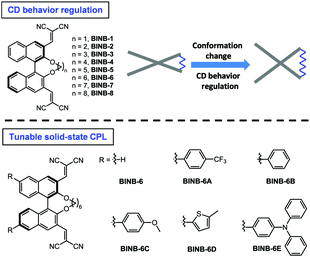
Regulation of circular dichroism behavior and construction of tunable solid-state circularly polarized luminescence based on BINOL derivatives†
Na
Zhao
 *a,
Wangwang
Gao‡
a,
Min
Zhang‡
a,
Junfang
Yang
b,
Xiaoyan
Zheng
*a,
Wangwang
Gao‡
a,
Min
Zhang‡
a,
Junfang
Yang
b,
Xiaoyan
Zheng
 *b,
Yue
Li
a,
Rongrong
Cui
a,
Wei
Yin
a and
Nan
Li
*b,
Yue
Li
a,
Rongrong
Cui
a,
Wei
Yin
a and
Nan
Li
 *a
*a
aKey Laboratory of Macromolecular Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry of Ministry of Education, Key Laboratory of the Ministry of Education for Medicinal Resources and Natural Pharmaceutical Chemistry, and School of Chemistry & Chemical Engineering, Shaanxi Normal University, 710119 Xi’an, China. E-mail: nzhao@snnu.edu.cn; nli@snnu.edu.cn
bBeijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Key Laboratory of Cluster Science of Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, 100081 Beijing, China. E-mail: xiaoyanzheng@bit.edu.cn
First published on 4th June 2019
Abstract
The development of chiral fluorescent materials has attracted great interest. [1,1′-binaphthalene]-2,2′-diol (BINOL), a privileged C2-symmetric molecular skeleton, is one of the most frequently used chiral optical backbones. However, a phenomenon of circular dichroism (CD) annihilation in the aggregated state was observed in BINOL based molecules, which limits the application of CD in the condensed state. In this work, by inserting various bridged alkyl chains into the hydroxyl groups of 3,3′-dicyanomethylene functionalized (R)-BINOL, a series of chiral luminogens (BINB-n, n = 1 to 8) was obtained. All BINB-n luminogens exhibited aggregation-induced emission (AIE) characteristics and their molecular conformation was significantly dependent on the length of the alkyl chain, which further regulated their CD behavior from annihilation to preservation in the aggregated state. Moreover, in virtue of the AIE characteristic of BINB-6, judiciously introducing suitable electron donors successfully realized enhanced and tunable solid-state circularly polarized luminescence with the maximum glum value of 1 × 10−2.
Introduction
Chirality is a ubiquitous phenomenon in nature. Chiral molecules play crucial roles in most fundmental processes including the synthesis of deoxyribonucleic acid (DNA) or proteins, exploring new drug candidates and developing chiral functional materials.1 Unlike the significant progress of chiral molecules made in biological science and the pharmaceutical industry, their applications in the field of material science, especially in the fabrication of chiral organic materials, are relatively less investigated. Indeed, owing to their distinct optical information and angular independence, chiral organic materials are of pivotal importance for opto-electronic applications and chiral recognition processes.2–4 In order to obtain fine chiral information to clarify the working mechanism and further optimize the performance of chiral materials, various optical methods have been established. Among these methods, circular dichroism (CD) is without doubt the most commonly used technique.5 CD spectrometry, which originated from ground-state electronic transition of π-conjugated chromophores, is extremely sensitive and accurate towards the chirality variations. Benefited by CD spectroscopy, the chirality information of chiral materials in different molecular states can be readily obtained.[1,1′-Binaphthalene]-2,2′-diol (BINOL), one of the most privileged C2-symmetric molecules, was employed as an ideal chiral source to fabricate chiral organic materials.6 In recent years, an abnormal effect termed aggregation-annihilated CD (AACD) was reported in BINOL based molecules. Further studies revealed that the variation of the torsion angle (θ) between two naphthalene rings after molecular aggregation is responsible for this AACD behavior.7 The AACD phenomenon obstructed the application of CD in the aggregated state. Some elegant strategies were reported recently to surpass the annihilation of CD in BINOL derived molecules.7a,8 Nevertheless, precisely regulating the CD response in the process of aggregation through rational molecular design and synthesis is still a formidable challenge.
In addition, circularly polarized luminescence (CPL) is another key parameter of chiral materials which provides the excited-state chiral information. The CPL active materials, especially solid-state CPL materials have potential applications in the fields of 3D organic light-emitting diodes and optical data recording devices.9–14 Recently, a concept of aggregation-induced emission (AIE) has opened up great opportunities to develop solid-state CPL materials.15 For instance, naturally chiral auxiliaries such as amino acids, sugars, and lipids were decorated into achiral AIE luminogens (AIEgens) to obtain an enhanced CPL response in the condensed state.16 Meanwhile, axial BINOL was used as a chiral source to construct solid-state CPL materials.17 Beside superior chiral characteristics, BINOL also possess excellent luminescence properties due to its favorable π-conjugated structure and most of the reported studies focused on its CPL properties in the solution state.18 In contrast, development of enhanced and tunable solid-state CPL materials using BINOL as a chiral fluorescent skeleton is still rare.
Herein, by inserting various bridged alkyl chains into the hydroxyl groups of 3,3′-dicyanomethylene functionalized (R)-BINOL, a series of chiral luminogens (BINB-n, n = 1 to 8) was obtained (Fig. 1). All BINB-n exhibited typical AIE features. Meanwhile, the molecular conformation of BINB-n was successfully controlled by the length of the alkyl chain, which resulted in the CD behavior converted from annihilation to preservation. Combining the experimental result with molecular dynamic simulation, the molecular conformation of BINB-n significantly influenced the change of θ during the aggregation process, and further affected their CD behaviors. Moreover, thanks to the AIE characteristic, introducing various electron donors into BINB-6 successfully yielded enhanced and tunable solid-state CPL.
Results and discussion
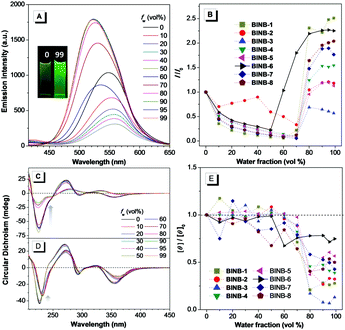
Following the molecular design concept, we began our investigation by introducing bridged alkyl chains with different lengths into the hydroxyl groups of 3,3′-dicyanomethylene functionalized (R)-BINOL to form a series of bridged structures termed as BINB-n (n = 1 to 8). All luminogens displayed a higher absorption peak at approximately 280 nm in acetonitrile solution, which can be attributed to the π–π* transition. The longest absorption band at around 340 nm was observed, which can be ascribed to intramolecular charge transfer (ICT) transition (Fig. S1A, ESI†). Meanwhile, a remarkable solvent effect was observed for BINB-6 as an example, which further confirmed their ICT characteristics (Fig. S1D, ESI†).19 After adding water into the acetonitrile solution, the emission intensity of all luminogens initially decreased owing to the intensive ICT process, but later increased and reached a maximum when the water fraction (fw) was up to 99%, demonstrating their typical AIE feature (Fig. 2A, B and Fig. S5, ESI†). The AIE effect was mainly attributed to the restriction of the free motion of dicyanomethylene groups.7b It is noteworthy that the emission in the aggregated state exhibited a blue shift compared to that in the solution state, which could be caused by the reduction of polarity inside the aggregates. Molecular aggregation was further confirmed by dynamic light scattering measurement (Fig. S8, ESI†).Because of the identical absolute configuration of BINOL, similar Cotton effects were observed for BINB-1 to BINB-8 at 230, 270, 320 and 350 nm in their solution state (Fig. 2C and Fig. S11, ESI†). The strong Cotton effects in the wavelength range from 230 to 320 nm can be attributed to the characteristic absorption of chiral binaphthyl moieties, while the CD peak at 350 nm originated from the whole molecular chirality.20 By increasing fw, the bridged chiral AIEgens presented distinct changes in CD response. Taking BINB-2 and BINB-6 as examples (Fig. 2C–E), almost no significant change in the CD spectra was detected when fw was less than 60%. However, with continuously increasing fw, the change of CD response exhibited a different tendency. For BINB-2, the CD signal dramatically decreased and approximately 25% intensity remained at 230 nm with the fw up to 99%. The decrease in CD response became weaker for BINB-6 while the CD intensity maintained more than 75% even at fw of 99%. Other BINB derivatives also displayed significantly alkyl chain dependent CD behavior with either preservation or annihilation. As described in Fig. 2E, BINB-6 displayed the utmost capability to preserve the CD intensity in the aggregated state. This remarkable conversion of CD behavior proved that the alkyl chain length can serve as an efficient way to modulate the CD property of chiral AIEgens in the process of molecular aggregation.
On the other hand, unbridged substituents with different steric hindrance, including methyl, tertbutyldimethylsilyl and tertbutyldiphenylsilyl groups, were introduced to generate BINUB-1 to BINUB-3 in order to investigate the influence of steric hindrance on the CD behavior (Scheme S3, ESI†). A similar AIE phenomenon was observed for the three unbridged luminogens (Fig. S2A, B, S6 and S9, ESI†). However, the CD signal gradually decreased with increasing fw, while the intensity at 230 nm dropped more than 55% at fw of 99% (Fig. S2C, D and S12, ESI†). This result suggested that, due to the flexible nature of the unbridged BINOL backbone, varying the degree of steric hinderance of the unbridged groups cannot effectively restrain the CD annihilation effect.
To determine the underlying reasons for the special optical behavior of these bridged chiral AIEgens, single crystals of the corresponding AIEgens were grown by slow evaporation from a suitable mixture of solvents. All single crystals of BINB-n were successfully obtained except BINB-3 and BINB-6 owing to their poor crystallization ability. Fortunately, a single crystal of the BINB-6 derivative that bears a trifluoromethylphenyl group at the 6,6′-position (BINB-6A), was obtained.21 The single crystal data are summarized in Tables S6 and S7 (ESI†). As shown in Fig. 3 and Fig. S14–S20 (ESI†), all corresponding molecules adopted highly nonplanar configurations inherited from BINOL while multiple intermolecular C–H⋯N interactions (2.472–2.645 Å) were found throughout most of the crystal lattice. The twisted structure combined with weak intermolecular interactions helped to rigidify the molecular conformation, prevented the molecules from forming π–π or dipole–dipole interactions, and endowed BINB-n with high solid-state emission efficiency.
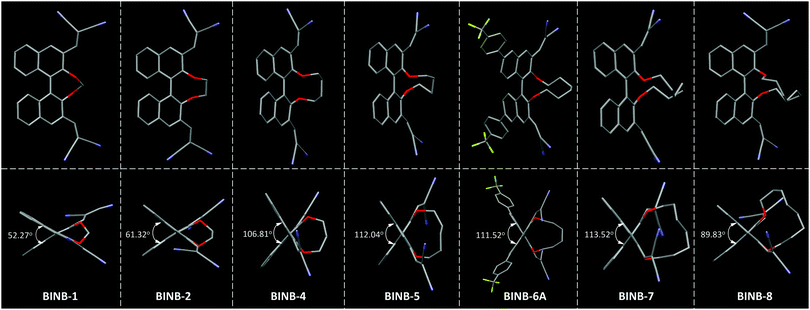 | ||
| Fig. 3 The view of (top) molecular conformation and (bottom) θ value for the corresponding BINB derivatives. Hydrogen atoms are omitted for clarity. | ||
Further analysis of the molecular conformation revealed that the different lengths of alkyl chain at the 2,2′-position of BINB-n generated various θ values in the crystal. From BINB-1 to BINB-4, the θ value increased from 52.27 to 106.81°. By extending the alkyl chain from n-pentyl to n-heptyl (BINB-5 to BINB-7), the θ value showed little difference which was more than 111° (111.52 to 113.52°). However, continuously prolonging the length of the alkyl chain (BINB-8), the θ value decreased to 89.83°. To understand the origin of alkyl chain-dependent CD behavior, molecular dynamics (MD) simulation of chiral AIEgens in a molecularly dissolved state was carried out by using BINB-2 and BINB-6A as representative examples (Fig. S21, ESI†). The simulation results indicated that the θ value was 71.00° and 111.00° for BINB-2 and BINB-6A in their dissolved state. The change of θ value between the dissolved and crystal state was 9.68° and 0.52° for BINB-2 and BINB-6A, respectively. Clearly, the variation of the θ value for BINB-6A is smaller than that of BINB-2, which resulted in the preservation of the CD response of BINB-6A rather than BINB-2 in their aggregated state.8,22
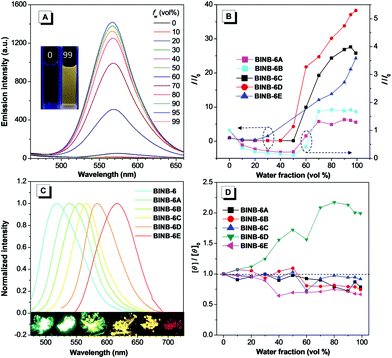
Since BINB-6 exhibited a relatively notable AIE effect, we expected to construct chiral AIEgens with tunable emission by introducing different donors into BINB-6 as suitable donor–acceptor systems can sufficiently control the separation of the highest occupied molecular orbital and lowest unoccupied molecular orbital.19,23 The electron-donating potential increased in the following order: 4-(trifluoromethyl)phenyl, phenyl, 4-methoxyphenyl, 5-methylthiophenyl, and 4-(diphenylamino)phenyl, which formed a series of chiral AIEgens (BINB-6A to BINB-6E). The ICT absorption bands exhibited typical bathochromic-shift from 340 to 350 nm, which is in line with the electron donating ability. Meanwhile, the longest CD response also shifted from 340 to 375 nm in the order from BINB-6 to BINB-6E. Importantly, the installed different electron donor has negligible influence on CD behavior since the CD intensity of BINB-6 derivatives is preserved in the process of aggregation. As shown in Fig. 4D and Fig. S13 (ESI†), the CD signals at around 260 nm for BINB-6 derivatives maintained more than 75% at fw of 99%. It is interesting that 2-fold enhancement of the CD intensity was observed for BINB-6D, which could be associated with its well-ordered aggregates.24
All BINB-6 derivatives exhibited enhanced emission in their aggregated state (Fig. 4A, B and Fig. S6, S9, ESI†). Meanwhile, their solid-state quantum yields were higher than that in solution, which is consistent with their AIE feature (Table 1). Owing to the gradual strengthening of the electron-donating ability, the significant red-shift in solid-state emission ranging from 522 to 623 nm was observed (Fig. 4C). The CIE chromaticity diagrams (Fig. S3 and Table S1, ESI†) demonstrated the same tunable emission color from green CIE (0.2634, 0.6195) to red CIE (0.646, 0.3536).
| Compd. | Solution statea | Solid state | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λ abs [nm] | λ em [nm] | ΦFb [%] | τ avg [ns] | λ em [nm] | ΦFb [%] | τ avg [ns] | λ CPL [nm] | g lum (10−3) | |
| a Acetonitrile solution (10 μM). b Absolute fluorescence quantum yield measured using the calibrated integrating sphere system. c Mean fluorescence lifetime (τavg) calculated using the equation τavg = A1τ1 + A2τ2. d Self-assembled microstructure. e No CPL was detected due to its microparticle form. | |||||||||
| BINB-6 | 338 | 547 | 8.6 | 4.98 | 518 | 11.2 | 5.38 | 547 | 0.6 |
| BINB-6A | 338 | 555 | 9.2 | 5.03 | 538 | 9.5 | 4.85 | 536 | −5.0 |
| BINB-6B | 342 | 562 | 6.6 | 4.35 | 555 | 7.9 | 6.34 | 557 | 4.0 |
| BINB-6C | 345 | 575 | 0.5 | 0.99 | 566 | 6.8 | 7.54 | 580 | 1.0 |
| BINB-6D | 338 | 577 | 0.7 | 3.59 | 584 | 4.0 | 6.15 | 597 | −10.0 |
| BINB-6E | 350 | 623 | 0.3 | 4.35 | 617 | 0.7 | 9.06 | —e | —e |
We further investigated the solid-state CPL properties of BINB-6 derivatives due to their AIE characteristics. Self-assembly of chiral organic molecules was reported to efficiently amplify the CPL signal because of the chirality transfer from small molecules to the self-assembled network.15d,16c,25 Therefore, we prepared self-assembled samples of BINB-6 derivatives on a glass substrate by slowly evaporating the solvent (acetonitrile or dichloromethane). As shown in Fig. 5, the scanning electron microscope (SEM) images revealed that all BINB-6 derivatives successfully self-organized into microstructures with different shapes except for BINB-6E which formed microparticles. Detailed morphological analysis indicated that irregular tiny microslices and microneedles were formed for BINB-6 and BINB-6A, while BINB-6B and BINB-6C assembled into microrods. The microflowers constituted by secondary microplates were observed for BINB-6D. Under the confocal fluorescence microscope, all self-assembled microstructures displayed intense emission. At the same time, the emission color ranged from green to orange (520 to 580 nm) following the order from self-assembled BINB-6 to BINB-6D (Fig. S4, ESI†).
 | ||
| Fig. 5 The SEM images (A–E) and confocal fluorescent images (F–J) of self-assembled BINB-6 to BINB-6D. Scale bar for F–J: 20 μm. | ||
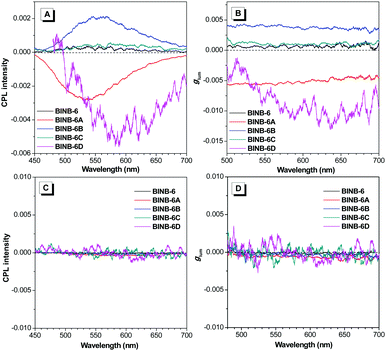
As expected, all self-assembled chiral AIEgens emitted CPL upon photoexcitation. As shown in Fig. 6A, the CPL peaks varied from 520 to 610 nm following the order from BINB-6 to BINB-6D, which cover the region from green to red. The magnitude of CPL was evaluated by the luminescence dissymmetry factor (glum), which is defined as glum = 2 × (IL − IR)/(IL + IR), where IL and IR refer to the intensity of left- and right-handed CPL, respectively. It is noted that the glum factor of all self-assembled AIEgens was greater than 10−4, and the maximum glum factor can reach up to 1 × 10−2 for BINB-6D (Fig. 6B). In sharp contrast, almost no CPL response was detected in their dissolved state while nearly zero glum was obtained (Fig. 6C and D). Intriguingly, BINB-6A and BINB-6D gave opposite CPL signals compared with those of BINB-6, BINB-6B and BINB-6C, which could be attributed to their different macroscopic chirality when they underwent a self-assembly process.
Conclusions
In conclusion, we developed a strategy to regulate CD behavior in the process of molecular aggregation through controlling the molecular conformation. By inserting different alkyl chains into the hydroxyl groups of BINOL derivatives, a series of chiral AIEgens (BINB-n) with different conformations was obtained. The conversion of the CD response from annihilation to preservation for BINOL derivatives was observed. Combining single crystal analysis and theoretical simulation, the different CD behavior was associated with their varied molecular conformation, which further influences the variation of θ value in the process of molecular aggregation. In addition, introducing diverse electron donors into the molecular backbone of BINB-6 yielded a series of chiral AIEgens with tunable emission. Moreover, these chiral AIEgens exhibited enhanced and tunable CPL after self-assembly. The maximum glum value reached up to 1 × 10−2. This work provides a general method to regulate the CD behavior for chiral luminogens as well as a facile approach to construct tunable solid-state CPL materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate State Key Laboratory of Luminescent Materials and Devices for circularly polarized luminescence acquisition. This work was supported by the National Natural Science Foundation of China (21672135, 51403122 and 21402115), the Fundamental Research Funds for the Central Universities (GK201902006 and GK201702002), the Natural Science Foundation of Shaanxi Province (2018JM5086) and Funded Projects for the Academic Leaders and Academic Backbones, Shaanxi Normal University (18QNGG007).Notes and references
- (a) J. E. Hein and D. G. Blackmond, Acc. Chem. Res., 2012, 45, 2045–2054 CrossRef CAS PubMed; (b) Z. Wang, W. Xu, L. Liu and T. F. Zhu, Nat. Chem., 2016, 8, 698–704 CrossRef CAS PubMed; (c) W. H. Brooks, W. C. Guida and K. G. Daniel, Curr. Top. Med. Chem., 2011, 11, 760–770 CrossRef CAS PubMed; (d) J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 0045 CrossRef CAS; (e) S. R. LaPlante, L. D. Fader, K. R. Fandrick, D. R. Fandrick, O. Hucke, R. Kemper, S. P. F. Miller and P. J. Edwards, J. Med. Chem., 2011, 54, 7005–7022 CrossRef CAS PubMed; (f) H. Xu, S. Ding, W. An, H. Wu and W. Wang, J. Am. Chem. Soc., 2016, 138, 11489–11492 CrossRef CAS PubMed.
- (a) J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1–16 CrossRef CAS; (b) G. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo and S. Abbate, Chirality, 2016, 28, 696–707 CrossRef CAS PubMed.
- (a) M. Li, S. H. Li, D. D. Zhang, M. H. Cai, L. Duan, M. K. Fung and C. F. Chen, Angew. Chem., Int. Ed., 2018, 57, 2889–2893 CrossRef CAS PubMed; (b) C. Liu, D. Yang, Q. Jin, L. Zhang and M. H. Liu, Adv. Mater., 2016, 28, 1644–1649 CrossRef CAS PubMed.
- (a) L. Pu, Acc. Chem. Res., 2017, 50, 1032–1040 CrossRef CAS PubMed; (b) X. Zhang, J. Yin and J. Yoon, Chem. Rev., 2014, 114, 4918–4959 CrossRef CAS PubMed; (c) L. Pu, Chem. Rev., 2004, 104, 1687–1716 CrossRef CAS PubMed.
- (a) C. Wolf and K. W. Bentley, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC; (b) H. H. Jo, C. Y. Lin and E. V. Anslyn, Acc. Chem. Res., 2014, 47, 2212–2221 CrossRef CAS PubMed; (c) G. Pescitelli, L. Di Bari and N. Berova, Chem. Soc. Rev., 2011, 40, 4603–4625 RSC; (d) N. Berova, L. Di Bari and G. Pescitelli, Chem. Soc. Rev., 2007, 36, 914–931 RSC.
- J. M. Brunel, Chem. Rev., 2007, 107, PR1–PR45 CrossRef CAS.
- (a) H. K. Zhang, H. K. Li, J. Wang, J. Z. Sun, A. J. Qin and B. Z. Tang, J. Mater. Chem. C, 2015, 3, 5162–5166 RSC; (b) N. Li, H. L. Feng, Q. Gong, C. X. Wu, H. Zhou, Z. Y. Huang, J. Yang, X. H. Chen and N. Zhao, J. Mater. Chem. C, 2015, 3, 11458–11463 RSC.
- H. K. Zhang, X. Zheng, R. T. K. Kwok, J. Wang, N. L. C. Leung, L. Shi, J. Z. Sun, Z. Y. Tang, J. W. Y. Lam, A. J. Qin and B. Z. Tang, Nat. Commun., 2018, 9, 4961 CrossRef PubMed.
- (a) J. M. Han, S. Guo, H. Lu, S. J. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 1800538 CrossRef; (b) H. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, K. Nobusawa, H. Tsumatori and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9266–9269 CrossRef CAS PubMed.
- (a) R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673–7686 RSC; (b) C. T. Yeung, K. H. Yim, H. Y. Wong, R. Pal, W. S. Lo, S. C. Yan, M. Y. Wong, D. Yufit, D. E. Smiles, L. J. McCormick, S. J. Teat, D. K. Shuh, W. T. Wong and G. L. Law, Nat. Commun., 2017, 8, 1128 CrossRef PubMed; (c) R. Aoki, R. Toyoda, J. F. Kögel, R. Sakamoto, J. Kumar, Y. Kitagawa, K. Harano, T. Kawai and H. Nishihara, J. Am. Chem. Soc., 2017, 139, 16024–16027 CrossRef CAS PubMed.
- (a) R. A. van Delden, N. P. M. Huck, J. J. Piet, J. M. Warman, S. C. J. Meskers, H. P. J. M. Dekkers and B. L. Feringa, J. Am. Chem. Soc., 2003, 125, 15659–15665 CrossRef CAS PubMed; (b) E. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, M. J. Ortiz, B. G. Vo, G. Muller and S. de la Moya, J. Am. Chem. Soc., 2014, 136, 3346–3349 CrossRef PubMed; (c) K. Dhbaibi, L. Favereau, M. Srebro-Hooper, M. Jean, N. Vanthuyne, F. Zinna, B. Jamoussi, L. D. Bari, J. Autschbach and J. Crassous, Chem. Sci., 2018, 9, 735–742 RSC; (d) H. Nishimura, K. Tanaka, Y. Morisaki, Y. Chujo, A. Wakamiya and Y. Murata, J. Org. Chem., 2017, 82, 5242–5249 CrossRef CAS PubMed; (e) S. Feuillastre, M. Pauton, L. H. Gao, A. Desmarchelier, A. J. Riives, D. Prim, D. Tondelier, B. Geffroy, G. Muller, G. Clavier and G. Pieters, J. Am. Chem. Soc., 2016, 138, 3990–3993 CrossRef CAS PubMed.
- (a) S. H. Chen, D. Katsis, A. W. Schmid, J. C. Mastrangelo, T. Tsutsui and T. N. Blanton, Nature, 1999, 397, 506–508 CrossRef CAS; (b) M. Oda, H. G. Nothofer, G. Lieser, U. Scherf, S. C. J. Meskers and D. Neher, Adv. Mater., 2000, 12, 362–365 CrossRef CAS.
- (a) F. D. Meng, Y. Z. Li, W. J. Zhang, S. H. Li, Y. W. Quan and Y. X. Cheng, Polym. Chem., 2017, 8, 1555–1561 RSC; (b) Y. Nagata, K. Takagi and M. Suginome, J. Am. Chem. Soc., 2014, 136, 9858–9861 CrossRef CAS PubMed.
- (a) D. Yang, P. F. Duan, L. Zhang and M. H. Liu, Nat. Commun., 2017, 8, 15727 CrossRef CAS PubMed; (b) J. L. Han, J. You, X. G. Li, P. F. Duan and M. H. Liu, Adv. Mater., 2017, 29, 1606503 CrossRef PubMed; (c) F. Vera, R. Tejedor, M. P. Romero, J. Barberá, M. B. Ros, J. L. Serrano and T. Sierra, Angew. Chem., Int. Ed., 2007, 46, 1873–1877 CrossRef CAS PubMed; (d) T. Goto, Y. Okazaki, M. Ueki, Y. Kuwahara, M. Takafuji, R. Oda and H. Ihara, Angew. Chem., Int. Ed., 2017, 56, 2989–2993 CrossRef CAS PubMed.
- (a) J. D. Luo, Z. L. Xie, J. W. Y. Lam, L. Cheng, H. Y. Chen, C. F. Qiu, H. S. Kwok, X. W. Zhan, Y. Q. Liu, D. B. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC; (b) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (c) J. Roose, B. Z. Tang and K. S. Wong, Small, 2016, 12, 6495–6512 CrossRef CAS PubMed; (d) J. B. Xiong, H. T. Feng, J. P. Sun, W. Z. Xie, D. Yang, M. H. Liu and Y. S. Zheng, J. Am. Chem. Soc., 2016, 138, 11469–11472 CrossRef CAS PubMed; (e) L. Shi, L. Y. Zhu, J. Guo, L. J. Zhang, Y. Shi, Y. Zhang, K. Hou, Y. L. Zheng, Y. F. Zhu, J. W. Lv, S. Q. Liu and Z. Y. Tang, Angew. Chem., Int. Ed., 2017, 56, 15397–15401 CrossRef CAS PubMed.
- (a) J. Z. Liu, H. M. Su, L. M. Meng, Y. H. Zhao, C. M. Deng, J. C. Y. Ng, P. Lu, M. Faisal, J. W. Y. Lam, X. H. Huang, H. K. Wu, K. S. Wong and B. Z. Tang, Chem. Sci., 2012, 3, 2737–2747 RSC; (b) Q. Ye, D. D. Zhu, H. X. Zhang, X. M. Lu and Q. H. Lu, J. Mater. Chem. C, 2015, 3, 6997–7003 RSC; (c) H. K. Li, J. Cheng, Y. H. Zhao, J. W. Y. Lam, K. S. Wong, H. K. Wu, B. S. Li and B. Z. Tang, Mater. Horiz., 2014, 1, 518521 Search PubMed.
- (a) S. W. Zhang, Y. X. Wang, F. D. Meng, C. H. Dai, Y. X. Cheng and C. J. Zhu, Chem. Commun., 2015, 51, 9014–9017 RSC; (b) J. Kumar, T. Nakashima, H. Tsumatori and T. Kawai, J. Phys. Chem. Lett., 2014, 5, 316–321 CrossRef CAS PubMed; (c) Y. Sheng, D. Shen, W. J. Zhang, H. X. Zhang, C. J. Zhu and C. X. Cheng, Chem. – Eur. J., 2015, 21, 13196–13200 CrossRef CAS PubMed; (d) F. Y. Song, Z. Xu, Q. S. Zhang, Z. Zhao, H. K. Zhang, W. J. Zhao, Z. J. Qiu, C. X. Qi, H. Zhang, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam, Z. J. Zhao, A. J. Qin, D. G. Ma and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1800051 CrossRef.
- (a) K. Takaishi, M. Yasui and T. Ema, J. Am. Chem. Soc., 2018, 140, 5334–5338 CrossRef CAS PubMed; (b) E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem. – Eur. J., 2015, 21, 13488–13500 CrossRef PubMed.
- J. N. Zhang, H. Kang, N. Li, S. M. Zhou, H. M. Sun, S. W. Yin, N. Zhao and B. Z. Tang, Chem. Sci., 2017, 8, 577–582 RSC.
- L. D. Bari, G. Pescitelli and P. Salvadori, J. Am. Chem. Soc., 1999, 121, 7998–8004 CrossRef.
- CCDC 1823469 (BINB-1), 1823470 (BINB-2), 1823471 (BINB-4), 1823472 (BINB-5), 1823473 (BINB-6A), 1856064 (BINB-7) and 1823474 (BINB-8)†.
- (a) S. F. Mason, R. H. Seal and D. R. Roberts, Tetrahedron, 1974, 30, 1671–1682 CrossRef CAS; (b) T. Kimoto, N. Tajima, M. Fujiki and Y. Imai, Chem. – Asian J., 2012, 7, 2836–2841 CrossRef CAS PubMed.
- Z. Y. Zhang, B. Xu, J. H. Su, L. P. Shen, Y. S. Xie and H. Tian, Angew. Chem., Int. Ed., 2011, 50, 11654–11657 CrossRef CAS PubMed.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed.
- (a) X. B. Shang, I. Song, H. Ohtsu, Y. H. Lee, T. M. Zhao, T. Kojima, J. H. Jung, M. Kawano and J. H. Oh, Adv. Mater., 2017, 29, 1605828 CrossRef PubMed; (b) G. Huang, R. Wen, Z. Wang, B. S. Li and B. Z. Tang, Mater. Chem. Front., 2018, 2, 1884–1892 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, optical spectra, crystal data and DFT calculations. CCDC 1823469–1823474 and 1856064. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qm00292h |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |