Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Future opportunities for bio-based adhesives – advantages beyond renewability
Lydia Alexandra
Heinrich

Fraunhofer WKI Institute for Wood Research, Bienroder Weg 54E, 38108 Braunschweig, Germany. E-mail: lydia.heinrich@wki.fraunhofer.de
First published on 25th March 2019
Abstract
Bio-based materials are attracting more and more attention in all fields due to their improved environmental footprint and due to the independence from petroleum resources that comes with their use. This is also true in the field of adhesives, where renewable materials from biopolymers to monomers derived from renewable resources are increasingly investigated. However, their sustainability is rarely a sufficient argument for their commercialisation, especially if the new materials cannot be implemented as drop-in replacements for existing technology. The aim of this review is therefore to point out the advantages that bio-based materials can bring to adhesives compared to their petroleum-based counterparts beyond their renewability. Specifically, new functionalities through novel molecular architectures, the advantages of vegetable oils such as hydrophobicity, reduced human and environmental toxicity and the performance of bio-based compared to petroleum-based adhesives are covered.
Introduction
Due to an increasing environmental awareness and the growing need to decrease dependence on petroleum resources, much attention has been paid to the possibilities of synthesising polymeric materials from bio-based, renewable resources.1 Where adhesive technology is concerned, this has led to a renewed interest in traditionally bio-based binders, such as starch or renewable rubber, but also to the application of more recent technologies such as the use of modified vegetable oils or lignin derivatives for binder synthesis.2A wide ground of adhesive technologies can today be covered using renewable materials, which may show an equal or better performance than their commercial, petroleum-based counterparts. However, the mere fact that renewables are used in a product is often not a sufficient argument for its commercialisation, especially when additional costs are associated with either the materials or their implementation, i.e. when they are not drop-in replacements for current technology.3
Notwithstanding, the use of plant-based materials can induce properties that were not previously possible, for example due to new structural elements, high monomer functionalities and the high molecular weight of starting materials, which favour the formation of densely cross-linked networks and adhesion to a variety of substrates.4
Examples for interesting bio-based building blocks include vegetable oils, which can increase adhesive hydrophobicity and therefore water resistance, but also biopolymers such as proteins, polysaccharides and lignin as well as bio-based monomers such as isosorbide and itaconic acid, which can improve the performance of petroleum-based adhesives in a variety of ways.
This short review aims to highlight the advantages bio-based materials can bring to adhesives beyond their renewability, and to provide a guide across the spectrum of adhesive types to match specific needs and opportunities.
Overview of different adhesive types
The term adhesive covers a wide range of materials, and while the function is always to bond separate substances, this is achieved through a variety of mechanisms and to very different specifications.5 The adhesive joint usually contains a polymeric substance that is connected to the substrate through chemical bonds, physiochemical attractions and physical interlinking. The fashion in which this polymer is applied is equally as important as its chemical composition because it determines the conditions under which the application must take place and therefore the possible end uses. It also influences factors such as the spreading of the adhesive on the substrate and the area of contact, which in turn have a big impact on the adhesive forces that can be developed.6 The different types of adhesives are summarised in Table 1.| Type | Mechanism of hardening | Examples for compounds used |
|---|---|---|
| Solution/dispersion | Evaporation of solvent/water | Polyvinyl acetates, polyurethanes, acrylates, rubber |
| Hotmelt | Cooling | Polyamides, saturated polyester, ethylene vinyl acetates |
| 1-Component | External impulse, i.e. water, temperature, UV-light | Polyurethanes, silanes, cyanoacrylates, condensation resins (phenol formaldehyde, urea formaldehyde, melamine formaldehyde), acrylates |
| 2-Component | Mixing of the components | Epoxides, polyurethanes, methacrylates |
| Pressure sensitive adhesives | Retain tackiness | Acrylates, rubber |
Some, though progressively fewer, adhesives are applied in solution. The solvent subsequently evaporates to give the final joint. Due to environmental concerns dispersions, in which the polymer is suspended in water, are becoming a popular alternative. Both solvent-based adhesives and dispersion adhesives can be based on polyvinyl acetates, polyurethanes, acrylates, and natural and synthetic rubber.7
Another method of application is used for hotmelt adhesives. The main advantage of hotmelt adhesives is the short time in which bonding is achieved, and they are therefore usually chosen for processes that require a high throughput. The polymer is melted and applied while hot, and the joint is hardened simply by the cooling of the adhesive. Hotmelt adhesives are generally based on thermoplastic polymers such as polyamides, saturated polyesters and ethylene-vinyl acetate copolymers.8
Finally, there are adhesives that are applied before the polymer is completely formed. The joint is hardened through a chemical reaction of the components, and the adhesives are therefore termed reactive adhesives. They are further characterised into 1-component reactive adhesives, in which all reactive components are present in one component, and 2-component reactive adhesives, in which the reactive substances are mixed only shortly prior to the application.9
In the former type, the crosslinking reaction that forms the adhesive joint is generally triggered by an external impulse, such as water for polyurethanes, silane adhesives and cyanoacrylates, the absence of air for anaerobic adhesives or high temperature for condensation resins such as phenol formaldehyde, urea formaldehyde or melamine formaldehyde adhesives. Condensation resins are frequently used in the wood construction industry for bonding wood and wood composite materials.10 Another example are acrylates, cured through ultra-violet light (UV), which activates a photo-initiator compound that starts a radical polymerisation reaction.
2-Component reactive adhesives can be based on epoxides, polyurethanes or methacrylates. While epoxides and polyurethanes undergo addition reactions, methacrylates like acrylates undergo radical polymerisation, in this case started by mixing with an initiator compound.11 The different adhesive classes are not always completely distinct, as there can be hybrids such as reactive hotmelt adhesives. These contain a mixture of polymers, one of which cools down quickly while the other undergoes further chemical reaction, combining the fast application of hotmelt adhesives with the superior cohesion and durability of reactive adhesives.5
A last, somewhat separate class of adhesives are pressure sensitive adhesives, which are differentiated because they do not harden but retain their tackiness throughout the service life, and rely heavily on non-covalent interactions with the substrate. Pressure sensitive adhesives are often based on acrylates, rubbers and UV-curing polymers, and are used for example in adhesive tapes and labels.12
Motivations for the use of sustainable adhesives
In recent years, there has been an increasing drive in the entire chemical industry to improve the sustainability of processes and products. This is due on one hand to the environmental awareness of customers and the ensuing regulations and on the other to the looming shortage of oil from which many chemicals are derived and the associated threat of petroleum price volatility. In the adhesive industry, this has manifested itself most notably in the switch from solvent- to water-based or high solid adhesives, and in the renewed interest in traditional natural adhesive materials such as polysaccharides and proteins.13Another incentive for producing adhesives based on renewable materials is the move towards a circular economy. Using bio-renewable or waste feedstock helps to reduce the carbon footprint. As a bonus, the inherent biodegradability of renewable materials such as starch, polyhydroxyalkanoates or cellulose is often higher than that of synthetic materials such as polypropylene and polyethylene.14
While the majority of adhesives are still petroleum-based, the recent classification of formaldehyde as a harmful substance is another incentive that drives the search for alternative adhesive solutions, especially in the wood industry.15 Many wood adhesives for both solid wood and wood composites are still based on formaldehyde-containing condensation resins. In order to avoid harmful emissions both during production and during the service life, alternative adhesives must be found. This is another factor that has accelerated research into greener and also renewable alternatives such as protein adhesives.
Aside from regulatory forces, another aspect in the adhesive market drives investment into new, sustainable products. As pointed out in a report by Frost & Sullivan from 2015, titled “Investment Analysis of the European Adhesives and Sealants Market”, the adhesives’ market is highly competitive, which necessitates the development of customised, specialty products to enable differentiation and increase loyalty.16
In the construction sector, also analysed in the report “North American and European Construction Adhesives and Sealants Market, Forecast to 2022”, there is a need for increasingly high performance products, especially regarding shock, heat, moisture and UV resistance.17 On the contrary, the market for non-structural adhesives is increasingly focused on technologies that are easy to use and allow flexibility in the formulation.16 In the automotive sector, the focus is on lightweight cars that can lower the carbon footprint. Therefore, there is a need to develop adhesive solutions for bonding lightweight materials, and for adhesives that ease recycling and are low in hazardous substances.
Some of these challenges can be met using the unique properties of renewable materials, and these market trends are therefore a good opportunity to investigate how sustainable adhesives can be introduced into portfolios to benefit the environment and generate profits.
Introducing bio-based materials into adhesives
There are several ways in which renewable materials can be introduced into adhesives. The most obvious route is to use natural products, i.e. biopolymers such as proteins, that already have adhesive characteristics. A second possibility is to use building blocks or monomers that can be derived from renewable sources, and combine them to make polymers closely resembling synthetic adhesives. While this route requires initially more efforts to generate the necessary structures, it presents a much easier drop-in solution at the application end as similar equipment can be used for the processing, and formulation components can remain largely unchanged. Lastly, bio-based materials can be introduced as additives into synthetic adhesive formulations.Proteins, natural rubber and polysaccharides, especially starch, natural gums and cellulose, are all renewable polymers that have been used as adhesives in the past. When glue laminated timber was first used for construction in the 19th century, bio-based adhesives based on the protein casein were used. These have now been replaced with synthetic phenolic resins which offer superior adhesive strength and water resistance.20 Animal glue, which is based on collagen obtained from burning animal connective tissue, has been used for over 3000 years.21 Due to its water solubility, it is nowadays only used in specialist applications, such as in conservation and for the construction of musical instruments.22 Aside from the historical accuracy that necessitates its use, it is also advantageous due to its brittleness, which causes it to break without damaging the attached wood, making it ideal for repair and reassembly.
Today, traditional bio-based adhesives are still employed in certain applications where they present an advantage over synthetic polymers. One example are stationary adhesives, especially glue sticks, which are usually based on modified starch and water. In many stationary applications, solvent-based adhesives are used because the water damages the paper, but in glue sticks, the necessary quantity of water is small enough not to cause problems.23 Pressure sensitive adhesives for office labels often still contain natural rubber, which has good cohesive properties and shear resistance, and is cheaper than the synthetic alternative.24
Corrugated cardboard is also still produced using starch. Sodium borate (Na2B4O7·10H2O) is used to connect the hydroxyl groups on the starch with those of the cellulose in the paper.23 Casein is widely used to attach labels to glass bottles due to its good adhesion and the ease of removing it under hot water when the bottles are recycled. Bio-based polysaccharides are also often used in medicinal applications such as plasters. In this case their advantage over synthetic adhesives is their non-toxicity, and in addition their capability to absorb moisture without losing adhesion.
These materials present a good basis for renewable adhesives. However, their structure and therefore their properties are adapted to their original natural environment. If they are to be used in different contexts, especially where high performance is required, modifications to these structures are necessary.25 For example, due to the many polar groups in both proteins and polysaccharides, water resistance is often the major hurdle to be overcome.
Several possibilities exist to improve the water resistance of adhesives based on biopolymers. For proteins, these include making the functional groups more available for crosslinking, for example through modification of the tertiary and quarternary structure, chemical crosslinking to create denser networks and mixing of the proteins with synthetic adhesives.26
Similar strategies can be employed where polysaccharides are concerned. Imam et al. for example formulated a wood adhesive based on corn starch, citric acid and polyvinyl alcohol with a molecular weight of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–146
000–146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da.27 The shear force required to de-bond hard wood pieces in an ASTM D-906-64 test was increased from 1000 kg to 2750 kg by crosslinking the adhesive with hexamethoxymethylmelamine. The water resistance as measured in % of veneer failure could further be improved from 70% to 99% by adding latex to the formulation.
000 Da.27 The shear force required to de-bond hard wood pieces in an ASTM D-906-64 test was increased from 1000 kg to 2750 kg by crosslinking the adhesive with hexamethoxymethylmelamine. The water resistance as measured in % of veneer failure could further be improved from 70% to 99% by adding latex to the formulation.
Another example for overcoming the inherent hydrolytic susceptibility of biopolymer adhesives is the work of Zheng et al., who created a wood adhesive entirely based on soy, more specifically defatted soy flour made up of 50% soy protein and 40% carbohydrates.28 The moisture resistance was increased by hydrolysation of the carbohydrates, causing self-crosslinking with the proteins in the formulation. The hydrolysation was performed for example by adding HCl to the carbohydrates at a concentration of 2% at 140 °C for 60 min. After soaking in water at 63 °C for 3 h, the shear strength could thus be increased from 0.6 MPa to 1.18 MPa.
An extensive list of all the works published concerning the modification of adhesive properties of biopolymers would be out of scope for this review. It should however be noted that numerous publications detail successful ways to improve moisture resistance, strength and durability and that consequentially, many promising strategies for the application of biopolymers as adhesives exist. For wood adhesives, which are one of the most important areas of application for biopolymer adhesives, these have been recently reviewed by He.26
A biopolymer with rather different properties to proteins, natural rubber and polysaccharides is lignin. Contrary to the aforementioned biopolymers, it consists of a densely crosslinked aromatic network, which shows low compatibility with most solvents and decomposes without melting. The challenge that needs to be overcome to enable its application in adhesives is therefore not its moisture resistance but rather the ability to process it. Due to structural similarities between lignin and condensation resins used for wood composite bonding, this has been the most prominent area of research into lignin adhesives.29–31 Thanks to numerous hydroxyl groups in the structure, it can however also be used as a polyol in polyurethanes.32
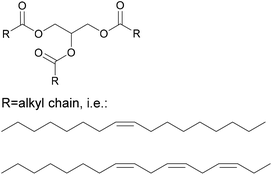
Another important product group are vegetable oils. Their components glycerol and fatty acids can either be used directly in polyester synthesis or converted to new building blocks.33 For example, the double bonds can be converted by epoxidation, followed by ring opening to create secondary hydroxyl groups, or converted to primary hydroxyl groups by ozonolysis or hydroformylation followed by hydrogenation. New dimers can also be created through thiol–ene click chemistry, such as shown in Fig. 1. In this case, a new amine functionality was introduced by Stemmelen et al. into grapeseed oil using cysteamine hydrochloride.34
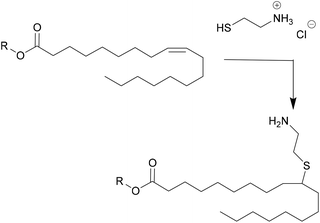 | ||
| Fig. 1 Thiol–ene reaction introducing new functionality in the vegetable oil published by Stemmelen et al. | ||
Due to these availabilities, the most common classes of adhesives to be synthesised from renewable building blocks from a chemical point of view are polyesters, polyurethanes and epoxy-based polymers.
One example in which different renewable building blocks were combined to create an adhesive is the work of Dai et al., who used both diacids and diols to make a polyester and cured it with a vegetable-oil-based crosslinker.35 The polyester was prepared from itaconic acid with ethylene glycol, 1,4-butanediol, 1,6-hexanediol and glycerol; the crosslinker from acrylated epoxidised soybean oil.
The combination of the two improved the adhesion to both tin and glass plates significantly from 0 to 5B according to the ASTM D3359-09 crosshatch adhesion test compared to the soybean material without polyester.
Of course, different renewable building blocks or biopolymers and bio-based monomers can be combined to benefit the adhesive properties. One example is the work of Jian et al., who cured epoxidised soybean oil with polybutylene succinate.36 Thus, a highly structured chain element could be introduced in the polymer. When the molecular weight of the polybutylene succinate was increased from 462 g mol−1 to 978 g mol−1, the melting points of the corresponding cured adhesives could be increased from 57 °C to 86 °C. The tensile strength was also increased from 0.6 MPa to 7.9 MPa.
Renewable building blocks can also be used in polyurethanes, and one such adhesive was recently prepared by Malik et al. from vegetable oils.37 Canola oil was first epoxidised, followed by ring opening, and then derivatised with different diisocyanates. The polyurethane was then used as an adhesive for teak wood. One problem with such bio-based polyurethanes is that the isocyanates used are usually non-renewable, limiting the overall renewable content of the polyurethane. Some bio-based diisocyanates are however also available, and have been tested for their adhesion strength with castor-oil-based polyols for instance by Sahoo et al.38
Natural fats and oils also find application as plasticisers. Recently, for example, a mixture of liquefied wood and depolymerised polyethylene terephthalate from waste streams was evaluated as a plasticiser for a polyvinyl acetate adhesive for flooring applications by Jasiukaitytė-Grojzdek et al.39
The motivations for including bio-based materials into adhesives and the methods with which it can be done are summarised in Table 2.
| Motivations | Ways to introduce bio-based content |
|---|---|
| • Customer environmental awareness | • Natural macromolecules with adhesive properties (proteins, natural rubber, polysaccharides, lignin) |
| • Regulations, i.e. classification of formaldehyde as a carcinogen | • Synthesis of polymers from renewable monomers (succinic acid, itaconic acid, 1,3-propanediol etc.) |
| • Oil shortage & associated price volatility | • Use of renewable compounds as additives (rosin, fats, oils) |
| • Differentiation through the development of customised/specialty products |
This review does not aim to include all examples of research in which renewable materials have been successfully introduced into adhesives. Instead, its purpose is to point out and exemplify the different advantages that can be gained by using those materials that have been discovered, in the hope of serving as a guide to potential users and thus of increasing the number and scale of bio-based adhesive applications.
New types of macromolecular architectures through bio-based starting materials
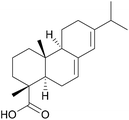
One important factor that differentiates bio-based adhesives from traditional adhesives based on petroleum-derived materials is their “molecular architecture”. Where biopolymers are used, macromolecules are already present. This eliminates the need for a polymerisation process. On top of this, the connections formed in nature, for example in lignin or in proteins, are often complex and not easily accessible by traditional polymerisation chemistry.Alternatively, monomers derived from renewable sources also contain combinations of functional groups that would not be economical if produced through petrochemical pathways. One example is isosorbide, a molecule derived from glucose that contains four stereo-centres, as shown in Fig. 3. These differences can be leveraged to improve adhesive performances in a number of ways, some illustrations of which are detailed below.
Additional bond formation due to high functionalities
The functionality of biopolymers is often much higher than that of traditional resins. One example for this is softwood Kraft lignin, which was recently analysed by Crestini et al.40 The lignin was fractionated according to solubility, glass transition temperature (Tg) and molecular weight, and the functionality was analysed for the different fractions.The highest molecular weight fraction, which was insoluble in acetone, was found to contain 3.5 mmol g−1 phenolic OH and 3 mmol g−1 aliphatic OH. At a molecular weight of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, this corresponds approximately to 43 phenolic and 37 aliphatic OH-groups per molecule. This is significantly higher than the concentration in most synthetic polyols, which contain only two to six OH functions as end groups.
200 g mol−1, this corresponds approximately to 43 phenolic and 37 aliphatic OH-groups per molecule. This is significantly higher than the concentration in most synthetic polyols, which contain only two to six OH functions as end groups.
This can become useful in two ways. On one hand, the speed of crosslinking of the resin during the curing can significantly increase, as a higher number of reactive groups are present. This was demonstrated for example by Ferdosian et al., who blended a lignin-based epoxy resin with a bisphenol-A-based epoxy resin to make a polymer matrix for fibre-reinforced plastics and coatings.41 The lignin-epoxy resin was prepared from depolymerised Kraft lignin and epichlorohydrin, and blended with a commercial bisphenol A epoxy resin at 25 wt%. A curing agent (4,4′-diaminodiphenylmethane) was then added to the mixture and curing was interrupted through quenching in an ice bath before the curing process was characterised by DSC.
Compared to the lignin-free resin, the activation energy of the lignin-containing resin could be lowered from 48 kJ mol−1 to 45 kJ mol−1, while its curing onset and end of curing reaction at a heating rate of 10 °C min−1 were lowered from 77 °C to 58 °C and 260 °C to 243 °C respectively. The authors attribute this speeding up of the curing reaction to the additional hydroxyl groups present in the lignin that can participate in the crosslinking reaction.
The higher density of functional groups in bio-based products can also lead to overall higher crosslinking densities. This was observed for example by Desai et al., who produced polyurethane adhesives from toluene diisocyanate and various bio-based polyols and tested their lap shear strength for wood bonding.25 The polyol was prepared by first performing a glycosylation of potato starch to obtain glycol glycosides, as shown in Fig. 4. These were then condensed with argemone or castor oil in different quantities to vary the hydroxyl value.
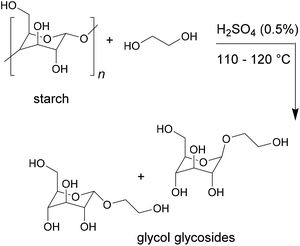 | ||
| Fig. 4 Glycolisation of starch to obtain glycol glycosides (Desai et al. 2003).25 | ||
The authors found that the highest hydroxyl values from adhesives based on both oils also resulted in the highest lap shear strength and the highest amounts of wood failure compared to cohesive and adhesive failures. The thus synthesised adhesive performed better than commercially available adhesives. Unfortunately, only the brand and not the exact type of commercial adhesive used for the comparison is specified.
A similar effect was reported by Mija et al., who mixed humins, a hydoxymethylfurfural biorefinery byproduct shown in Fig. 5, into a polyfurfuryl alcohol resin.42 Cellulose composites were impregnated with the resulting resin and cured, and the tensile strength and Young's modulus was evaluated. The tensile strength increased from 15 MPa to 30 MPa and the Young's modulus increased from 3.5 GPa to 4 GPa with the addition of the humins compared to pure polyfuryl alcohol resins.
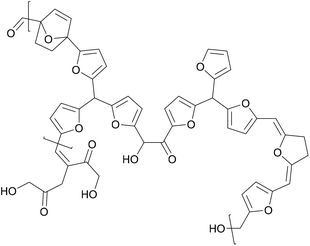 | ||
| Fig. 5 Structure of humins (Mija et al. 2017).42 | ||
This improvement is probably related to the fact that humins already possess a crosslinked structure, which therefore contributes to an increased crosslinking density, but it is also due to another positive aspect that is often associated with biopolymers. Specifically, the good compatibility between the cellulose substrate and the humins also leads to a strong interfacial adhesion, overall strengthening the composites.
This effect was also leveraged by Liu et al. in the design of a water resistant adhesive based on soy protein.43 Calcium carbonate was first introduced into the soy protein to make a nanocomposite. The nanocomposite adhesive was then used to bond plywood samples, and its adhesion performance was evaluated. Due to the strong interactions between the calcium carbonate crystals and the free functional groups of the soy protein polypeptides, the shear strength could be increased from 1.7 MPa without calcium carbonate to above 5 MPa.
Lastly, the large amount of functional groups in many biopolymers allows for a great variety in modification. In addition to reacting with traditional crosslinking agents, such as poly(methylenediphenylisocyanates) (pMDI) and formaldehyde, the hydroxyl functionality can also be modified for example using silane reagents. This was done by Li et al., who doubled the wet shear strength of a soy-protein-based adhesive for plywood by adding 3% of an epoxy-silane coupling agent.44
New properties due to novel monomer architecture
Bio-based monomers differ in their structure from traditional, petroleum-based monomers due to the different pathways in which they are synthesised. The full potential of bio-based materials can be tapped if their advantages over petroleum-based equivalents are identified and subsequently applied in areas in which those advantageous properties can be fully exploited, rather than where a one-to-one replacement of current materials is attempted.A well-known example of this is the compound L-3,4-dihydroxyphenylalanine (L-DOPA), which is an active part of the substance used by mussels to achieve high bond strength even under water.
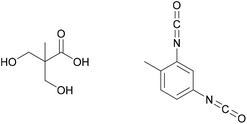
Its bonding effect is due on one hand to the oxidative crosslinking that can take place between the aromatic rings, and on the other hand to the chelating effect on metals.45 If L-DOPA is incorporated into adhesive formulations, these mechanisms can be used to increase underwater strength, or adhesion to metallic substrates. Incorporation can happen for example through derivatisation with a diol and p-toluenesulfonic acid as done by Manolakis et al. and shown in Fig. 6, followed by incorporation into a polyamide.46
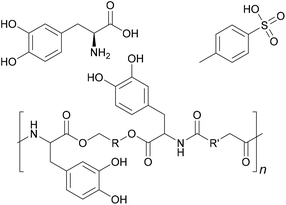 | ||
| Fig. 6 L-DOPA, polyamide with incorporated L-DOPA and p-toluenesulfonic acid, which was used as counterion (Manolakis et al. 2014).46 | ||
Another monomer with potential to induce novel properties into adhesives is 2-pyrone-4,6-dicarboxylic acid, shown in Fig. 7. A chemically stable metabolic intermediate of lignin, it can be obtained from lignin through bacterial transformations.47
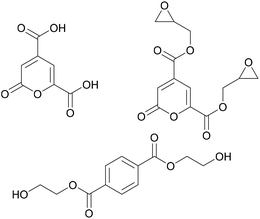 | ||
| Fig. 7 Lignin derived 2-pyrone-4,6-dicarboxylic acid, its diepoxy derivative (Hasegawa et al. 2009)48 and bis(2-hydroxyethyl)terephthalate (Michinobu et al. 2008). | ||
Hasegawa et al. synthesised an epoxy adhesive based on this monomer by adding two epoxy groups and then curing it with different anhydrides.48 The adhesive was tested on metal substrates and necessitated shorter curing times and lower temperatures compared to the petroleum-derived reference adhesive based on bisphenol A. Furthermore, the tensile strength of the lignin-based adhesive was observed to be higher. This was explained by the authors by the high polarity of the monomer as well as by possible chelation effects on the metal surface.
Another interesting effect of the monomer architecture is that it enables tuning of the degradation behaviour. Michinobu et al. incorporated 2-pyrone-4,6-dicarboxylic acid into a polyester, and found that both thermal and hydrolytic degradation were influenced compared to a polyethylene terephthalate reference material.49 Thermal degradation in thermogravimetric analysis (TGA) started at 250 °C, corresponding to the breaking of the lactone cycle, instead of at 400 °C, when the backbone ester bonds start to degrade. Hydrolytic degradation of a copolymer from bis(2-hydroxyethyl)terephthalate and 2-pyrone-4,6-dicarboxylic acid, which was also considered to proceed via opening of the lactone cycle, could be adjusted through the amount of the renewable monomer.
A film containing 50% of the terephthalate was observed to lose over 90% of its weight after 30 days in a 0.1 M aqueous sodium hydroxide solution, while a film containing 70% of the terephthalate lost below 10% in the same conditions. An adhesive material with tuneable biodegradability would be especially interesting to applications with a short life span, for which the degradation must be prevented for the duration of its use, but enabled after it is thrown away.
An interesting property was also discovered by Fan et al., who planned to make a thermoplastic resin from a citric acid derivative and observed a slow self-crosslinking reaction after short storage times that turned the resin into a thermoset that could be used for example for 1-component adhesive applications.50 Citric acid was converted to methyl-3-(methoxycarbonyl)furan-2-acetate (MCFA) via conversion to dimethyl-1,3-acetonedicarboxylate (DMAD) followed by reaction with chloroacetaldehyde, as shown in Fig. 8.
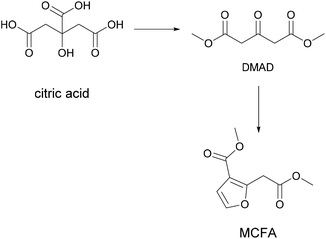 | ||
| Fig. 8 Conversion of citric acid to methyl-3-(methoxycarbonyl)furan-2-acetate (Fan et al. 2016).50 | ||
The MCFA was then transesterified with various linear diols. A kind of crosslinking, causing the thermosetting nature of the final product, was produced by a ring opening of the furan followed by the formation of enols due to the strong electron withdrawing effects of the ester groups as shown in Fig. 9, and the formation of hydrogen bonds between the enol group and the ester and aldehyde groups in other chains.
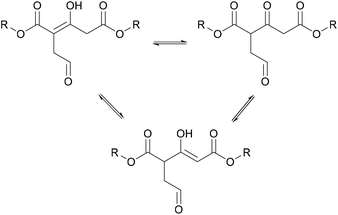 | ||
| Fig. 9 Enol formation on citric acid-based polymer (Fan et al. 2016).50 | ||
Other than the chelating ability, polarity and reactivity of the monomers, the positions in which chains are connected can also differentiate bio-based materials from traditional polymers. One example for such an effect was reported by Pawlik et al. for a polyol based on palm oil.51 It was used to make a polyurethane foam in this case, but the observations can likely be extrapolated to polyurethane adhesives. Only quantities of up to 15% of the bio-based polyol were used to avoid having to make modifications to the formulation. The tensile strength of the resulting foam could however be increased from 48 kPa to up to 86 kPa through the addition of the bio-based material. This was thought to be because the hydroxyl groups of the bio-based polyol were in the middle of the chain instead of in the end as it is the case for the petroleum-based polyol. For an equivalent molecular weight of the polymer, the soft segments were therefore shorter, improving the mechanical properties.
Another example of a novel structure with interesting properties are the hyperbranched epoxy resins synthesised by Duarah et al. from starch, epichlorohydrin and bisphenol A.52 Due to the globular shape of the hyperbranched polymer, a low viscosity resin was obtained. Unlike adhesives based on starch and traditional epoxy resins, the resulting adhesives showed both excellent chemical resistance and biodegradability. An adhesive containing 20% starch showed only 0.0017% weight loss in 10% aqueous HCl compared to 0.0025% weight loss that was measured for a starch free diglycidyl ether epoxy of bisphenol A (DGEBA) that was used as a reference, and also outperformed the reference in NaOH, NaCl and ethanol solutions as well as in water. Furthermore, around 25% weight loss upon exposure to bacteria was observed for the 20% starch resin compared to below 5% for the DGEBA reference.
Of course, it may be possible to achieve the same effect that was seen for bio-based monomers using petroleum-based compounds that also achieve mid-chain crosslinking, possess high polarities or can chelate metals. However, it is worth considering whether using the bio-based monomers may be a more direct route to the desired properties, and whether in combination the advantages, such as sustainability and higher bond strength than a standard petroleum-based polyol, constitute a sufficient argument for their implementation.
Components with multiple functionalities
Another advantage of many bio-based molecules is the possibility to use them for more than one function in a formulation. This was demonstrated for example by Qi et al., who mixed different commercial latex adhesives, including a urea formaldehyde resin, with modified soy protein.53 The addition of 40% of the modified soy protein to the urea formaldehyde resin resulted in equal dry strength and improved the wet shear strength in the bonded veneer samples from 4.7 MPa to 6.4 MPa.One cause for this improvement was the participation of the modified soy protein in the crosslinking reaction, via bond formation between free hydroxyl groups on the urea formaldehyde resin and carboxylic acid, hydroxyl and amine groups on the protein. This was evidenced for example through the appearance of new ester groups in the adhesive IR spectrum, and new peaks in the thermal analysis. A further basis for the improvement can be found in the fact that the protein acidified the adhesive, acting as a catalyst in the curing reaction. Additionally, the presence of the modified soy protein also lowered the overall viscosity and therefore improved the spreading and processability of the adhesive.
A similar effect was observed by Desai et al., who made an adhesive based on potato starch glycol glycosides and castor oil.54 The replacement of the trimethylolpropane, shown in Fig. 10, with glycol glycosides in the formulation resulted both in an increase in lap shear strength from 43 × 105 N m−2 to 60 × 105 N m−2 and in a decrease of the viscosity from 30 poise to 3.7 poise. The glycol glycosides therefore acted as both crosslinker and viscosity modifier. Unfortunately, it also decreased the resistance to hot water, lowering the peel strength from 7.4 kN m−1 to 6.7 kN m−1 after treatment. This was probably due to the increased polarity caused by the numerous hydroxyl groups.
Where extracted tannins, natural polyphenols present in most plants, are used, the opposite effect can be observed. Hydrocolloid gums are often also present in tannin extracts, and can serve to increase the viscosity of adhesives that are made from tannins or introduce thixotropic behaviour.55
On example of a bio-based monomer that can be used in many different functions is citric acid. It can be a catalyst, a crosslinker with different functionalities, a dispersing agent or a monomer. For example, Sridach et al. used citric acid as a catalyst for crosslinking an adhesive based on polyvinyl alcohol, starch and hexamethoxymethylmelamine.56 Yang et al. also used citric acid as a crosslinking agent, this time for an adhesive based only on cotton.57 They found that in addition to its function as an acidic crosslinker, the free OH-group could react with other anhydrides, creating a tetrafunctional monomer as shown in Fig. 11.
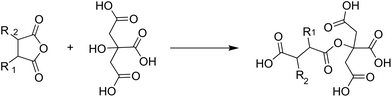 | ||
| Fig. 11 Reaction of citric acid with polymaleic anhydride to give a tetrafunctional monomer (Yang et al. 1997).57 | ||
In contrast, Nordqvist et al. used citric acid as a dispersing agent for dispersions of wheat gluten and soy protein isolate in a study designed to evaluate the differences in bonding performance between the two products.58 Lastly, citric acid was used as a comonomer together with sucrose to make an adhesive for particle boards by Umemura et al.59
In another study, the concept was even taken a step further, where it was used without any other components. It was mixed with wood powder and pressed at 200 °C and 4 MPa pressure for 10 minutes.60 At a citric acid content of 20 wt%, an impact strength of 0.9 kJ m−2 was observed.
A different case are nanocellulosic materials. These are generally used to reinforce the mechanical properties of materials, but recently, they have also been investigated as binders in adhesive formulations, for example for particleboards.61 Amini et al. prepared particle boards containing 15% or 20% of cellulose nanofibrils as binder, which passed modulus of rupture and modulus of elasticity industry requirements for low-density grades based on ANSI A208.1 (2016), i.e. for boards with densities less than 0.64 g cm−3.62 The requirements for medium density particle boards, i.e. with densities between 0,64 g cm−3 and 0,8 g cm−3 could however not be met.
Some further problems, such as the water content of the nanocellulose, which is generally as high as 97%, as well as their price, remain to be overcome.63 A solution for the first appears to be the cold pressing of the water after a slurry with wood particles has been produced, while a solution to the second could be the use of lignocellulose nanofibers extracted from recycled particleboard.64 If these developments are successful, the nanocellulose could fill a function both as structural reinforcement and as adhesive material in the product.
Modification of protein adhesion
A specific advantage of protein-based adhesives is the dependence of their adhesive strength on their different levels of structure. As macromolecules, proteins can be processed in similar fashion to petroleum-based macromolecular adhesives, but are more complicated in their make-up.The folding of the protein chains, for example, can be influenced by crosslinking reactions or by the addition of compounds that interact with the chains. Manipulation of the adhesive strength through modification of the tertiary structure was for example demonstrated by Cheng et al., comparing cottonseed and soy protein as adhesives for maple veneer.65 They disrupted the structure by adding guanidine hydrochloride, sodium dodecyl sulfate, sodium hydroxide or urea, all shown in Fig. 12. In the case of soy protein, the tensile strength was measured at 230 lb in−2 (1.6 MPa) after 10 minutes pressing time at 100 °C. When the soy protein was modified with sodium dodecyl sulfate, sodium hydroxide and guanidine hydrochloride, the tensile strength increased to 250 lb in−2 (1.7 MPa), 260 lb in−2 (1.8 MPa) and 280 lb in−2 (1.9 MPa) respectively.
In the case of the cottonseed protein, however, the tensile strength was lowered from 490 lb in−2 (3.4 MPa) for the unmodified protein to 450 lb in−2 (3.1 MPa), 310 lb in−2 (2.1 MPa), 190 lb in−2 (1.3 MPa) and 70 lb in−2 (0.48 MPa) for the samples modified with sodium dodecyl sulfate, urea, sodium hydroxide and guanidine hydrochloride. Thus, the properties of the protein can significantly change through simple measures. If the mechanisms are properly understood, they could be leveraged for example to make adhesives that are switchable in their properties as needed for specific applications or in different situations.
Another mechanism by which proteins can be influenced is through hydrolysis of their bonds and application of heat. Both short heating to 50–90 °C and enzymatic hydrolysis were found by Nordqvist et al. to improve the bond strength and water resistance of wheat gluten adhesive used for bonding beech wood panels.66 Hydrolysis to a degree of 0.8% with the serine protease Alcalase for example decreased the viscosity of a 23% dispersion to 900 mPas compared to 432![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mPas for the unhydrolysed sample.
000 mPas for the unhydrolysed sample.
On the other hand, hydrolysis to a degree of 0.3% increased the viscosity to 542![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mPas, and also increased the tensile strength from below 10 MPa to above 10 MPa in D1 and D2 tests according to EN 204. The D1 test requires 7 days storage in standard atmosphere, while the D2 tests requires 7 days in standard atmosphere followed by 3 hours in water followed by another 7 day stretch of storage in standard atmosphere.
000 mPas, and also increased the tensile strength from below 10 MPa to above 10 MPa in D1 and D2 tests according to EN 204. The D1 test requires 7 days storage in standard atmosphere, while the D2 tests requires 7 days in standard atmosphere followed by 3 hours in water followed by another 7 day stretch of storage in standard atmosphere.
Heat treatment at 90 °C for 4 hours increased the viscosity of a 20% dispersion to 332![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mPas compared to 810 mPas for the untreated wheat gluten. It also increased the tensile strength in the D2 test from around 4 MPa to above 8 MPa. The tensile strength in the D1 test remained similar.
000 mPas compared to 810 mPas for the untreated wheat gluten. It also increased the tensile strength in the D2 test from around 4 MPa to above 8 MPa. The tensile strength in the D1 test remained similar.
The adhesive strength can also change with the pH, as demonstrated by Park et al. for a protein adhesive based on meat and bone meal.67 The strength required to break glued joints between two pieces of wood reached a peak of 65–78 kg around a pH of 7 depending on the temperature of the adhesive treatment before bonding. Less strength was required for pH 5 (42–70 kg) and pH 9 (55–65 kg).
Generally, the highest adhesive strength for proteins is observed close to their isoelectric point. Near this point, their solubility is also lowered, which can increase the interaction between protein chains, and therefore their hydrophobicity.68,69 This could for example be used to tune the water resistance of protein adhesives.
Substrate compatibility
As society is moving to a more bio-based economy, renewable components are employed not only in adhesives but also as bulk building materials for example in construction contexts. Therefore, compatibilities with these new materials can be an essential advantage of bio-based adhesives. This can apply either where the building materials are based on the same substance as the adhesive, or more generally, where compatibility is achieved due to similar functional groups, polymer structure or functional group distribution.This was for example utilised by Freire et al., who developed new materials based on cellulose.70 They observed that adhesives based on polysaccharides show high potential for nanocellulose materials due to the chemical similarity, and that the two substances can therefore easily be combined, resulting in good mechanical strength and adhesive properties.
One example was a composite from the homopolysaccharide pullulan with nanocellulose. Due to the high compatibility, no aggregates were formed for up to 40% nanocellulose content. Similar composites from pullulan and nanofibrillated cellulose also showed high homogeneity such that they were transparent at 40% of the reinforcing cellulose element.
The different advantages that can be gained due to the specific architecture of renewable materials are summarised in Table 3.
| Property | Advantage | Example |
|---|---|---|
| High functionality | Faster crosslinking | Lignin-epoxy resin41 |
| • Additional bond formation | Higher crosslinking density | Potato starch polyurethane,25 humin polyfurfuryl alcohol resin42 |
| • Many free functional groups | Strong interaction with minerals i.e. calcium carbonate | Soy protein adhesive with CaCO3 filler43 |
| • Many possibilities for modification | Customisability and versatility | Soy protein adhesive with epoxy-silane functionality44 |
| Chelate-structure and high polarity | Underwater and metal adhesion | L-DOPA adhesive46 |
| High adhesive strength | 2-Pyrone-4,6-dicarboxylic acid adhesive48 | |
| Additional degradation pathways, i.e. breaking of lactone cycle | Tuneable biodegradability | 2-Pyrone-4,6-dicarboxylic acid adhesive49 |
| Mid-chain functionality | Increased tensile strength | Palm oil polyurethane51 |
| Multiple functionalities | Binder, acid catalyst and viscosity reduction | Protein and urea formaldehyde53 |
| Binder and viscosity reduction | Potato starch and castor oil adhesive54 | |
| Binder and viscosity increase | Adhesives from tannins containing hydrocolloid gums55 | |
| Catalyst,56 crosslinker,57 dispersing agent,58 monomer59 | Citric acid | |
| Mechanical reinforcement and binder | Nanocellulose62 | |
| Tertiary structure | Manipulation of adhesion by disrupting the structure,65 changing pH,67–69 temperature66 | Protein adhesives |
| Similarity to renewable materials | Easy combination, i.e. high contents of nanocellulose possible in binder while maintaining transparency | Pullulan nanocellulose composite70 |
Hydrophobicity and other properties induced by vegetable oils
Vegetable oils are one of the most traditional renewable resources used in binders. They have for example been components in alkyd resins for wood coating applications since the 1920s, and have been used in printing inks for more than 500 years.71 They can be employed either as an oil, in their triglyceride form shown in Fig. 13, which will be hydrolysed during the reaction to give glycerol and fatty acids, or directly as fatty acids.Where the oils are used directly, a mixture of fatty acid chains, which may vary in length and degrees of saturation, will be present. In adhesives, the specific properties of vegetable oils can be leveraged in several different ways. The most important is the hydrophobicity that can be introduced through the long alkyl chain of the fatty acids. The hydrophobicity benefits that have been observed by different authors are summarized in Table 4.
| Vegetable oil adhesive | Reference | Test used | Property vegetable oil | Property reference |
|---|---|---|---|---|
| Low density fibre boards (wheat straw) with soybean oil – MDI adhesive72 | Low density fibre board with MDI adhesive | 2 h in water | 26% | 50% |
| Mass absorption Thickness swelling | 24% | 41% | ||
| 2-Component polyurethane adhesive from canola oil and pMDI73 | Three commercial polyurethane adhesives | Lap shear strength after storage in hot water | 5.4 MPa | 3.9 MPa, 3.2 MPa and 4.6 MPa |
| Polyurethane dispersion shoe adhesive from natural rubber seed oil74 | Polyurethane dispersions in general | Water uptake of films after 7 days | 10%–25% | 1000% |
| UV-curable polyurethane binder for textile printing with 20% soybean oil75 | Polyurethane without soybean oil | Contact angle with water | 91° | 65° |
| Water absorption | 8.5% | 22% | ||
| Thermoset coating from soybean oil and polyester35 | — | Water sorption | 0.6%–0.9% | — |
Hydrophobicity
The hydrophobicity induced by vegetable oils was utilized for example by Sitz et al. in the production of low density fibre boards.72 The boards were produced from fibres either made from wheat or from soy straw and from an adhesive based on the epoxidised sucrose ester of soybean oil fatty acid and 4,4′-methylenediphenyldiisocyanate (MDI). In the case of wheat fibreboards, the addition of the soybean resin decreased the mass absorption of water after 2 h from 50% to 26% and the thickness swelling after 2 h from 41% to 24%.This effect was however not observed in boards made from soybean straw. In those, the mass absorption after 2 h increased from 74% to 122% after addition of the resin, while the thickness swelling after 2 h was unchanged. The authors expect that adjusting the formulation, for example in terms of particle size, could improve the performance of the soybean straws to also reflect these advantages.
Kong et al. also observed good hydrolysis resistance for an adhesive based on epoxidised canola oil.73 The oil was ring opened with 1,3-propanediol and the polyol thus produced was then used in a 2-component adhesive formulation with pMDI. The ratio of isocyanate to hydroxyl groups used was between 1.2 and 1.8. The adhesive was tested against a variety of commercial adhesives such as Henkel Macroplast SIA 116, Gorilla glue and Titebond PU glue, all of which are 1-component polyurethane adhesives.
The hydrolysis resistance in hot water was found to be superior for the vegetable-oil-based adhesive in a lap shear strength test. The bio-based adhesive showed a lap shear strength of 5.4 MPa, compared to strengths of 3.9 MPa, 3.2 MPa and 4.6 MPa shown by the commercial adhesives. This was attributed to the alkyl chain branches, i.e. the vegetable oil chains, present in the bio-based adhesive. The fact that the commercial adhesives were all 1-component systems, while the bio-based system was tested as a 2-component system, could however also have influenced the result. A potential for good hydrolysis resistance was nevertheless demonstrated for this vegetable-oil-based adhesive.
A similar result was obtained by Saetung et al., who made a polyurethane dispersion to be used as an adhesive for shoes.74 The dispersion was made from mixtures of hydroxytelechelic natural rubber (HTNR) and hydroxylated rubber seed oil (HRSO), dimethylolpropionic acid (DMPA) and toluene-2,4-diisocyanate (TDI), shown in Fig. 14. It was then tested for water uptake in film form. The water uptake was between 10% and 25% after 7 days, which is significantly lower than that of around 1000% usually observed for more hydrophilic polyurethane dispersions. Due to the ester groups in the HRSO, the water uptake increased with increasing HRSO and decreasing HTNR contents.
The hydrophobic effect of the oil was demonstrated more directly by Li et al. for a soybean-oil-based UV-curable polyurethane acrylate binder for textile printing.75 They found that with higher soybean oil contents, the contact angle of the water increased, resulting also in lower water absorption. By adding 20% of soybean oil, the contact angle was increased from 65° to above 91°, while the water absorption decreased from 22% to below 8.5%. As mentioned in the introduction, Dai et al. synthesised a thermosetting coating based on epoxidised soybean oil crosslinked with an itaconic-acid-based polyester.35 They found that due to the hydrophobic oil segments, the coatings showed very low water sorption of 0.6% to 0.9%.
Other advantages of vegetable-oil-based structures
Aside from the hydrophobicity that vegetable oils can induce in adhesive formulations, they can be useful for a number of other reasons. These advantages have been summarised in Table 5. Epoxidised vegetable oils can be used, for example, to improve compatibility with epoxy substrates.| Vegetable oil property | Advantage |
|---|---|
| Epoxy groups on epoxidised vegetable oils | Good compatibility with other epoxy compounds such as epoxy-crosslinkers76 |
| Network formation of secondary hydroxyl groups | Dangling chain ends serve as plasticizer78 |
| Free carboxylic acid group | Use as ionic segment in polyurethane dispersions79 |
This was utilized for example by Li et al., who combined a polyol based on epoxidised soybean oil and a crosslinker based on ring-opened dihydroxy-soybean oil.76 The result was a structure with highly flexible crosslinks, and good compatibility between the reagents.
Aside from polyol and crosslinker, vegetable oils can be used in a variety of different functions. The C–C double bonds in the alkyl chains can be converted to hydroxyl groups either via epoxidation and ring opening, which generates secondary hydroxyl groups, or through hydroformylation and hydrogenation, which generates primary hydroxyl groups.77 Where secondary hydroxyl groups are used to form a network, dangling chain ends are created that can serve as a kind of plasticiser.78
The possibility to create different types of structures from the same monomer is also interesting. It could potentially be used to design adhesives with new properties from vegetable oils. If for example the hydroxyl groups were generated post polymer synthesis, combinations of primary and secondary hydroxyl groups otherwise inaccessible could be generated.
Lastly, vegetable oils can also be used as ionic segments in polyurethane dispersions. This was done for example by Chen et al., who used the free carboxylic acid group of linseed oil for charge stabilisation in an anionic polyurethane dispersion.79
A slightly different path to a similar end was followed by Fu et al., who added mercaptopropionic acid, shown in Fig. 15, to the castor oil double bond. This could then be incorporated as the ionic group into a polyurethane dispersion, which showed only 1.8% water absorption.80 This suggests that good hydrophobic properties and a high resistance to degradation by water can be expected.
Reduced human and environmental toxicity
The primary drive for the introduction of biobased materials has been their positive impact on climate change. While this has to be verified on a product to product basis for example through life cycle analyses, it can be said in general that bio-based materials do not contribute to the depletion of fossil fuels and that, prior to processing, they inherently have a low carbon footprint due to the capture of CO2 by the plants used to grow them.81Their environmental benefits, however, go beyond these factors to their comparative toxicity to humans and the environment and to their biodegradability. Both are attractive features for adhesives, not only due to sustainability, but also because lower toxicity and higher biodegradability can increase product appeal and lower costs associated with health, safety and environmental regulations.
While it would be misleading to state that natural products are less toxic than synthetic ones, as indeed the number of natural toxins and carcinogens resembles that of synthetically derived ones, bio-based polymeric resins are frequently less toxic than the petroleum-based products they were designed to replace.82 Examples include the replacement of toxic phenol with benign lignin, the replacement of the corrosive monomers acrylic and methacrylic acid with non-corrosive itaconic acid, and the replacement of oestrogen-mimic bisphenol A with isosorbide.30,83
As with its toxicity, the biodegradability of a compound depends on many factors and predictions consequentially have to be treated with caution. A general rule of thumb that has been established based on a number of degradation studies conducted in the last century states among other things that halogens, polycyclic residues, heterocyclic residues, and aliphatic ether bonds decrease biodegradability, while groups susceptible to enzymatic hydrolysis, such as esters and other oxygen containing functional groups including hydroxyl, aldehydes and carboxylic acid groups increase it.84 Most halogens and polycyclic products are traditionally derived from non-renewable resources. Furthermore, the oxygen content of the currently available bio-based feedstock is significantly higher than that of the petroleum-based feedstock.85 If follows that the number of easily degradable groups in and the polarity of bio-based products is higher, making them more easily degradable than their petroleum-based equivalents. Additionally, for some bio-based compounds nature has already developed tailored degradation mechanisms, such as the white rot fungi in the case of lignin.
Lastly, bio-based adhesives are generally designed with sustainability in mind, so that the solutions that have been developed are less likely to include toxic and harmful chemicals, and more likely to be synthesised in accordance with green chemistry principles.
In summary, bio-based adhesives in general have the following advantages compared to petrol-based adhesives with regards to human and environmental toxicity:
• Low carbon footprint.
• Lower human toxicity.
• Higher biodegradability.
• Sustainable design.
Biocompatibility with the human body
Due to their similarity with the extracellular matrix and with polymers found in the human body, bio-based polymers, especially polysaccharides, can be used in a variety of adhesives for biomedical applications.86 Properties common in certain bio-based polymers such as pseudoplastic behaviour, gelation ability, water binding capacity and biodegradability are also very valuable in this area.87 The most traditional example of this is fibrin glue, which derives from fibrinogen, a protein present in human blood, and thrombin, a bovine enzyme.88 Fibrin glue is used in a variety of surgical applications, such as to control bleeding, speed up wound healing and seal holes.89Not only blood-, but also plant-based polymers can be employed. Hoffmann et al. for example developed a 2-component adhesive for bones based on dextran and chitosan.90 Dextran was oxidised with periodic acid to generate aldehyde groups, which could then crosslink with both chitosan amine groups and free amine groups on the bone. The adhesive strength on bovine bones was measured as 0.41 MPa, which was more than twice as high as fibrin glue at 0.14 MPa, but not as high as cyanoacrylate glue at 1.5 MPa. While cyanoacrylates are however not resorbable and thus inhibit endogenous bone repair, the new adhesive exhibited excellent biocompatibility. Cells seeded on an adhesive sample proliferated, increasing their number from below 100 per high power field to above 400 after 6 days.
Reducing the harmfulness to humans also assumes particular importance in applications such as adhesive for fixing of theatrical props to skin. The usefulness of bio-based adhesives to such an application was shown by Kim et al., who replaced the spirit gum that served as a tackifier in a cosmetic adhesive formulation based on polyvinyl alcohol and polybutylene with renewable guar gum.91 This reduced the skin irritation, and also resulted in superior adhesion. The tensile strength of the cosmetic adhesive containing guar gum was 16 MPa, compared to under 10 MPa for spirit gum.
Lower emissions and toxicity
As mentioned above, the classification of formaldehyde as a carcinogenic product is a major driver for research into alternative adhesive systems for wood and wood composite products. The alternative solutions in this context are developed to avoid the use of volatile formaldehyde. One example is the adhesive for medium density fibreboards (MDF) designed by Trosa et al. as an alternative to urea formaldehyde adhesives.92It was based on tannins and crosslinked with tris(hydroxymethyl)nitromethane. Tannins are polyphenols naturally occurring in most plants as protein binders. Their structure is built on the base units gallic acid, flavone and phloroglucinol, shown below in Fig. 16.
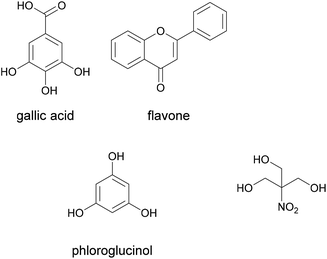 | ||
| Fig. 16 Tannin base units and tris(hydroxymethyl)nitromethane (Trosa et al. 2001).92 | ||
The tannin adhesive not only reduced emissions of formaldehyde to the level that is found in wood, but also showed promising mechanical properties.
In an industrial plant trial, in which an MDF manufactured with a traditional urea formaldehyde resin was tested against one manufactured with 9% tannin adhesive, the tannin MDF showed an internal bond strength of 1.8 MPa and bending strength of 38 MPa compared to 1.8 MPa and 30 MPa in the control.
Another subject of replacement efforts in wood adhesives because of its toxicity is the phenol in phenol formaldehyde resins. A popular alternative is lignin, shown in Fig. 17. Research on the use of lignin as an adhesive for different wood products has been conducted for over 50 years.93 While a lignin content of 15–30% was originally found to be the limit after which properties deteriorate compared to the original adhesives, more progress has been made recently.94 One example was presented by Kalami et al., who used corn stow lignin and replaced 100% of the phenol in a plywood adhesive with no impact on the lap shear performance.30
The two strategies above were combined by Rhazi et al., who used both tannins and lignosulfonates to make plywood adhesives.95 The lignosulfonates were glyoxalated prior to use, so that the use of free formaldehyde was avoided and formaldehyde emissions from the adhesive could be eliminated.
Lower quantities of adhesive and avoidance of organic solvents
Environmental benefits are not only achieved when the toxic substances are eliminated from the adhesive formulation, but also when the overall amount of adhesive needed is decreased. This can reduce the energy needed for production, transport and application of the adhesive and lessen any damage and toxicity associated with these steps.Umemura et al. demonstrated that bio-based adhesives can in some cases achieve the same effect as petroleum-based adhesives even when applied in smaller quantities.96 Adhesives were prepared from konjac glucomannan, a water-soluble polysaccharide extracted from the tuber of the Amorphophallus konjac, or devil's tongue plant, and from chitosan, a polysaccharide prepared through deacetylation of chitin, a compound that can be extracted from the epidermis of crustaceans such as crabs and shrimps.
These adhesives were then compared to urea formaldehyde adhesives for plywood bonding. While the dry shear strength of the konjac glucomannan adhesive was lower than that of the urea formaldehyde adhesive (1.4 MPa vs. 1.8 MPa), the chitosan performed comparatively well (2.1 MPa). Impressively, only 8 g m−2 of the konjac glucomannan adhesive and 16 g m−2 of the chitosan adhesive were used compared to 74 g m−2 of the urea formaldehyde adhesive.
The wet bonding of both adhesives was however much lower than that observed in the urea formaldehyde, and would need to be improved so that the advantage of the low required quantities can be fully exploited.
A similar emission-reducing effect is achieved when water can be used instead of organic solvents. This is the case for example for the bacterial polysaccharide FucoPol. FucoPol is synthesised by the bacterium Enterobacter A47 from glycerol, and was tested as an adhesive by Araújo et al.97 It was prepared as a 7.6% solution in deionised water, and used for bonding wood-wood, glass-glass, cellulose acetate-cellulose acetate and cardboard-cardboard joints. The shear strength of the adhesive was then evaluated and compared to that of UHU universal glue.
The polysaccharide showed a similar shear strength to the UHU reference for wood and cardboard joints. For wood, the joint was either not broken at 742 kPa, which was the maximum strength of the used equipment, or wood failure was observed. For cardboard, the FucoPol adhesive showed a shear bond strength of 416 kPa compared to 425 kPa shown by UHU universal glue.
However, cohesive failure was observed for glass and cellulose acetate substrates, even though the shear strength of 115 kPa and 153 kPa respectively was superior to the 68 kPa and 79 kPa displayed by the reference. While this kind of adhesive would be limited to certain applications, the low quantity of adhesive needed and the fact that water can be used as a solvent indicate a high environmental compatibility.
The water solubility of natural products, in this case whey protein, a by-product of cheese making, was also used by Gao et al. to make an aqueous adhesive for glue laminated timber.98 The whey protein could be dissolved in water to be reacted with the polyisocyanate pMDI and thus partially replaced the polyvinyl acetate emulsions usually employed in emulsion polymerisation isocyanate (EPI) adhesives. Due to the water solubility, no emulsifying agents were necessary for the whey protein. After formulation with polyvinyl acetate, polyvinyl alcohol and calcium carbonate filler, the adhesive containing 55% of whey protein isolate showed a dry strength of 13 MPa and wet strength of 6.8 MPa. This is comparable to the dry and wet strengths of 13 MPa and 6.4 MPa measured for an unspecified commercial EPI adhesive.
Biodegradability
Especially for protein-based adhesives, the susceptibility to hydrolysis and therefore degradation of the polymer backbone structure in contact with water is high. This can often be a problem when exposure to high humidity during the service life is expected, but it can also be an advantage where recyclability of the bonded products is required. This is especially true for products such as adhesive tapes, which can potentially be made fully degradable if renewable, polyester-based materials are used.99 The same cannot be achieved for example with tapes based on polyolefin or acrylic adhesives.A biodegradable adhesive for aluminium, steel and Teflon bonding was developed by Jenkins et al., aiming for easy recyclability of the bonded products.100 The adhesive was based on polylactic acid, a polymer both bio-based and biodegradable, and 3,4-(methylenedioxy)mandelic acid oligomers, which served as L-DOPA mimics after acid treatment.101 3,4-(methylenedioxy)mandelic acid is shown in Fig. 18. Adhesive strengths of 2.6 MPa, 1.7 MPa and 0.32 MPa were observed for the different substrates.
Choi et al. developed a biodegradable hotmelt adhesive based on coconut oil, polycaprolactone and soy protein isolate.102 The presence of the soy protein increased the softening point from 60 °C with no soy protein isolate to above 75 °C when it was added at a concentration of 40% to the polycaprolactone. Unfortunately, the tensile strength simultaneously decreased from 11 MPa to 2.9 MPa.
The recyclability of urethane linkages has also been addressed in a bio-based polyurethane product. Schneiderman et al. synthesised a polyurethane based on the renewable β-methyl-δ-valerolactone (MVL), as shown in Fig. 19.103
After crosslinking, this polyurethane can be easily reverted to the monomer by heating, providing a high quality recycling strategy for products in which it is used. While the polymer was so far only evaluated for foam and thermoplastic applications, it could also be useful for creating crosslinked and yet recyclable adhesives.
An overview over the different advantages regarding their human and environmental toxicity that bio-based adhesives can introduce is given in Table 6.
| Application | Advantage | Example |
|---|---|---|
| Biomedical adhesives | Chemical similarity to human body, often pseudoplastic behaviour, gelation ability, water binding capacity, biodegradability | Polysaccharides,86 fibrin glue88 |
| Bone glue | Biocompatibility enables endogenous bone repair | 2-Component adhesive from dextran and chitosan90 |
| Adhesive for theatrical props on skin | Reduced skin irritation | Polyvinyl alcohol, polybutylene and guar gum adhesive91 |
| Medium density fibre boards adhesive | Lower formaldehyde emissions (level found in wood) | Tannin-tris(hydroxmethyl)nitromethane adhesive92 |
| Plywood adhesive | No toxic phenol, lower formaldehyde emissions | Lignin adhesive,30 adhesive from glyoxylated lignosulfonates and tannins95 |
| Plywood adhesive | Lower quantity needed: 8 g m−2 instead of 74 g m−2 | Konjac glucomannan adhesive96 |
| Wood, glass, cellulose acetate and cardboard adhesive | Replacement of organic solvents with water, reduction of solvent emissions | FucoPol bacterial polysaccharide97 |
| Adhesive for glue laminated timber | Water soluble, no need for emulsifying agents used in EPI adhesives | Whey protein-MDI adhesive98 |
| Aluminium, steel, Teflon adhesive | Easy recycling | Adhesive from polylactic acid and 3,4-(methylenedioxy)mandelic acid,100 polyurethane from β-methyl-δ-valerolactone103 |
| Hotmelt adhesive | Biodegradability | Coconut oil, polycaprolactone and soy protein adhesive102 |
Performance of bio-based adhesives compared to traditional solutions
While additional functionality, sustainability and environmental compatibility can be strong advantages for new adhesives, their performance remains a critical factor that decides if the developed adhesive can be commercialised successfully. Therefore, this last section is dedicated to the assessment of bonding performances and furthermore to other factors that affect the performance, such as price, handling and ease of application.Adhesive strength of bio-based products compared to traditional adhesives
There are several bio-based adhesives reported in the literature not only with comparable but with superior adhesive strength compared to commercial adhesives. It seems that especially polyurethane adhesives based on vegetable oil polyols have a high potential. One example is the bio-based polyol described above that was developed by Kong et al. from canola oil and 1,3-propanediol and crosslinked with pMDI.73 It showed comparable adhesion but better chemical resistance to hot water compared to the commercial polyurethane adhesives Henkel MACROPLAST SIA-116, Gorilla glue and Titebond polyurethane glue. Unfortunately, the fact that only the name of the supplier and no exact product reference is given for the latter two commercial adhesives raises the question whether these results could easily be replicated.In an example published by Ang et al., a polyester polyol was made from epoxidised palm oil and phthalic acid.104 It was then crosslinked with pMDI and glycerol at an excess of isocyanate groups to hydroxyl groups of 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The lap shear strength was found to be 5.3 MPa and therefore nearly twice as high as that of two commercial adhesives, Titebond and Weldbond, with which it was compared. Furthermore, the commercial adhesives displayed cohesive or adhesive failures, while the developed adhesive only showed substrate failure. While this is impressive, it is probably due to the fact that both commercial adhesives tested were polyvinyl-acetate-based and not crosslinked polyurethanes like the developed product. The comparison with a commercial polyurethane would have perhaps been more revealing regarding the potential of the bio-based product.
1. The lap shear strength was found to be 5.3 MPa and therefore nearly twice as high as that of two commercial adhesives, Titebond and Weldbond, with which it was compared. Furthermore, the commercial adhesives displayed cohesive or adhesive failures, while the developed adhesive only showed substrate failure. While this is impressive, it is probably due to the fact that both commercial adhesives tested were polyvinyl-acetate-based and not crosslinked polyurethanes like the developed product. The comparison with a commercial polyurethane would have perhaps been more revealing regarding the potential of the bio-based product.
The assessments of other authors show similar problems. Sahoo et al. claim that their adhesive made from partially bio-based isocyanate and castor oil shows a lap shear strength 2 to 4 times greater than that of commercial adhesives. The commercial adhesives in question were however not examined in the same lab. Instead, their data was taken from a separate publication.38 The article referenced again examined the commercial polyvinyl acetate adhesives Titebond and Weldbond.105
Somani et al. also found that the lap shear strength of their adhesive based on castor oil, different glycols and diisocyanates was ten times higher than that of a commercial adhesive.106 The assessment of the commercial adhesive however seems to come from a publication written 5 years prior.107 In this publication, again only the name of the suppliers, specifically Dunlop, Superchem, Chandra's and Fevibond, are given, but the exact nature of the adhesive is not unmistakeably specified.
Malik et al. also find the lap shear strength of their adhesive based on a canola oil polyether–polyester to be significantly higher than that of a commercial wood adhesive, without specifying which commercial adhesive was tested.37
Another example in which an adhesive based on a bio-based monomer showed better properties than the previous, non-bio-based solution was reported by Berlanga Duarte et al. and concerned dental fillers.108 The bio-based monomer isosorbide was modified with isophorone diisocyanate and hydroxyethylmethacrylate (HEMA) and compared to the traditional matrix material bisphenol A glycerolate dimethacrylate (bis-GMA) as shown in Fig. 20 and 21.
The isosorbide-based alternative showed overall adequate properties as well as 24% lower volume reduction than the reference and water sorption of only 17 μg mm−3 compared to 25 μg mm−3 for the reference.
However, this effect was apparently due to the fact that oligomers were formed prior to the hardening of the matrix, so that fewer double bonds were available, resulting in higher conversion and a higher crosslinking density, and not due to intrinsic properties of the bio-based monomer.
Protein-based adhesives are more commonly compared to the necessary standards for certain wood products than to commercial adhesives. When mixed with those commercial adhesives, they can however be used to improve their performance.
This was for example patented by Breyer et al., who modified a urea formaldehyde resin for particle boards with soy protein.109 When pressing times of 270 s or 300 s were used, the presence of 25% soy protein improved the internal bond strength of the boards from 85 psi to 145 psi and from 60 psi to 160 psi respectively. The internal bond strength was not improved when pressing times of 240 s were used, leading the authors to theorise that the protein needs longer periods to develop its full strength.
Overall, the reporting of superior performances of bio-based adhesives has some room for improvement. It seems clear however that they have potential to rival or improve traditional adhesives where performance is concerned.
Other commercially relevant advantages
Apart from their performance, bio-based adhesives can offer other commercially relevant advantages. The most important one is pricing. Especially proteins, such as waste animal protein, can be obtained at low cost.One such example was published by Hse et al., who hydrolysed soy flour in alkaline medium in the presence of phenol, and incorporated it into a phenol formaldehyde resin for the manufacture of oriental strand board.110 Based on an estimated price of soy flour of $0.12 per lb and of phenol of $0.40 per lb, the authors estimate a 30% substitution of the phenol to result in a 20% material cost saving while maintaining a comparable performance.
Imam et al. also formulated an adhesive based on starch, polyvinyl alcohol and hexamethoxymethylmelamine with citric acid as a catalyst.27 This adhesive was not only formaldehyde-free but due to the fact that starch is derived from a commodity crop and produced in surplus, the authors expect it to cost less than the phenol formaldehyde resin it was designed to replace.
Another advantage is easier handling. Protein adhesives need lower press temperatures than formaldehyde resins, and can bind to wood with higher moisture contents.111
This can also be true for other types of adhesives. Monisha et al. for example synthesised benzoxazine resins both based on bisphenol A and bio-based cardanol.112 Due to the lower viscosity of the cardanol-based resin, the use of solvent was avoided both in the synthesis and during the application.
A summary of the performance-related advantages of bio-based adhesives is presented in Table 7.
| Advantage | Example |
|---|---|
| Higher adhesive strength | Polyurethane from palm oil polyol,104 polyurethane from partially bio-based isocyanate and castor oil,38 polyurethane based on castor oil,106 polyether-polyester based on canola oil,37 protein-urea formaldehyde mixture for particle boards109 |
| Better chemical resistance | Polyurethane from canola oil and 1,3-propanediol polyol73 |
| Lower volume reduction | Isosorbide-based dental adhesive108 |
| Low cost | Soy flour protein adhesive for oriental strand board,110 starch – polyvinyl alcohol – melamine adhesive27 |
| Easier handling due to lower press temperatures and higher moisture tolerance | Protein adhesives111 |
| Avoiding solvents due to lower viscosity | Cardanol-benzoxazine resin112 |
Perspective
This last section aims to give a perspective on the future of bio-based adhesives by addressing some remaining challenges and giving examples of interesting recent progress. As challenges are often consistent for each raw material across different types of adhesive applications, the sections are sorted by types of renewable source. Challenges and recent progress in the field of adhesives based on polysaccharides, proteins, lignin, vegetable oils and small molecule monomers based on renewable materials will be addressed in turn, as well as some recent developments regarding adhesive-free bonding. A summary is given in Table 8.| Renewable resource | Challenges | Recent progress |
|---|---|---|
| Polysaccharides and proteins | Decrease susceptibility to hydrolytic degradation111,113–116 | Statistical design,117 enzyme layer deposition,119 amphiphilic battery binder,121 seaweed120,122 |
| Lignin | Increase reactivity to condensation reactions,123,124 improve reproducibility and definition of structure125 | Vitrimer with reversible bonding126 |
| Vegetable oils and renewable monomers | Non-food sources127 | Flame retardant polymers,128,129 lactic acid – vegetable oil combination,130 nylon – vegetable oil combination,131 reversible crosslinking,132 self-healing133 |
Polysaccharides and proteins
Examples are the crosslinking of a protein produced by Bacillus subtilis using glutaraldehyde, shown in Fig. 22, by Cuesta-Garrote et al., which resulted in an increase of the molecular weight from 32 kDa to up to 250 kDa, the crosslinking of a soy protein with a bisphenol-A-epoxy resin by Xu et al., which resulted in an increase of the wet shear strength by 55%, as well as the incorporation of L-DOPA into a soy protein for plywood bonding by Liu et al., which increased the wet shear strength from below 1 MPa to above 3 MPa.111,113,114
An interesting solution was recently proposed by Paiva et al., who oxidised xanthan gum using sodium metaperiodate (NaIO4) before testing it as an adhesive for cork.115 The oxidation was observed to increase the tensile strength of a cork joint glued with 6% adhesive from around 1 MPa to 1.8 MPa.
A solution for the water susceptibility of protein adhesives was also proposed by Zhang et al. for plywood applications.116 A soybean soluble polysaccharide was first oxidised using sodium metaperiodate and then turned into a hyperbranched structure by reaction with a polyamide based on succinic anhydride and diethylenetriamine. 3 g of this hyperbranched modified polysaccharide was added to a solution containing 12 g soy protein isolate, 83 g water and 2 g triglycidyl amine. Compared to pure soy protein isolate, this increased the wet shear strength from 0.4 MPa to 1.1 MPa.
An interesting method for the application of protein adhesives was put forward by Ruediger et al. concerning the protein casein.118,119 The enzyme chymosin, an aspartic protease, was adsorbed onto a glass surface and used to destabilise casein micelles, resulting in the deposition of a casein layer at the surface. Where two surfaces were placed at a distance below 375 μm from each other, an adhesive casein layer formed between them. In an Epprecht twistometer, the adhesive strength was determined to be 7.8 MPa for glass surfaces. While this is only about a quarter of the strength that can be achieved using synthetic adhesives such as polyurethane or epoxy based adhesives in a comparable situation, it represents a pressure-free application method and an interesting way to control the adhesive layer.
For polysaccharide-based adhesives, an important area of future developments is the use of new sources for polysaccharides, such as marine plants. Algae present a good source of renewable materials because of their abundance and relative irrelevance for food production. One example of using marine-based polysaccharides for adhesive applications was presented by Chhatbar et al., grafted polyvinylpyrrolidone onto a sulfated seaweed polysaccharide in a weight ratio of 1–2.5 to 1 under microwave irradiation.120 A 5% aqueous dispersion was applied onto paper and wood samples and dried for 24 h. In general terms, adhesive properties of the polymer were confirmed. Unfortunately, no evaluation of the adhesive strength was done except for stating that the pieces could not be pulled apart afterwards.
Alginates were also used as a binder by Lacoste et al. for a composite material based on wood fibres and recycled cotton fibres.122 With thermal conductivities between 0.078 and 0.089 W m−1 K−1, the composites possessed good insulating properties. Crosslinking of the alginate binder with 8% glutaraldehyde increased the bending Young's modulus from 10 MPa to 17 MPa.
Lastly, an important application area for bio-based adhesives is the tailoring to special requirements of different application areas. One example of this are the binders used in lithium ion batteries. The anodes contain both silicon and carbon conductive materials such as graphite, and in order to achieve a homogeneous distribution, the binder must be compatible with both substances. Kim et al. designed an amphiphilic adhesive inspired by the protein mucin, which contains both a hydrophobic protein backbone and hydrophilic oligosaccharide branches.121
Low molecular weight DNA from salmon sperm was dissolved in water, heated to 80 °C for 10–15 min in order to achieve denaturation and combined with an alginate solution to produce the binder. The binder was successfully used to achieve homogeneous distributions of the different anode components, improving the cyclability of the electrode. As stated by the authors themselves, salmon sperm is a not suitable resource for the production of battery binders in practical terms, but the design principle of orientating binder design on nature and using bio-based alginates as a resource is a promising concept for the future of adhesives.
Lignin
 | ||
| Fig. 23 Conversion of phenolic OH to aliphatic aldeyhde (Foyer et al. 2016)123 and n-dodecylmercaptan. | ||
Another method to increase the lignin reactivity to condensation reactions is its demethylation.124 Li et al. used sulphur, NaSH, Na2SO3 and n-docecylmercaptan to turn the methoxy groups into phenolic hydroxyl groups. It was then used to substitute 30% of phenol in a phenol formaldehyde resin for plywood. The demethylation with Na2SO3 increased the bond strength of the resin compared to a resin with unmodified lignin from 0.9 MPa to 1.1 MPa, and decreased the formaldehyde emissions from 0.9 mg L−1 to 0.4 mg L−1, indicating a more thorough crosslinking reaction.
A second problem that remains with lignin-based adhesives is the heterogeneity of the lignin and the associated difficulty of creating well-defined and reproducible structures. A possible approach to overcome this is solvent fractionation of the lignin prior to its use, though the impact on product cost, sustainability and industrial applicability need to be evaluated.134 Gioia et al. demonstrated that the molecular weight as well as the quantity of phenolic hydroxyl groups, aliphatic hydroxyl groups, carboxylic acid groups and condensed phenol moieties can be controlled via extractions with organic solvents such as ethyl acetate, ethanol, methanol and acetone.125 These properties in turn had an effect on the tensile strength of epoxy resins based on the different lignin fractions.
Griffini et al. also demonstrated that polyurethane coatings with good adhesion to different substrates such as wood, glass and metal can be made from the 2-methyltetrahydrofuran extracted fraction of Kraft lignin.135
Vegetable oils and small molecule renewable monomers
Similarly, Jian et al. improved the tensile strength of a soybean oil based adhesive by adding a castor oil-based nylon oligomer to reduce the brittleness.131 The tensile strength increased from 0.4 MPa for the control sample containing no nylon oligomer to 26 MPa for the sample containing the highest molecular weight nylon oligomer (1408 g mol−1).
A promising way to introduce renewable monomers into adhesives is where they provide additional functionality and therefore added value at the same time. A recent example of such a case is the development of flame-retardant adhesives from bio-based monomers. Flame retardant adhesive tapes have been developed by Wang et al. based on soybean oil, and a flame retardant epoxy resin has been developed by Wang et al. based on vanillin, in both cases through the covalent incorporation of phosphorous compounds into a polymer based on the renewable monomer.128,129
Another recent example where new functionality was provided through the renewable monomers are the thermosetting resins proposed by Duval et al. based on furan-functionalised tannins and bis-maleimides.132 As the network was formed using a Diels–Alder reaction between the furan-moieties on the tannin and the bis-maleimide crosslinker, shown in Fig. 24, the crosslinking could be reversed at a temperature of 120 °C, providing a good recycling route.
In a similar example, a self-healing polyurethane was synthesised by Ghosh et al. from a bio-based dimer acid and glycerol.133 A hyperbranched structure was first obtained from esterification of the vegetable oil-based dimer acid and glycerol, which was then reacted with polycaprolactone and an excess of different diisocyanates. The resulting polyurethane showed an intrinsic self-healing of 100% under microwave irradiation.
Bonding without adhesives
An important research area into increasing the sustainability of adhesives for lignocellulosic products that should be mentioned is bonding without adhesives. This means that the surface of the wood is modified to activate adhesive forces without adding any polymeric resins. One example of this type of research is the work of Nakaya et al., who used ionic liquids to depolymerise some of the wood components at the surface, such as polysaccharides and lignin, and repolymerised them to generate adhesion for application in plywood.136 Imidazole hydrochloride was used as an ionic liquid, and water and glucose were also added to the solution in a ratio of 9/3/2.About 83 g m−2 of the ionic liquid was then applied to both surfaces of the core layer of a three-ply plywood, and hot-pressed at 2.9 MPa for 60 min. So far, only comparatively low bond strengths of 0,6 MPa could be achieved. It was however observed that in addition to the solubilisation and repolymerisation of the wood components, which achieved chemical bonding between the plywood layers, softening of the cell walls and the resulting entwining during the hot press process also contributed to the adhesive forces developed in this experiment.
Conclusions
Overall, bio-based adhesives have a number of advantages beyond their renewability, a short summary of which is given in Table 9. Renewable materials can be used in adhesive applications in many ways. These include the incorporation of biopolymers that already have adhesive properties into adhesives and the use of bio-based monomers in the synthesis of new adhesive polymer structures. Both the complete replacement of petroleum-based adhesives and the mixing of bio-based adhesives with petroleum-based adhesives are promising venues.| Property | Advantage |
|---|---|
| Molecular architecture differs from petroleum-based adhesives | Performance benefits and new functionalities |
| Lipophilic chains (vegetable oils) | Water resistance |
| Low toxicity, biodegradability | Medicinal applications and environmental benefits |
| Biodegradability and low toxicity | Easy recycling, environmental benefits |
| Low cost raw materials, easier handling | Cost savings |
While a large pool of experience with biopolymers in adhesives exists due to their historic applications, the variety and availability of different renewable materials, monomers or biopolymers, is also growing alongside the demand for their use. Outside of the field of adhesives, knowledge about the properties of bio-based polymeric materials can also be transferred from coatings, foams and plastics.
The advantages conferred by bio-based adhesives can be leveraged in different areas. High functionalities, as for example in the case of lignin, can serve to increase curing speed and strengthen the adhesive bond. Long alkyl chains as found in vegetable oils can be used to promote water resistance. Inherent biodegradability of many bio-based materials can ease the recycling process, and low toxicity as well as better compatibility with the environment can save efforts and costs in terms of health and safety regulations, as well where future restrictions are expected. Furthermore, new functionalities can be introduced in adhesives using bio-based components. Examples for this are proteins, which change not only their primary, but also secondary and tertiary structure depending on the conditions, or nanocellulose, which can act both as a binder and as a structural reinforcement. Lastly, increasing amounts of renewable materials such as wood and natural fibre-reinforced composites are used in construction, the automotive sector and consumer goods. The compatibility of bio-based adhesives based on similar resources with these materials offers further advantages. Altogether, bio-based adhesives can be introduced in many different functions and applications.
Even though the market for bio-based adhesives is still small, and while the reporting of bio-based adhesive performance could be in some cases improved, it has been shown that they can compete with petroleum-based adhesives. In conclusion, bio-based adhesives present an interesting alternative to traditional petroleum-based products that have great potential to enter new applications and experience further growth.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author would like to thank Dr Tobias Robert, Dr Niall O'Toole and Dr Stefan Friebel for their advice and input writing this review.Notes and references
- (a) F. J. Díaz López and C. Montalvo, J. Cleaner Prod., 2015, 102, 30–43 CrossRef; (b) Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354 CrossRef CAS PubMed.
- (a) M. R. Finlay, J. Ind. Ecol., 2003, 7, 33–46 CrossRef; (b) L. C. Speight, Bio-Based Feedstocks for Adhesives and Sealants: Everything Old is New Again, available at: https: //www.adhesivesmag.com/articles/93912-bio-based-feedstocks-for-adhesives-and-sealants-everything-old-is-new-again, accessed 1 November 2018.
- D. Grago, Biobasierte Beschichtungen: Nachhaltigkeit 2.0, available at: https: //www.farbeundlack.de/Wissenschaft-Technik/Rohstoffe/Biobasierte-Beschichtungen-Nachhaltigkeit-2.0, accessed 1 November 2018.
- J. Gesthuizen and T. Robert, Nachwachsende Rohstoffe: “Es gibt viele Monomere, die über neuartige Strukturelemente verfügen”, available at: https: //www.farbeundlack.de/Wissenschaft-Technik/Nachwachsende-Rohstoffe-Es-gibt-viele-Monomere-die-ueber-neuartige-Strukturelemente-verfuegen, accessed 1 November 2018.
- B. Müller and W. Rath, Formulierung von Kleb- und Dichtstoffen. Das kompetente Lehrbuch für Studium und Praxis, Vincentz, Hannover, 2004 Search PubMed.
- G. Habenicht, Kleben. Leitfaden für die praktische Anwendung und Ausbildung, Vieweg + Teubner Verlag, Wiesbaden, 1995 Search PubMed.
- (a) S. K. De, in Handbook of Adhesive Technology, ed. A. Pizzi and K. L. Mittal, 2003, vol. 23, pp. 508–511 Search PubMed; (b) W. F. Harrington, in Handbook of Adhesive Technology, ed. A. Pizzi and K. L. Mittal, 2003, vol. 24, pp. 512–527 Search PubMed; (c) K. Geddes, in Handbook of Adhesive Technology, ed. A. Pizzi and K. L. Mittal, 2003, vol. 35, pp. 712–722 Search PubMed.
- A. Pizzi, in Handbook of Adhesive Technology, ed. A. Pizzi and K. L. Mittal, 2003, vol. 37, pp. 730–736 Search PubMed.
- D. G. Lay and P. Cranley, in Handbook of Adhesive Technology, ed. A. Pizzi and K. L. Mittal, 2003, vol. 34, pp. 688–711 Search PubMed.
- F. Ferdosian, Z. Pan, G. Gao and B. Zhao, Polymers, 2017, 9, 70 CrossRef.
- D. J. Damico, in Handbook of Adhesive Technology, ed. A. Pizzi and K. L. Mittal, 2003, vol. 38, pp. 737–749 Search PubMed.
- R. Vendamme, N. Schüwer and W. Eevers, J. Appl. Polym. Sci., 2014, 131, 40669 CrossRef.
- C. R. Frihart, C. G. Hunt and M. J. Birkeland, in Recent Advances in Adhesion Science and Technology in Honor of Dr. Kash Mittal, ed. W. Gutowski and H. Dodiuk, Taylor and Francis, Hoboken, 2013, vol. 16, pp. 277–291 Search PubMed.
- E. F. Gómez and F. C. Michel, Polym. Degrad. Stab., 2013, 98, 2583–2591 CrossRef.
- Formaldehyde – Brief Profile – ECHA, available at: https: //echa.europa.eu/brief-profile/-/briefprofile/100.000.002, accessed 12 January 2018.
- S. Krishnan, Investment Analysis of European Adhesives and Sealants Market, Frost & Sullivan, 2015 Search PubMed.
- Frost & Sullivan , North American and European Construction Adhesives and Sealants Market, Forecast to 2022. Emphasis on Light Weighting in Building Components to Drive Revenue Growth.
- W. Maaßen, Echt oder falsch? - das ist hier die Frage! Fälschungen und Fälscher in der Philatelie, Phil Creativ, Schwalmtal, 2003, Bd. 3 Search PubMed.
- W. Maaßen, in Echt oder falsch? - das ist hier die Frage! Fälschungen und Fälscher in der Philatelie, Phil Creativ, Schwalmtal, 2003, pp. 234–237 Search PubMed.
- C. R. Frihart, For. Prod. J., 2015, 65, 4–8 Search PubMed.
- H. B. Sweatt, J. Chem. Educ., 1946, 23, 192 CrossRef CAS.
- N. C. Schellmann, Stud. Conserv., 2013, 52, 55–66 CrossRef.
- R. Quack and C. Yaacoub, Klebstoffe aus nachwachsenden Rohstoffen. Perspektiven und Grenzen am Beispiel der Bereiche Konsumerprodukte, Papierherstellung und -verarbeitung, Lebensmittelverpackung, Pharmazie und Medizin: Studie, Franz-Patat-Zentrum, Braunschweig, 1998 Search PubMed.
- I. Khan and B. T. Poh, J. Polym. Environ., 2011, 19, 793–811 CrossRef CAS https://link.springer.com/content/pdf/10.1007%2Fs10924-011-0299-z.pdf .
- S. D. Desai, J. V. Patel and V. K. Sinha, Int. J. Adhes. Adhes., 2003, 23, 393–399 CrossRef CAS.
- Z. He, Bio-based Wood Adhesives. Preparation, Characterization, and Testing, CRC Press, Boca Raton, 2017 Search PubMed.
- S. H. Imam, S. H. Gordon, L. Mao and L. Chen, Polym. Degrad. Stab., 2001, 73, 529–533 CrossRef CAS.
- P. Zheng, Y. Li, F. Li, Y. Ou, Q. Lin and N. Chen, Polymers, 2017, 9, 153 CrossRef.
- M. Fleckenstein, V. Biziks, C. Mai and H. Militz, Eur. J. Wood Wood Prod., 2017, 419, 161 Search PubMed.
- S. Kalami, M. Arefmanesh, E. Master and M. Nejad, J. Appl. Polym. Sci., 2017, 134, 45124 CrossRef.
- M. Wang, M. Leitch and C. Xu, Eur. Polym. J., 2009, 45, 3380–3388 CrossRef CAS.
- Q. Zhang, G. Zhang, J. Xu, C. Gao and Y. Wu, Rev. Adv. Mater. Sci., 2015, 2015, 146–154 Search PubMed http://www.ipme.ru/e-journals/RAMS/no_24015/04_24015_zhang.pdf .
- C. Zhang, T. F. Garrison, S. A. Madbouly and M. R. Kessler, Prog. Polym. Sci., 2017, 71, 91–143 CrossRef CAS.
- M. Stemmelen, F. Pessel, V. Lapinte, S. Caillol, J.-P. Habas and J.-J. Robin, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2434–2444 CrossRef CAS.
- J. Dai, S. Ma, Y. Wu, L. Han, L. Zhang, J. Zhu and X. Liu, Green Chem., 2015, 17, 2383–2392 RSC.
- X.-Y. Jian, Y. He, Y.-D. Li, M. Wang and J.-B. Zeng, Chem. Eng. J., 2017, 326, 875–885 CrossRef CAS.
- M. Malik and R. Kaur, Polym. Eng. Sci., 2017, 46, 3771 Search PubMed.
- S. Sahoo, H. Kalita, S. Mohanty and S. K. Nayak, Macromol. Res., 2017, 772–778 CrossRef CAS.
- E. Jasiukaitytė-Grojzdek, M. Kunaver, D. Kukanja and D. Moderc, Int. J. Adhes. Adhes., 2013, 46, 56–61 CrossRef.
- C. Crestini, H. Lange, M. Sette and D. S. Argyropoulos, Green Chem., 2017, 19, 4104–4121 RSC.
- F. Ferdosian, Y. Zhang, Z. Yuan, M. Anderson and C. Xu, Eur. Polym. J., 2016, 82, 153–165 CrossRef CAS.
- A. Mija, J. C. van der Waal, J.-M. Pin, N. Guigo and E. d. Jong, Constr. Build. Mater., 2017, 139, 594–601 CrossRef CAS.
- D. Liu, H. Chen, P. R. Chang, Q. Wu, K. Li and L. Guan, Bioresour. Technol., 2010, 101, 6235–6241 CrossRef CAS PubMed.
- C. Li, H. Li, S. Zhang and J. Li, BioResources, 2014, 9, 5448–5460 Search PubMed.
- R. J. Stewart, T. C. Ransom and V. Hlady, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 757–771 CrossRef CAS PubMed.
- I. Manolakis, B. A. J. Noordover, R. Vendamme and W. Eevers, Macromol. Rapid Commun., 2014, 35, 71–76 CrossRef CAS PubMed.
- Y. Otsuka, M. Nakamura, K. Shigehara, K. Sugimura, E. Masai, S. Ohara and Y. Katayama, Appl. Microbiol. Biotechnol., 2006, 71, 608–614 CrossRef CAS PubMed.
- Y. Hasegawa, K. Shikinaka, Y. Katayama, S. Kajita, E. Masai, M. Nakamura, Y. Otsuka, S. Ohara and K. Shigehara, Sen-i Gakkaishi, 2009, 65, 359–362 CrossRef CAS.
- T. Michinobu, M. Hishida, M. Sato, Y. Katayama, E. Masai, M. Nakamura, Y. Otsuka, S. Ohara and K. Shigehara, Polym. J., 2008, 40, 68 CrossRef CAS.
- C. Fan, C. Pang, X. Liu, J. Ma and H. Gao, Green Chem., 2016, 18, 6320–6328 RSC.
- H. Pawlik and A. Prociak, J. Polym. Environ., 2012, 20, 438–445 CrossRef CAS.
- R. Duarah and N. Karak, RSC Adv., 2015, 5, 64456–64465 RSC.
- G. Qi and X. S. Sun, J. Am. Oil Chem. Soc., 2011, 88, 271–281 CrossRef CAS.
- S. D. Desai, A. L. Emanuel and V. K. Sinha, J. Polym. Res., 2003, 10, 275–281 CrossRef CAS.
- S. Garnier, A. Pizzi, O. C. Vorster and L. Halasz, J. Appl. Polym. Sci., 2001, 81, 1634–1642 CrossRef CAS.
- W. Sridach, S. Jonjankiat and T. Wittaya, J. Adhes. Sci. Technol., 2013, 27, 1727–1738 CrossRef CAS.
- C. Q. Yang, X. Wang and I.-S. Kang, Text. Res. J., 1997, 67, 334–342 CrossRef CAS.
- P. Nordqvist, N. Nordgren, F. Khabbaz and E. Malmström, Ind. Crops Prod., 2013, 44, 246–252 CrossRef CAS https://ac.els-cdn.com/S0926669012006103/1-s2.0-S0926669012006103-main.pdf?_tid=2ea61a22-f06a-11e7–9de1–00000aab0f01&acdnat=1514972630_20ca50eedc849553a9e42e5877e37885 .
- K. Umemura, O. Sugihara and S. Kawai, J. Wood Sci., 2013, 59, 203–208 CrossRef CAS.
- K. Umemura, T. Ueda and S. Kawai, J. Wood Sci., 2012, 58, 38–45 CrossRef CAS https://link.springer.com/content/pdf/10.1007%2Fs10086-011-1214-x.pdf .
- M. Tajvidi, D. J. Gardner and D. W. Bousfield, J. Renewable Mater., 2016, 4, 365–376 CrossRef CAS.
- E. Amini, M. Tajvidi, D. J. Gardner and D. W. Bousfield, BioResources, 2017, 12(2), 4093–4110 CrossRef CAS.
- A. H. Tayeb, E. Amini, S. Ghasemi and M. Tajvidi, Molecules, 2018, 23, 2684 CrossRef PubMed.
- Y. Kojima, A. Isa, H. Kobori, S. Suzuki, H. Ito, R. Makise and M. Okamoto, Materials, 2014, 7, 6853–6864 CrossRef CAS PubMed.
- H. N. Cheng, M. K. Dowd and Z. He, Ind. Crops Prod., 2013, 46, 399–403 CrossRef CAS https://ac.els-cdn.com/S0926669013001015/1-s2.0-S0926669013001015-main.pdf?_tid=91c6eb16-e19e-11e7-bfaa-00000aab0f26&acdnat=1513345871_8afe31a0239e604133e7e6526b3bba79 .
- P. Nordqvist, E. Johansson, F. Khabbaz and E. Malmström, Ind. Crops Prod., 2013, 51, 51–61 CrossRef CAS.
- S. K. Park, D. H. Bae and N. S. Hettiarachchy, J. Am. Oil Chem. Soc., 2000, 77, 1223–1227 CrossRef CAS.
- J. M. S. Renkema, H. Gruppen and T. van Vliet, J. Agric. Food Chem., 2002, 50, 6064–6071 CrossRef CAS PubMed.
- D. Wang, X. S. Sun, G. Yang and Y. Wang, Trans. ASABE, 2009, 52, 173–177 CAS.
- C. S. Freire, S. C. Fernandes, A. J. Silvestre and C. Pascoal Neto, Holzforschung, 2013, 67, 603–612 CAS.
- T. Robert, Prog. Org. Coat., 2015, 78, 287–292 CrossRef CAS.
- E. D. Sitz, D. S. Bajwa, D. C. Webster, E. M. Monono, D. P. Wiesenborn and S. G. Bajwa, Ind. Crops Prod., 2017, 400–408 CrossRef CAS.
- X. Kong, G. Liu and J. M. Curtis, Int. J. Adhes. Adhes., 2011, 31, 559–564 CrossRef CAS.
- A. Saetung, A. Rungvichaniwat, P. Tsupphayakorn-ake, P. Bannob, T. Tulyapituk and N. Saetung, J. Polym. Res., 2016, 23, 264 CrossRef.
- C. Li, H. Xiao, X. Wang and T. Zhao, J. Cleaner Prod., 2018, 180, 272–279 CrossRef CAS.
- Y. Li, S.-H. Chou, W. Qian, J. Sung, S. I. Chang and X. S. Sun, J. Am. Oil Chem. Soc., 2017, 94, 713–721 CrossRef CAS.
- Y. Li, X. Luo and S. Hu, in Bio-based Polyols and Polyurethanes, ed. Y. Li, S. Hu and X. Luo, Springer International Publishing, Cham, 2015, pp. 15–43 Search PubMed.
- Z. Petrovic, Polym. Rev., 2008, 48, 109–155 CrossRef CAS.
- R. Chen, C. Zhang and M. R. Kessler, RSC Adv., 2014, 4, 35476–35483 RSC.
- C. Fu, Z. Zheng, Z. Yang, Y. Chen and L. Shen, Prog. Org. Coat., 2014, 77, 53–60 CrossRef CAS.
- M. L. Broeren, M. C. Zijp, S. L. Waaijers-van der Loop, E. H. Heugens, L. Posthuma, E. Worrell and L. Shen, Biofuels, Bioprod. Biorefin., 2017, 11, 701–718 CrossRef CAS.
- B. N. Ames, M. Profet and L. S. Gold, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 7782–7786 CrossRef CAS.
- (a) P. Li, S. Ma, J. Dai, X. Liu, Y. Jiang, S. Wang, J. Wei, J. Chen and J. Zhu, ACS Sustainable Chem. Eng., 2016, 5, 1228–1236 CrossRef; (b) T. Robert and S. Friebel, Green Chem., 2016, 18, 2922–2934 RSC; (c) S.-A. Park, J. Choi, S. Ju, J. Jegal, K. M. Lee, S. Y. Hwang, D. X. Oh and J. Park, Polymer, 2017, 116, 153–159 CrossRef CAS.
- R. S. Boethling, E. Sommer and D. DiFiore, Chem. Rev., 2007, 107, 2207–2227 CrossRef CAS PubMed https://pubs.acs.org/doi/pdf/10.1021/cr050952t .
- (a) B. E. Dale, J. Chem. Technol. Biotechnol., 2003, 78, 1093–1103 CrossRef CAS; (b) C. J. Arntzen and B. E. Dale, Range of Biobased Products, in Biobased industrial products. Priorities for research and commercialization, 1996, vol. 3, pp. 55–73 Search PubMed.
- R. L. Reis, Natural-based polymers for biomedical applications, 2008 Search PubMed.
- R. L. Reis, N. M. Neves, J. F. Mano, M. E. Gomes, A. P. Marques and H. S. Azevedo, in Natural-based polymers for biomedical applications, ed. R. L. Reis and N. M. Neves, Woodhead, Cambridge, 2008, pp. xxiii–xxv Search PubMed.
- V. Bhagat and M. L. Becker, Biomacromolecules, 2017, 18, 3009–3039 CrossRef CAS PubMed https://pubs.acs.org/doi/pdf/10.1021/acs.biomac.7b00969 .
- Fibrin Sealants – test, blood, complications, time, infection, risk, rate, Definition, available at: https://www.surgeryencyclopedia.com/Ce-Fi/Fibrin-Sealants.html, accessed 14 November 2018.
- B. Hoffmann, E. Volkmer, A. Kokott, P. Augat, M. Ohnmacht, N. Sedlmayr, M. Schieker, L. Claes, W. Mutschler and G. Ziegler, J. Mater. Sci.: Mater. Med., 2009, 20, 2001–2009 CrossRef CAS PubMed.
- J. H. Kim, H. J. Min, K. Park and J. Kim, Korean J. Chem. Eng., 2017, 34, 2236–2240 CrossRef CAS.
- A. Trosa and A. Pizzi, Holz Roh- Werkst., 2001, 59, 266–271 CrossRef CAS.
- (a) E. Roffael and W. Rauch, Holzforschung, 1973, 27, 214–217 CrossRef CAS; (b) E. Roffael and B. Dix, Holz Roh- Werkst., 1991, 49, 199 CrossRef CAS.
- (a) N. S. Çetin and N. Özmen, Int. J. Adhes. Adhes., 2002, 22, 481–486 CrossRef; (b) N. S. Çetin and N. Özmen, Int. J. Adhes. Adhes., 2002, 22, 477–480 CrossRef.
- N. Rhazi, M. Oumam, A. Sesbou, H. Hannache, F. Charrier-El Bouhtoury, J.-M. Nunzi, R. Bennacer and M. El Ganaoui, Eur. Phys. J.: Appl. Phys., 2017, 78, 34813 CrossRef.
- K. Umemura, A. Inoue and S. Kawai, J. Wood Sci., 2003, 49, 221–226 CrossRef CAS.
- D. Araújo, V. D. Alves, J. Campos, I. Coelhoso, C. Sevrin, C. Grandfils, F. Freitas and M. A. M. Reis, Int. J. Biol. Macromol., 2016, 92, 383–389 CrossRef PubMed.
- Z. Gao, W. Wang, Z. Zhao and M. Guo, J. Appl. Polym. Sci., 2011, 120, 220–225 CrossRef CAS.
- R. Vendamme, K. Olaerts, M. Gomes, M. Degens, T. Shigematsu and W. Eevers, Biomacromolecules, 2012, 13, 1933–1944 CrossRef CAS PubMed.
- C. L. Jenkins, H. M. Siebert and J. J. Wilker, Macromolecules, 2017, 50, 561–568 CrossRef CAS.
- F. T. Garrison, A. Murawski and L. R. Quirino, Bio-Based Polymers with Potential for Biodegradability, 2016, vol. 8 Search PubMed.
- W. Y. Choi, C. M. Lee and H. J. Park, LWT – Food Sci. Technol., 2006, 39, 591–597 CrossRef CAS.
- D. K. Schneiderman, M. E. Vanderlaan, A. M. Mannion, T. R. Panthani, D. C. Batiste, J. Z. Wang, F. S. Bates, C. W. Macosko and M. A. Hillmyer, ACS Macro Lett., 2016, 5, 515–518 CrossRef CAS.
- K. P. Ang, C. S. Lee, S. F. Cheng and C. H. Chuah, J. Appl. Polym. Sci., 2014, 131, 39967 CrossRef.
- A. Khoon Poh, L. Choy Sin, C. Sit Foon and C. Cheng Hock, J. Adhes. Sci. Technol., 2014, 28, 1020–1033 CrossRef.
- K. P. Somani, S. S. Kansara, N. K. Patel and A. K. Rakshit, Int. J. Adhes. Adhes., 2003, 23, 269–275 CrossRef CAS.
- N. John and R. Joseph, J. Appl. Polym. Sci., 1998, 68, 1185–1189 CrossRef CAS.
- M. L. Berlanga Duarte, L. A. Reyna Medina, P. T. Reyes, S. E. González Pérez and A. M. Herrera González, J. Appl. Polym. Sci., 2017, 134, 433 CrossRef.
- R. A. Breyer, R. H. Carey, X. S. Sun, E.-Z. M. Cheng and J. D. Rivers, US20060234077, 2006.
- C.-Y. Hse, F. Fu and B. S. Bryant, Proceedings of wood adhesives, 2000, http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.446.8268&rep=rep1&type=pdf.
- Y. Liu and K. Li, Macromol. Rapid Commun., 2002, 23, 739–742 CrossRef CAS.
- M. Monisha, S. Shukla and B. Lochab, Green Mater., 2017, 5, 1–30 CrossRef.
- N. Cuesta-Garrote, M. J. Escoto-Palacios, F. Arán-Ais and C. Orgilés-Barceló, Proc. IMechE, 2014, 228, 115–124 CrossRef CAS.
- H. Xu, J. Luo, Q. Gao, S. Zhang and J. Li, BioResources, 2014, 9, 4667–4678 Search PubMed.
- D. Paiva, C. Goncalves, I. Vale, M. M. S. M. Bastos and F. D. Magalhaes, Polymers, 2016, 8, 259 CrossRef.
- Y. Zhang, M. Zhang, M. Chen, J. Luo, X. Li, Q. Gao and J. Li, Chem. Eng. J., 2018, 354, 1032–1041 CrossRef CAS.
- R. Gu, B. Mu and Y. Yang, BioResources, 2016, 11, 8166–8177 CrossRef CAS.
- (a) O. I. Strube, A. A. Ruediger and W. Bremser, Int. J. Adhes. Adhes., 2015, 63, 9–13 CrossRef CAS; (b) A. A. Ruediger, W. Bremser and O. I. Strube, J. Coat. Technol. Res., 2016, 13, 597–611 CrossRef CAS.
- A. A. Ruediger, Dissertation, Universität Paderborn, 2017.
- M. U. Chhatbar and A. K. Siddhanta, J. Appl. Polym. Sci., 2015, 132, 42383 CrossRef.
- S. Kim, Y. K. Jeong, Y. Wang, H. Lee and J. W. Choi, Adv. Mater., 2018, 30, 1707594 CrossRef PubMed.
- C. Lacoste, R. El Hage, A. Bergeret, S. Corn and P. Lacroix, Carbohydr. Polym., 2018, 184, 1–8 CrossRef CAS PubMed.
- G. Foyer, B.-H. Chanfi, B. Boutevin, S. Caillol and G. David, Eur. Polym. J., 2016, 74, 296–309 CrossRef CAS.
- J. Li, W. Wang, S. Zhang, Q. Gao, W. Zhang and J. Li, RSC Adv., 2016, 6, 67435–67443 RSC.
- C. Gioia, G. Lo Re, M. Lawoko and L. Berglund, J. Am. Chem. Soc., 2018, 140, 4054–4061 CrossRef CAS PubMed.
- S. Zhang, T. Liu, C. Hao, L. Wang, J. Han, H. Liu and J. Zhang, Green Chem., 2018, 20, 2995–3000 RSC.
- N. Kim, Y. Li and X. S. Sun, Ind. Crops Prod., 2015, 64, 1–8 CrossRef CAS.
- S. Wang, S. Ma, C. Xu, Y. Liu, J. Dai, Z. Wang, X. Liu, J. Chen, X. Shen, J. Wei and J. Zhu, Macromolecules, 2017, 50, 1892–1901 CrossRef CAS.
- X.-L. Wang, L. Chen, J.-N. Wu, T. Fu and Y.-Z. Wang, ACS Sustainable Chem. Eng., 2017, 5, 3353–3361 CrossRef CAS.
- Y. Li, D. Wang and X. S. Sun, RSC Adv., 2015, 5, 27256–27265 RSC.
- X.-Y. Jian, X.-P. An, Y.-D. Li, J.-H. Chen, M. Wang and J.-B. Zeng, Macromolecules, 2017, 50, 5729–5738 CrossRef CAS.
- A. Duval, G. Couture, S. Caillol and L. Averous, ACS Sustainable Chem. Eng., 2017, 5, 1199–1207 CrossRef CAS.
- T. Ghosh and N. Karak, ACS Sustainable Chem. Eng., 2018, 6, 4370–4381 CrossRef CAS.
- J. Domínguez-Robles, T. Tamminen, T. Liitiä, M. S. Peresin, A. Rodríguez and A.-S. Jääskeläinen, Int. J. Biol. Macromol., 2018, 106, 979–987 CrossRef PubMed.
- G. Griffini, V. Passoni, R. Suriano, M. Levi and S. Turri, ACS Sustainable Chem. Eng., 2015, 3, 1145–1154 CrossRef CAS.
- N. Nakaya, T. Hosoya and H. Miyafuji, J. Wood Sci., 2018, 64, 794–801 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |