic rate in the TME are necessary, as they show optimal catalytic activity under strong acidic conditions (pH 2 to 4). In recent years, extensive research has been devoted to alternative or complementary CDT agents, including Cu-containing complexes (Cu–PECs, Cu–cysteines) on account of their superior Fenton-like activity under mild acidic conditions like the TME. Mn- and Co-containing complexes have also been reported, and they will provide meaningful and challenging insights that may facilitate future developments. However, further research is necessary to mitigate limitations associated with CDT, such as adverse cytotoxicity by the toxic metals leftover after the treatment and uncertainty in terms of their bioapplicability. Other agents are very rare and are still in the early stages of development.
As highlighted above, researchers have employed these types of CDT agents to enhance the therapeutic effects and safety through varying approaches, such as enhanced tumor targeting, stimuli-responsive catalyst delivery, combination with different cancer therapies, regulation of the reaction condition, and stimulation by exogenous energy. Moreover, multimodal approaches allow for the theranostic combination of CDT with fluorescence, SPECT, MR, or CL imaging.
The development of metal–organic complex-based CDT agents is a comparatively young field, and no established clinical uses exist for these agents, thus far. To direct these findings to clinical use, several obstacles need to be overcome. Further detailed mechanistic studies with these agents, as well as efforts to amplify the CDT efficiency and cellular susceptibility to ROS, will render this approach viable. Additionally, further investigations to optimize the delivery to appropriate regions, as well as appropriately conceived combination strategies with other modalities (therapeutic or diagnostic), would be greatly beneficial. In the course of the next years, the utility of these avenues will likely be the subject of intense study.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Hyupsung University Research Grant of 2019.Notes and references
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS PubMed.
- H. Ranji-Burachaloo, P. A. Gurr, D. E. Dunstan and G. G. Qiao, ACS Nano, 2018, 12, 11819–11837 CrossRef CAS.
- J. C. Reed and M. Pellecchia, Cell, 2012, 149, 963–965 CrossRef CAS PubMed.
- M. Li, H. Zhang, Y. Hou, X. Wang, C. Xue, W. Li, K. Cai, Y. Zhao and Z. Luo, Nanoscale Horiz., 2020, 5, 202–217 RSC.
- B. Kumar, S. Koul, L. Khandrika, R. B. Meacham and H. K. Koul, Cancer Res., 2008, 68, 1777–1785 CrossRef CAS PubMed.
- Q. Chen, D. Yang, L. Yu, X. Jing and Y. Chen, Mater. Horiz., 2020, 7, 317–337 RSC.
- Z. Shen, J. Song, B. C. Yung, Z. Zhou, A. Wu and X. Chen, Adv. Mater., 2018, 30, 1704007 CrossRef PubMed.
- A. Casas, C. Perotti, G. Di Venosa and A. Batlle, Mechanisms of resistance to photodynamic therapy: an update, ed. V. Rapozzi and G. Jori, Springer, Switzerland, 2015, pp. 29–63 Search PubMed.
- S.-J. Choi, J. K. Lee, J. Jeong and J.-H. Choy, Mol. Cell. Toxicol., 2013, 9, 205–210 CrossRef CAS.
- H. Xiang, W. Feng and Y. Chen, Adv. Mater., 2020, 32, 1905994 CrossRef CAS PubMed.
- Z. Dong, L. Feng, Y. Chao, Y. Hao, M. Chen, F. Gong, X. Han, R. Zhang, L. Cheng and Z. Liu, Nano Lett., 2019, 19, 805–815 CrossRef.
- S. Zhang, C. Cao, X. Lv, H. Dai, Z. Zhong, C. Liang, W. Wang, W. Huang, X. Song and X. Dong, Chem. Sci., 2020, 11, 1926–1934 RSC.
- M. Hermanek, R. Zboril, I. Medrik, J. Pechousek and C. Gregor, J. Am. Chem. Soc., 2007, 129, 10929–10936 CrossRef CAS PubMed.
- B. Wang, J.-J. Yin, X. Zhou, I. Kurash, Z. Chai, Y. Zhao and W. Feng, J. Phys. Chem. C, 2013, 117, 383–392 CrossRef CAS.
- T. Xue, C. Xu, Y. Wang, Y. Wang, H. Tian and Y. Zhang, Biomater. Sci., 2019, 7, 4615–4623 RSC.
- W. Xuan, Y. Xia, T. Li, L. Wang, Y. Liu and W. Tan, J. Am. Chem. Soc., 2020, 142, 937–944 CrossRef CAS PubMed.
- J. L. Arias, Mini-Rev. Med. Chem., 2011, 11, 1–17 CrossRef CAS PubMed.
- X. Liu, Y. Sang, H. Yin, A. Lin, Z. Guo and Z. Liu, MOJ Eco. Environ. Sci., 2018, 3, 00060 Search PubMed.
- M. Munoz, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2015, 176–177, 249–265 CrossRef CAS.
- V. Trujillo-Alonso, E. C. Pratt, H. Zong, A. Lara-Martinez, C. Kaittanis, M. O. Rabie, V. Longo, M. W. Becker, G. J. Roboz, J. Grimm and M. L. Guzman, Nat. Nanotechnol., 2019, 14, 616–622 CrossRef CAS PubMed.
- K. Ni, T. Aung, S. Li, N. Fatuzzo, X. Liang and W. Lin, Chem, 2019, 5, 1892–1913 CAS.
- H. Ranji-Burachaloo, F. Karimi, K. Xie, Q. Fu, P. A. Gurr, D. E. Dunstan and G. G. Qiao, ACS Appl. Mater. Interfaces, 2017, 9, 33599–33608 CrossRef CAS PubMed.
- J. He, Y. Zhang, X. Zhang and Y. Huang, Sci. Rep., 2018, 8, 5159 CrossRef PubMed.
- H. Ranji-Burachaloo, Q. Fu, P. A. Gurr, D. E. Dunstan and G. G. Qiao, Aust. J. Chem., 2018, 71, 826–836 CrossRef CAS.
- V. N. Babin, Yu. A. Belousov, V. I. Borisov, V. V. Gumenyuk, Yu. S. Nekrasov, L. A. Ostrovskaya, I. K. Sviridova, N. S. Sergeeva, A. A. Simenel and L. V. Snegur, Russ. Chem. Bull., 2014, 63, 2405–2422 CrossRef CAS.
- H. Hagen, P. Marzenell, E. Jentzsch, F. Wenz, M. R. Veldwijk and A. Mokhir, J. Med. Chem., 2012, 55, 924–934 CrossRef CAS PubMed.
- Z. Lei, X. Zhang, X. Zheng, S. Liu and Z. Xie, Org. Biomol. Chem., 2018, 16, 8613–8619 RSC.
- S. Lei, J. Chen, K. Zeng, M. Wang and X. Ge, Nano Res., 2019, 12, 1071–1082 CrossRef CAS.
- T. Xue, S. Jiang, Y. Qu, Q. Su, R. Cheng, S. Dubin, C.-Y. Chiu, R. Kaner, Y. Huang and X. Duan, Angew. Chem., Int. Ed., 2012, 51, 3822–3825 CrossRef CAS PubMed.
- B. Jiang, Y. Yao, R. Xie, D. Dai, W. Lu, W. Chen and L. Zhang, Appl. Catal., B, 2016, 183, 291–297 CrossRef CAS.
- Z. Li, B. Tian, W. Zhen, Y. Wu and G. Lu, Appl. Catal., B, 2017, 203, 408–415 CrossRef CAS.
- D. Nowis, M. Legat, T. Grzela, J. Niderla, E. Wilczek, G. M. Wilczynski, E. Głodkowska, P. Mrówka, T. Issat, J. Dulak, A. Józkowicz, H. Waś, M. Adamek, A. Wrzosek, S. Nazarewski, M. Makowski, T. Stokłosa, M. Jakóbisiak and J. Gołab, Oncogene, 2006, 25, 3365–3374 CrossRef CAS.
- Y. Yao, Y. Mao, Q. Huang, L. Wang, Z. Huang, W. Lu and W. Chen, J. Hazard. Mater., 2014, 264, 323–331 CrossRef CAS PubMed.
- H. Yi, M. Jiang, D. Huang, G. Zeng, C. Lai, L. Qin, C. Zhou, B. Li, X. Liu, M. Cheng, W. Xue, P. Xu and C. Zhang, J. Taiwan Inst. Chem. Eng., 2018, 93, 184–192 CrossRef CAS.
- Y. Wang, L. Shi, Z. Ye, K. Guan, L. Teng, J. Wu, X. Yin, G. Song and X.-B. Zhang, Nano Lett., 2020, 20, 176–183 CrossRef CAS PubMed.
- H. Chen and Y. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 21021–21034 CrossRef CAS PubMed.
- Q. Jin, W. Zhu, D. Jiang, R. Zhang, C. J. Kutyreff, J. W. Engle, P. Huang, W. Cai, Z. Liu and L. Cheng, Nanoscale, 2017, 9, 12609–12617 RSC.
- E. Brillas, M. A. Baños, S. Camps, C. Arias, P.-L. Cabot, J. A. Garrido and R. M. Rodríguez, New J. Chem., 2004, 28, 314–322 RSC.
- T. Soltani and B.-K. Lee, Chem. Eng. J., 2017, 313, 1258–1268 CrossRef CAS.
- L. Cheng, W. He, H. Gong, C. Wang, Q. Chen, Z. Cheng and Z. Liu, Adv. Funct. Mater., 2013, 23, 5893–5902 CrossRef CAS.
- C. Yue, P. Liu, M. Zheng, P. Zhao, Y. Wang, Y. Ma and L. Cai, Biomaterials, 2013, 34, 6853–6861 CrossRef CAS PubMed.
- S. Luo, X. Tan, S. Fang, Y. Wang, T. Liu, X. Wang, Y. Yuan, H. Sun, Q. Qi and C. Shi, Adv. Funct. Mater., 2016, 26, 2975 CrossRef.
- B. Ma, S. Wang, F. Liu, S. Zhang, J. Duan, Z. Li, Y. Kong, Y. Sang, H. Liu, W. Bu and L. Li, J. Am. Chem. Soc., 2019, 141, 849–857 CrossRef CAS PubMed.
- J. Zhang, Z. Liu, P. Lian, J. Qian, X. Li, L. Wang, W. Fu, L. Chen, X. Wei and C. Li, Chem. Sci., 2016, 7, 5995–6005 RSC.
- P. F. Gordon and P. Gregory, Organic Chemistry in Colour, Springer-Verlag, Berlin, Heidelberg and London, 1983 Search PubMed.
- X. Peng, Q. Pan, B. Zhang, S. Wan, S. Li, K. Luo, Y. Pu and B. He, Biomacromolecules, 2019, 20, 2372–2383 CrossRef CAS PubMed.
- A. Xu, K. Shao, W. Wu, J. Fan, J. Cui and G. Yin, Chin. J. Catal., 2010, 31, 1031–1036 CAS.
- S. Cao, J. Fan, W. Sun, F. Li, K. Li, X. Tai and X. Peng, Chem. Commun., 2019, 55, 12956–12959 RSC.
- C. Liu, D. Wang, S. Zhang, Y. Cheng, F. Yang, Y. Xing, T. Xu, H. Dong and X. Zhang, ACS Nano, 2019, 13, 4267–4277 CrossRef CAS PubMed.
- L. Leyssens, B. Vinck, C. Van Der Straeten, F. Wuyts and L. Maes, Toxicology, 2017, 387, 43–56 CrossRef CAS PubMed.
- G. Zhong, D. Liu and J. Zhang, J. Mater. Chem. A, 2018, 6, 1887–1899 RSC.
- S. Gao, Y. Jin, K. Ge, Z. Li, H. Liu, X. Dai, Y. Zhang, S. Chen, X. Liang and J. Zhang, Adv. Sci., 2019, 6, 1902137 CrossRef CAS PubMed.
- Y. Sang, F. Cao, W. Li, L. Zhang, Y. You, Q. Deng, K. Dong, J. Ren and X. Qu, J. Am. Chem. Soc., 2020, 142, 5177–5183 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |